Riboceine Rescues Auranofin-Induced Craniofacial Defects in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Husbandry
2.2. Auranofin (AFN) and Riboceine (RBC) Exposure
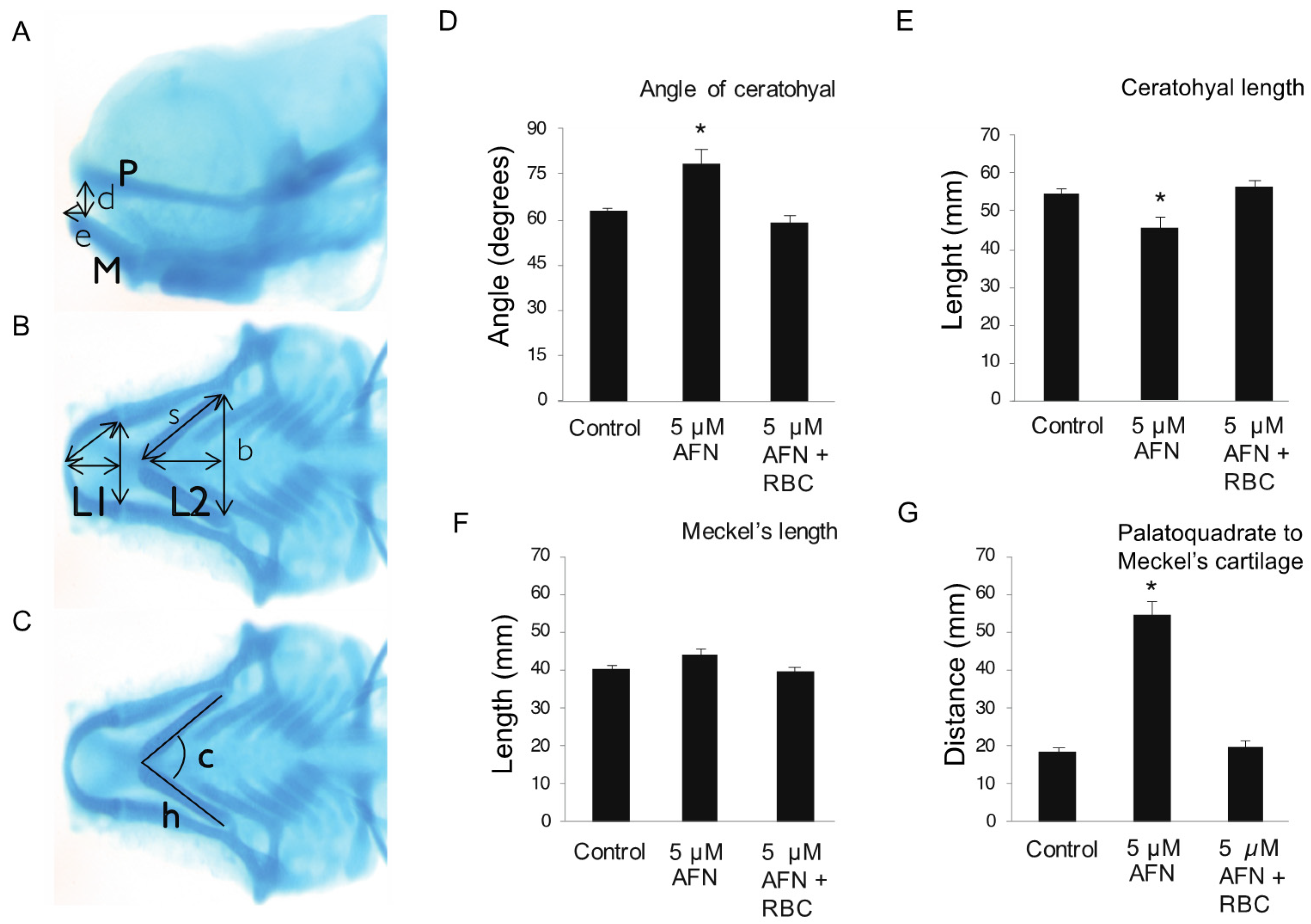
2.3. Cranial Cartilage Measurements
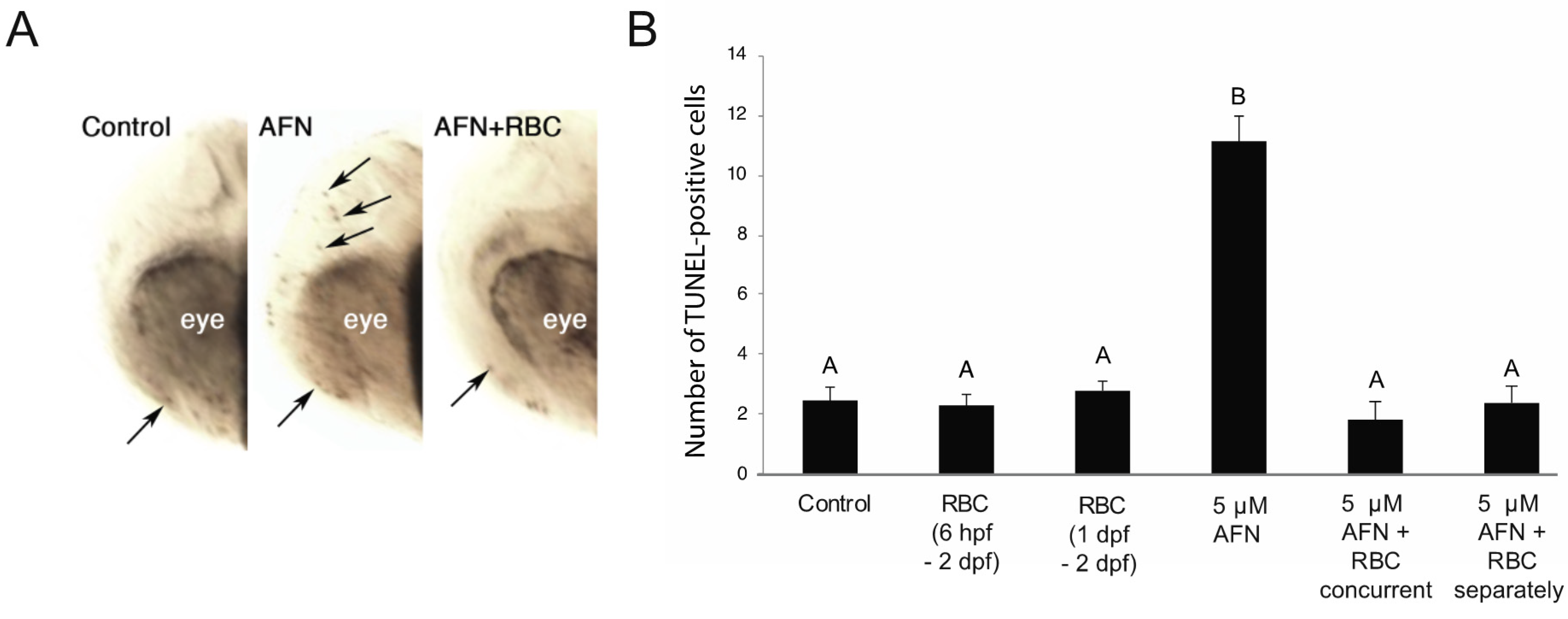
2.4. Detection of Cell Death
2.5. Antioxidant Gene Expression
2.6. Statistical Analysis
3. Results
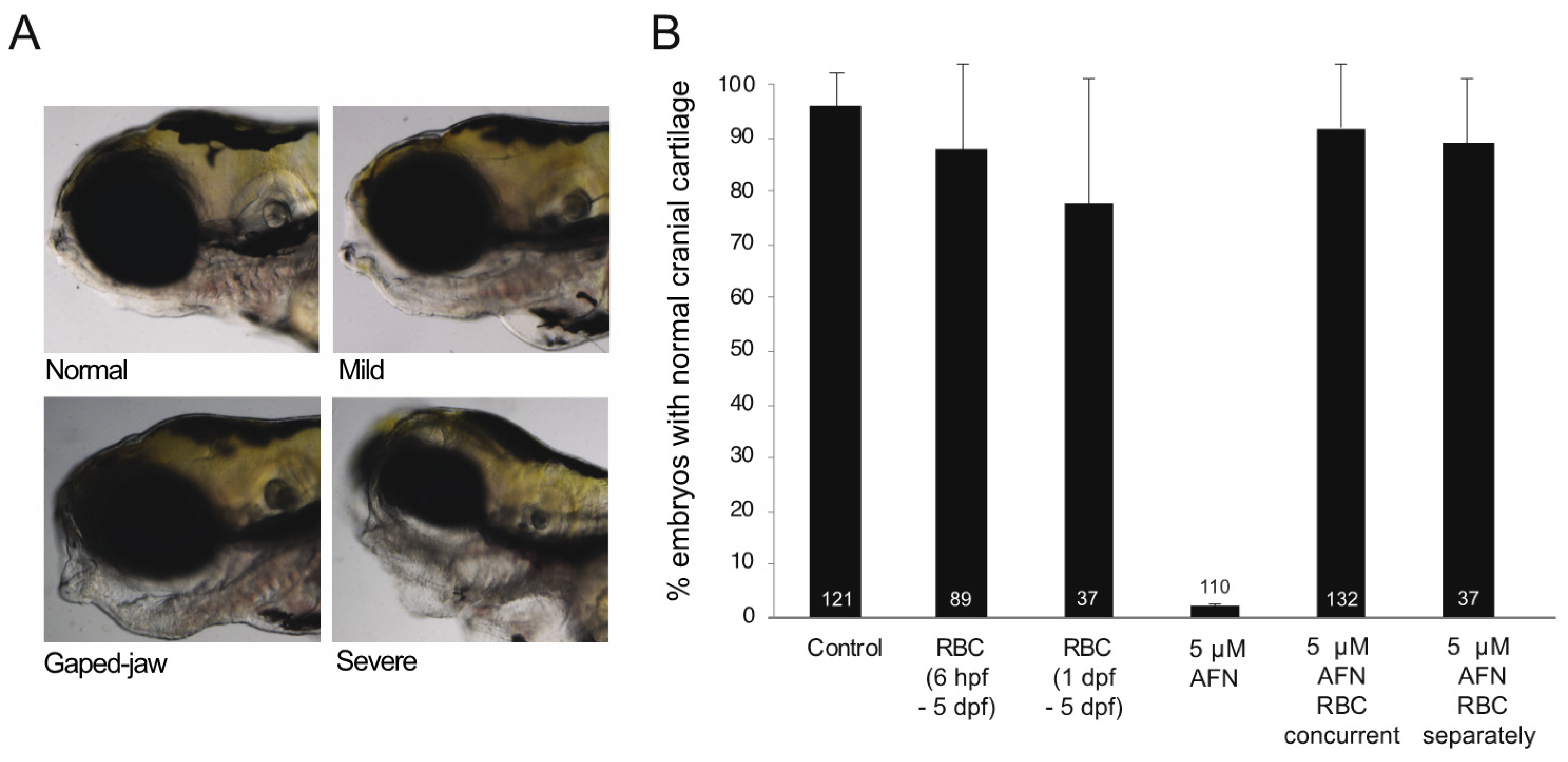
3.1. AFN Causes Craniofacial Defects Which Are Rescued by RBC
3.2. AFN Exposure Results in Apoptosis and Is Rescued by RBC
3.3. RBC Treatment Alters Antioxidant Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, J.; Cardy, A.; Munger, R.G. Tobacco smoking and oral clefts: A meta-analysis. Bull. World Health Organ. 2004, 82, 213. [Google Scholar]
- Mossey, P.A.; Little, J.; Munger, R.G.; Dixon, M.J.; Shaw, W.C. Cleft lip and palate. Lancet 2009, 374, 1773–1785. [Google Scholar] [CrossRef]
- Sabbagh, H.J.; Hassan, M.H.A.; Innes, N.P.T.; Elkodary, H.M.; Little, J.; Mossey, P. Passive smoking in the etiology of non-syndromic orofacial clefts: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0116963. [Google Scholar]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Sulik, K.K. Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol. Clin. Exp. Res. 1996, 20, 1071–1076. [Google Scholar] [CrossRef]
- Sakai, D.; Dixon, J.; Achilleos, A.; Dixon, M.; Trainor, P. Prevention of Treacher Collins syndrome craniofacial anomalies in mouse models via maternal antioxidant supplementation. Nat. Commun. 2016, 7, 10328. [Google Scholar] [CrossRef]
- Van Der Vaart, H.; Postma, D.S.; Timens, W.; Hacken, N.H.T.T. Acute effects of cigarette smoke on inflammation and oxidative stress: A review. Thorax 2004, 59, 713–721. [Google Scholar] [CrossRef]
