Tunneling in the Hydrogen-Transfer Reaction from a Vitamin E Analog to an Inclusion Complex of 2,2-Diphenyl-1-picrylhydrazyl Radical with β-Cyclodextrin in an Aqueous Buffer Solution at Ambient Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spectral and Kinetic Measurements
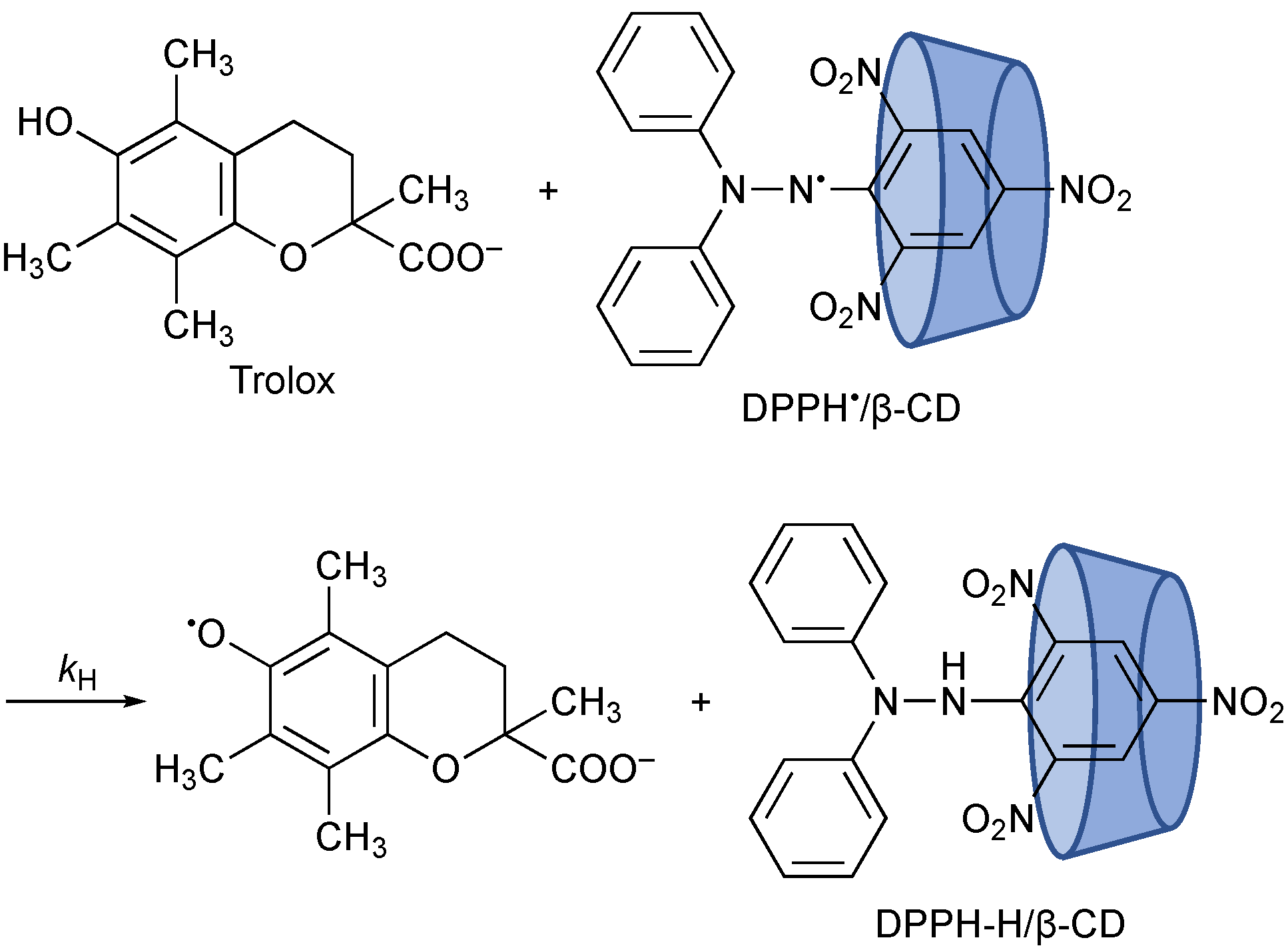
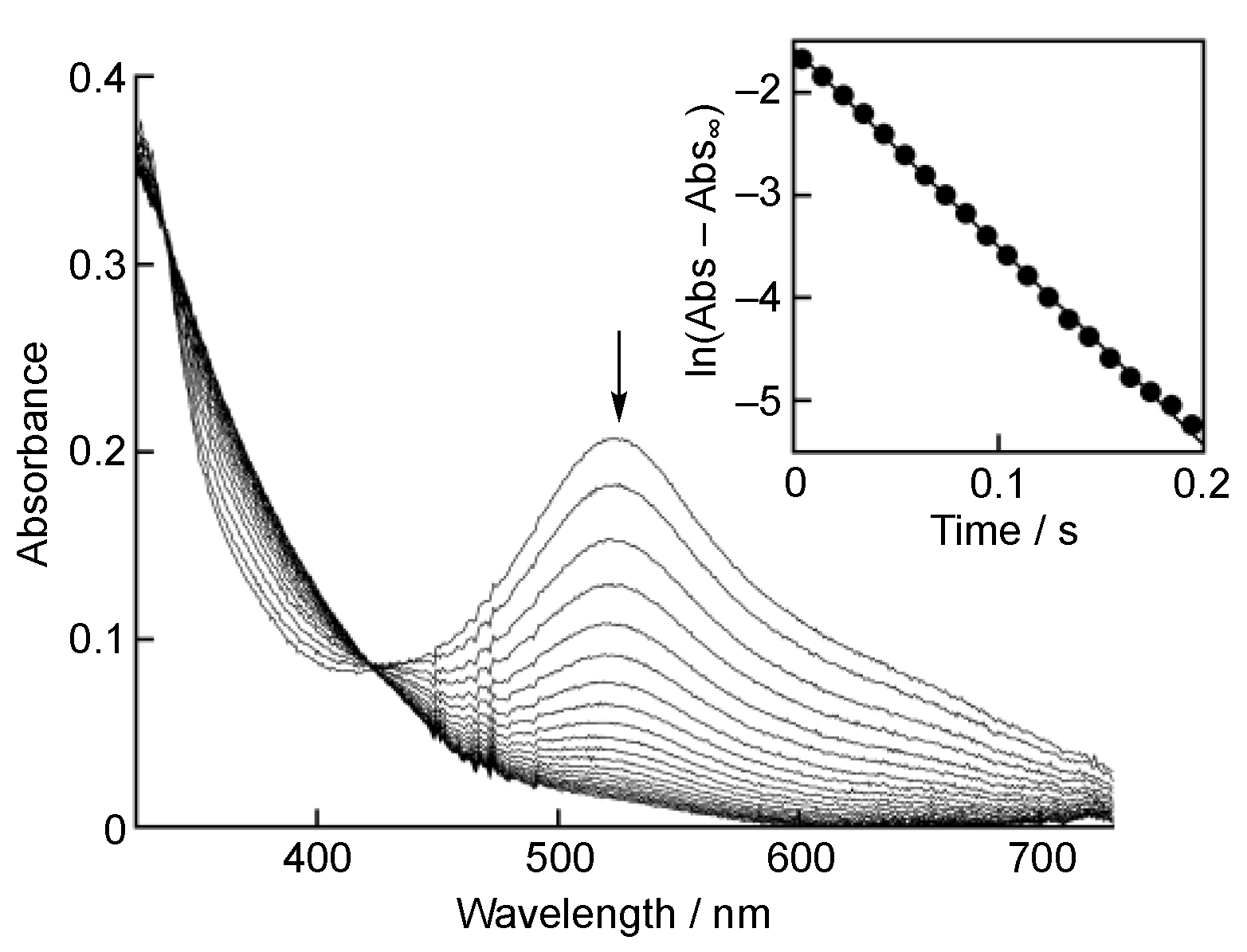
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McFadden, J.; Al-Khalili, J. The origins of quantum biology. Proc. R. Soc. A 2018, 474, 20180674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisner, J.; Kätner, J. Atom tunneling in chemistry. Angew. Chem. Int. Ed. 2016, 55, 5400–5413. [Google Scholar] [CrossRef]
- Schreiner, P.R. Tunneling control of chemical reactions: The third reactivity paradigm. J. Am. Chem. Soc. 2017, 139, 15276–15283. [Google Scholar] [CrossRef] [PubMed]
- Klinman, J.P.; Offenbacher, A.R. Understanding biological hydrogen transfer through the lens of temperature dependent kinetic isotope effects. Acc. Chem. Res. 2018, 51, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Nagel, Z.D.; Klinman, J.P. A 21st century revisionist’s view at a turning point in enzymology. Nat. Chem. Biol. 2009, 5, 543–550. [Google Scholar] [CrossRef]
- Nagaoka, S.; Inoue, M.; Nishioka, C.; Nishioku, Y.; Tsunoda, S.; Ohguchi, C.; Ohara, K.; Mukai, K.; Nagashima, U. Tunneling effect in antioxidant, prooxidant, and regeneration reaction of vitamin E. J. Phys. Chem. B 2000, 104, 856–862. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Foti, M.C. Use and abuse of the DPPH• radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Schaichi, K.M. Re-evaluation of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, I.; Ohkubo, K.; Imai, K.; Kamibayashi, M.; Yoshihashi, Y.; Matsumoto, K.; Fukuhara, K.; Terada, K.; Itoh, S.; Ozawa, T.; et al. Solubilisation of a 2,2-diphenyl-1-picrylhydrazyl radical in water by β-cyclodextrin to evaluate the radical-scavenging activity of antioxidants in aqueous media. Chem. Commun. 2015, 51, 8311–8314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, I.; Ohkubo, K.; Kamibayashi, M.; Ogawa, Y.; Ozawa, T.; Matsumoto, K.; Fukuzumi, S. Reactivity of 2,2-diphenyl-1-picrylhydrazyl solubilized in water by β-cyclodextrin and its methylated derivative. ChemistrySelect 2016, 1, 3367–3370. [Google Scholar] [CrossRef]
- Sekine-Suzuki, E.; Nakanishi, I.; Imai, K.; Ueno, M.; Shimokawa, T.; Matsumoto, K.; Fukuhara, K. Efficient protective activity of a planar catechin analogue against radiation-induced apoptosis in rat thymocytes. RSC Adv. 2018, 8, 10158–10162. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Fukuhara, K.; Ozawa, T.; Matsumoto, K.; Fukuzumi, S. Effects of reaction environments on radical-scavenging mechanisms of ascorbic acid. J. Clin. Biochem. Nutr. 2021, 68, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Covington, A.K.; Paabo, M.; Robinson, R.A.; Bates, R.G. Use of the glass electrode in deuterium oxide and the relation between the standardized pD (paD) scale and the operational pH in heavy water. Anal. Chem. 1968, 40, 700–706. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R. Membrane peroxidation: Inhibiting effects of water-soluble antioxidants on phospholipids of different charge types. Free Radical Biol. Med. 1994, 16, 779–788. [Google Scholar] [CrossRef]
- Bell, R.P. The Tunnel Effect in Chemistry; Chapman & Hall: London, UK, 1980. [Google Scholar]
- Nagaoka, S.; Nitta, A.; Suemitsu, A.; Mukai, K. Tunneling effect in vitamin E recycling by green tea. RSC Adv. 2016, 6, 47325–47336. [Google Scholar] [CrossRef]
- Sajenko, I.; Pilepić, V.; Jakobušić Brala, C.; Uršić, S. Solvent dependence of the kinetic isotope effect in the reaction of ascorbate with the 2,2,6,6-tetramethylpiperidine-1-oxyl radical: Tunneling in a small molecule reaction. J. Phys. Chem. A 2010, 114, 3423–3430. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Jakobušić Brala, C.; Pilepić, V.; Uršić, S. Kinetic isotope effects and hydrogen tunneling in PCET oxidations of ascorbate: New insight into aqueous chemistry? Molecules 2020, 25, 1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Ozawa, T.; Matsumoto, K.; Fukuzumi, S. A large kinetic isotope effect in the reaction of ascorbic acid with 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO•) in aqueous buffer solutions. Chem. Commun. 2020, 56, 11505–11507. [Google Scholar] [CrossRef] [PubMed]

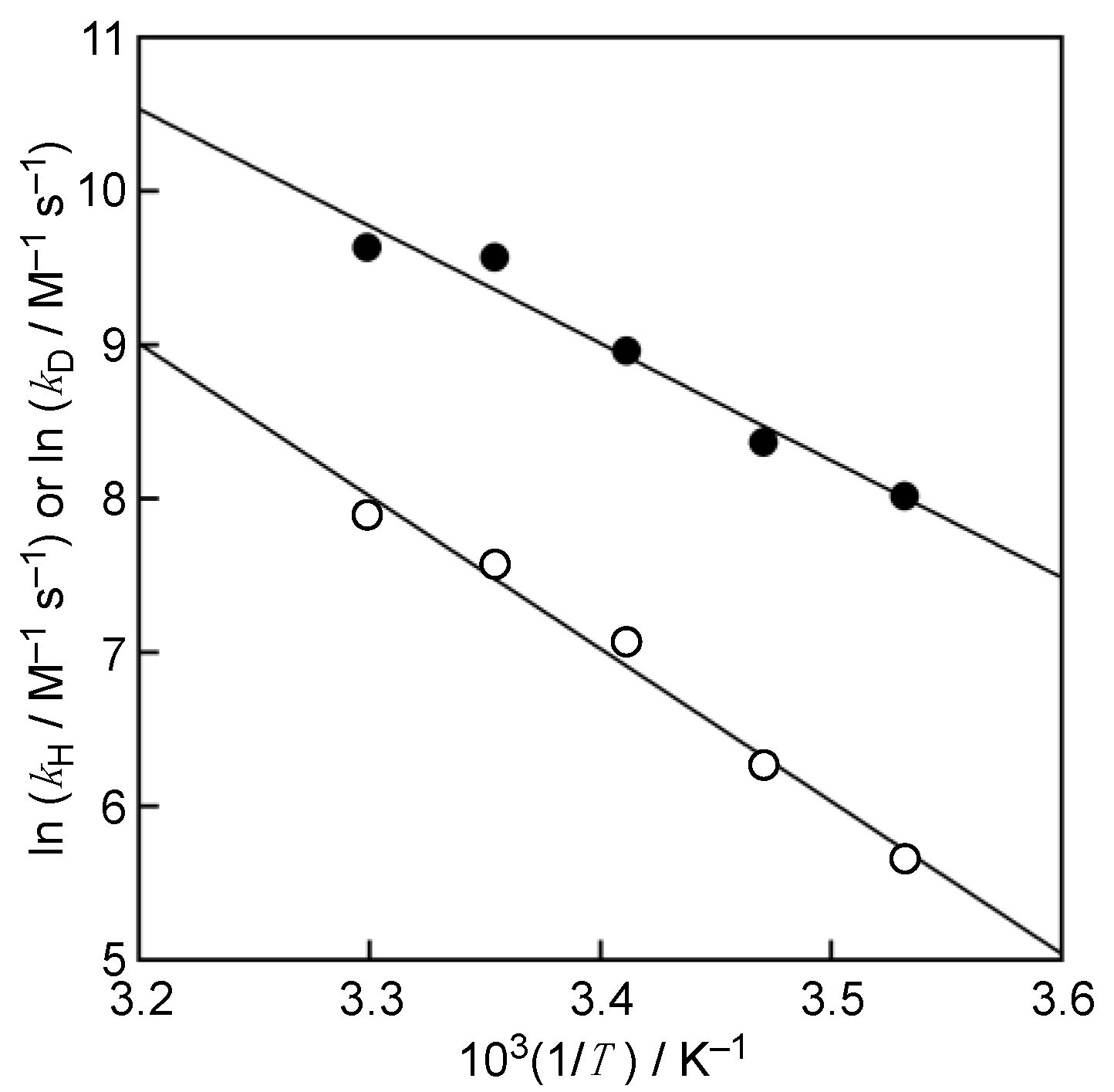
| T/K | kH/M−1 s−1 | kD/M−1 s−1 | kH/kD |
|---|---|---|---|
| 283 | 3.0 × 103 | 2.9 × 102 | 11 |
| 288 | 4.3 × 103 | 5.3 × 102 | 8.2 |