- Ornoy, A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod. Toxicol. 2007, 24, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Dennery, P.A. Effects of oxidative stress on embryonic development. Birth Defects Res. C Embryo Today 2007, 81, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Trainor, P.A. Specification and patterning of neural crest cells during craniofacial development. Brain Behav. Evol. 2005, 66, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Shen, W.-B.; Reece, E.A.; Yang, P. Deficiency of the oxidative stress-responsive kinase p70S6K1 restores autophagy and ameliorates neural tube defects in diabetic embryopathy. Am. J. Obstet. Gynecol. 2020, 223, 753.e1–753.e14. [Google Scholar] [CrossRef]
- Han, D.; Schomacher, L.; Schüle, K.M.; Mallick, M.; Musheev, M.U.; Karaulanov, E.; Krebs, L.; Von Seggern, A.; Niehrs, C. NEIL1 and NEIL2 DNA glycosylases protect neural crest development against mitochondrial oxidative stress. Elife 2019, 8, 8. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, S.; Wang, G.; Ma, Z.-L.; Chuai, M.; Cao, L.; Yang, X. High glucose environment inhibits cranial neural crest survival by activating excessive autophagy in the chick embryo. Sci. Rep. 2015, 5, 18321. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free. Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Pompella, A.; Visvikis, A.; Paolicchi, A.; De Tata, V.; Casini, A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharm. 2003, 66, 1499–1503. [Google Scholar] [CrossRef]
- Berners-Price, S.J.; Filipovska, A. Gold compounds as therapeutic agents for human diseases. Metallomics 2011, 3, 863–873. [Google Scholar] [CrossRef]
- Newman, T.A.; Carleton, C.R.; Leeke, B.; Hampton, M.B.; Horsfield, J.A. Embryonic oxidative stress results in reproductive impairment for adult zebrafish. Redox Biol. 2015, 6, 648–655. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, M.E.; Meister, A. Transport and direct utilization of gamma-glutamylcyst(e)ine for glutathione synthesis. Proc. Natl. Acad. Sci. USA 1983, 80, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Kader, T.; Porteous, C.M.; Williams, M.; Gieseg, S.P.; McCormick, S.P. Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein (a) mice. Atherosclerosis 2014, 237, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Harada, D.; Anraku, M.; Fukuda, H.; Naito, S.; Harada, K.; Suenaga, A.; Otagiri, M. Kinetic studies of covalent binding between N-acetyl-L-cysteine and human serum albumin through a mixed-disulfide using an N-methylpyridinium polymer-based column. Drug Metab. Pharmacokinet. 2004, 19, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Borgstrom, L.; Kagedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharm. 1986, 31, 217–222. [Google Scholar] [CrossRef]
- Timme-Laragy, A.R.; Van Tiem, L.A.; Linney, E.A.; Di Giulio, R.T. Antioxidant responses and NRF2 in synergistic developmental toxicity of PAHs in zebrafish. Toxicol. Sci. 2009, 109, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, H.; Zhang, T.; Liu, J.-X. Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis. Cell Commun. Signal. 2020, 18, 45. [Google Scholar] [CrossRef]
- Costa-Silva, D.G.D.; Leandro, L.P.; Vieira, P.d.B.; Carvalho, N.R.d.; Lopes, A.R.; Schimith, L.E.; Nunes, M.E.M.; Mello, R.S.d.; Martins, I.K.; Paula, A.A.D. N-acetylcysteine inhibits Mancozeb-induced impairments to the normal development of zebrafish embryos. Neurotoxicol. Teratol. 2018, 68, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.; Prats, E.; Gómez-Canela, C.; Hsu, C.-Y.; Arick, M.A.; Bedrossiantz, J.; Orozco, M.; Garcia-Reyero, N.; Ziv, T.; Ben-Lulu, S.; et al. Therapeutic potential of N-acetylcysteine in acrylamide acute neurotoxicity in adult zebrafish. Sci. Rep. 2019, 9, 16467. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Rodgers, J.; Robinson, B.; Kanungo, J. N-acetylcysteine prevents verapamil-induced cardiotoxicity with no effect on the noradrenergic arch-associated neurons in zebrafish. Food Chem. Toxicol. 2020, 144, 111559. [Google Scholar] [CrossRef]