| 293 | 7.8 × 103 | 1.2 × 103 | 6.6 |
| 298 | 1.4 × 104 | 2.0 × 103 | 7.4 |
| 303 | 1.5 × 104 | 2.7 × 103 | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, I.; Shoji, Y.; Ohkubo, K.; Fukuzumi, S. Tunneling in the Hydrogen-Transfer Reaction from a Vitamin E Analog to an Inclusion Complex of 2,2-Diphenyl-1-picrylhydrazyl Radical with β-Cyclodextrin in an Aqueous Buffer Solution at Ambient Temperature. Antioxidants 2021, 10, 1966. https://doi.org/10.3390/antiox10121966
Nakanishi I, Shoji Y, Ohkubo K, Fukuzumi S. Tunneling in the Hydrogen-Transfer Reaction from a Vitamin E Analog to an Inclusion Complex of 2,2-Diphenyl-1-picrylhydrazyl Radical with β-Cyclodextrin in an Aqueous Buffer Solution at Ambient Temperature. Antioxidants. 2021; 10(12):1966. https://doi.org/10.3390/antiox10121966
Chicago/Turabian StyleNakanishi, Ikuo, Yoshimi Shoji, Kei Ohkubo, and Shunichi Fukuzumi. 2021. "Tunneling in the Hydrogen-Transfer Reaction from a Vitamin E Analog to an Inclusion Complex of 2,2-Diphenyl-1-picrylhydrazyl Radical with β-Cyclodextrin in an Aqueous Buffer Solution at Ambient Temperature" Antioxidants 10, no. 12: 1966. https://doi.org/10.3390/antiox10121966
APA StyleNakanishi, I., Shoji, Y., Ohkubo, K., & Fukuzumi, S. (2021). Tunneling in the Hydrogen-Transfer Reaction from a Vitamin E Analog to an Inclusion Complex of 2,2-Diphenyl-1-picrylhydrazyl Radical with β-Cyclodextrin in an Aqueous Buffer Solution at Ambient Temperature. Antioxidants, 10(12), 1966. https://doi.org/10.3390/antiox10121966