- Westerfield, M. A guide for the laboratory use of zebrafish (Danio rerio). In The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 2000; Volume 2, pp. 4–5. [Google Scholar]
- Mukhi, S.; Patiño, R. Effects of prolonged exposure to perchlorate on thyroid and reproductive function in zebrafish. Toxicol. Sci. 2007, 96, 246–254. [Google Scholar] [CrossRef]
- Schilling, T.F. Genetic analysis of craniofacial development in the vertebrate embryo. Bioessays 1997, 19, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, J.; Chen, S.Y. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br. J. Pharm. 2013, 169, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.Y.; Li, K.; Jiang, L.-L.; He, M.-F.; Pu, C.-H.; Kang, D.; Xie, J. Developmental toxicity of auranofin in zebrafish embryos. J. Appl. Toxicol. 2017, 37, 602–610. [Google Scholar] [CrossRef]
- Komoike, Y.; Matsuoka, M. In vitro and in vivo studies of oxidative stress responses against acrylamide toxicity in zebrafish. J. Hazard. Mater. 2019, 365, 430–439. [Google Scholar] [CrossRef]
- Oretti, C.; Marino, S.; Mosca, F.; Colnaghi, M.R.; De Iudicibus, S.; Drigo, I.; Stocco, G.; Bartoli, F.; Decorti, G.; Demarini, S. Glutathione-S-transferase-P1 I105V polymorphism and response to antenatal betamethasone in the prevention of respiratory distress syndrome. Eur. J. Clin. Pharm. 2009, 65, 483–491. [Google Scholar] [CrossRef][Green Version]
- Lau, M.C.; Kwong, E.M.L.; Lai, K.P.; Li, J.-W.; Ho, J.C.H.; Chan, T.-F.; Wong, C.K.C.; Jiang, Y.-J.; Tse, W.K.F. Pathogenesis of POLR1C-dependent Type 3 Treacher Collins Syndrome revealed by a zebrafish model. Biochim. Biophys. Acta 2016, 1862, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Rosas, M.G.; Lorenzatti, A.; de Peralta, M.S.P.; Calcaterra, N.B.; Coux, G. Proteasomal inhibition attenuates craniofacial malformations in a zebrafish model of Treacher Collins Syndrome. Biochem. Pharm. 2019, 163, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Cukrov, D.; Newman, T.; Leask, M.; Leeke, B.; Sarogni, P.; Patimo, A.; Kline, A.D.; Krantz, I.D.; A Horsfield, J.; Musio, A. Antioxidant treatment ameliorates phenotypic features of SMC1A-mutated Cornelia de Lange syndrome in vitro and in vivo. Hum. Mol. Genet. 2018, 27, 3002–3011. [Google Scholar] [CrossRef]
- Xu, B.; Lee, K.K.; Zhang, L.; Gerton, J.L. Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome. PLoS Genet. 2013, 9, e1003857. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Sowa, N.; Cardenas, M.E.; Gerton, J.L. L-leucine partially rescues translational and developmental defects associated with zebrafish models of Cornelia de Lange syndrome. Hum. Mol. Genet. 2015, 24, 1540–1555. [Google Scholar] [CrossRef]
- Revenkova, E.; Focarelli, M.L.; Susani, L.; Paulis, M.; Bassi, M.T.; Mannini, L.; Frattini, A.; Delia, D.; Krantz, I.; Vezzoni, P.; et al. Cornelia de Lange syndrome mutations in SMC1A or SMC3 affect binding to DNA. Hum. Mol. Genet. 2009, 18, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, S.; Clark, D.; Kline, A.D.; Jackson, L.G.; Pie, J.; Siu, V.; Ramos, F.J.; Krantz, I.D.; Deardorff, M.A. Facial diagnosis of mild and variant CdLS: Insights from a dysmorphologist survey. Am. J. Med. Genet. A 2010, 152, 1641–1653. [Google Scholar] [CrossRef]
- Gimigliano, A.; Mannini, L.; Bianchi, L.; Puglia, M.; Deardorff, M.A.; Menga, S.; Krantz, I.D.; Musio, A.; Bini, L. Proteomic profile identifies dysregulated pathways in Cornelia de Lange syndrome cells with distinct mutations in SMC1A and SMC3 genes. J. Proteome. Res. 2012, 11, 6111–6123. [Google Scholar] [CrossRef]
- Wood, K.A.; Rowlands, C.F.; Thomas, H.B.; Woods, S.; Flaherty, J.; Douzgou, S.; Kimber, S.J.; Newman, W.G.; Keefe, R.T. Modelling the developmental spliceosomal craniofacial disorder Burn-McKeown syndrome using induced pluripotent stem cells. PLoS ONE 2020, 15, e0233582. [Google Scholar]
- Knapp, K.M.; Sullivan, R.; Murray, J.; Gimenez, G.; Arn, P.; D’Souza, P.; Gezdirici, A.; Wilson, W.G.; Jackson, A.P.; Ferreira, C.; et al. Linked-read genome sequencing identifies biallelic pathogenic variants in DONSON as a novel cause of Meier-Gorlin syndrome. J. Med. Genet. 2020, 57, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Mottola, F.; Santonastaso, M.; Iovine, C.; Rossetti, C.; Ronga, V.; Rocco, L. DNA Damage in Human Amniotic Cells: Antigenotoxic Potential of Curcumin and alpha-Lipoic Acid. Antioxidants 2021, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Singh, N.; Ahsan, K.; Beiriger, A.; Prince, V.E. Neural crest development: Insights from the zebrafish. Dev. Dyn. 2020, 249, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.-C.; Lin, Y.-C.; Chang, Y.-C.; Lin, H.-J.; Tsai, Y.-R.; Kang, H.-Y. Limited relationships between reactive oxygen species levels in culture media and zygote and embryo development. J. Assist. Reprod. Genet. 2019, 36, 325–334. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leask, M.; Carleton, C.; Leeke, B.; Newman, T.; Antoun, J.; Farella, M.; Horsfield, J. Riboceine Rescues Auranofin-Induced Craniofacial Defects in Zebrafish. Antioxidants 2021, 10, 1964. https://doi.org/10.3390/antiox10121964
Leask M, Carleton C, Leeke B, Newman T, Antoun J, Farella M, Horsfield J. Riboceine Rescues Auranofin-Induced Craniofacial Defects in Zebrafish. Antioxidants. 2021; 10(12):1964. https://doi.org/10.3390/antiox10121964
Chicago/Turabian StyleLeask, Megan, Catherine Carleton, Bryony Leeke, Trent Newman, Joseph Antoun, Mauro Farella, and Julia Horsfield. 2021. "Riboceine Rescues Auranofin-Induced Craniofacial Defects in Zebrafish" Antioxidants 10, no. 12: 1964. https://doi.org/10.3390/antiox10121964
APA StyleLeask, M., Carleton, C., Leeke, B., Newman, T., Antoun, J., Farella, M., & Horsfield, J. (2021). Riboceine Rescues Auranofin-Induced Craniofacial Defects in Zebrafish. Antioxidants, 10(12), 1964. https://doi.org/10.3390/antiox10121964







