Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review
Abstract
1. Introduction
2. Ageing-Related Gut Microbiota Dysbiosis and the Role of Lactobacillus
| Microbiota Diversity of Experimental Animals during Age-Related Conditions at Different Levels before Probiotic Supplementation | Strains of the Genus Lactobacillus Supplemented in the Studies | Microbiota Diversity after the Probiotic Supplementation | Ref: | |||
|---|---|---|---|---|---|---|
| Phyla | Increase | Decrease | Increase | Decrease | ||
| Firmicutes | √ | √ | L. plantarum CCFM10, L. delbrueckii subsp. Bulgaricus 2038, L. acidophilus DDS-1, L. helveticus OFS 1515 and L. fermentumDR9, L. casei LC122 | √ | [36,38,40,41,42] | |
| [36,37,39] | [38,40,41,42] | |||||
| L. paracasei KW3110, L. plantarum TWK10 | √ | [37,39] | ||||
| Bacteroidetes | √ | √ | L. delbrueckiisubsp. Bulgaricus 2038, L. casei LC122, L. plantarum TWK10 | √ | [38,39,42] | |
| [38,41] | [36,37,39,40,42] | L. plantarum CCFM10, L. acidophilus DDS-1, L. paracasei KW3110, L. helveticusOFS 1515 and L. fermentumDR9 | √ | [36,37,40,41] | ||
| F/B ratio | √ | √ | L. plantarum CCFM10, L. acidophilus DDS-1 | √ | [36,40] | |
| [36,37,39,42] | [38,40,41] | |||||
| L. paracasei KW3110, L. delbrueckiisubsp. Bulgaricus 2038, L. helveticus OFS 1515 and L. fermentum DR9, Lactobacillus casei LC122, L. plantarum TWK10 | √ | [37,38,39,41,42] | ||||
| TM7 | √ | √ | L. delbrueckiisubsp. Bulgaricus 2038, | √ | [38] | |
| [36] | [38] | |||||
| L. plantarum CCFM10 | √ | [36] | ||||
| Proteobacteria | √ | √ | L. plantarum CCFM10, L. delbrueckii subsp. Bulgaricus 2038, L. paracasei KW3110, L. casei LC122 | √ | [36,37,38,42] | |
| [36,39,40] | [37,42] | |||||
| L. acidophilus DDS-1, L. plantarum TWK10 | √ | [39,40] | ||||
| Deferrebacteres | √ | L. paracasei KW3110 | √ | [37] | ||
| [40] | [37] | |||||
| L. acidophilus DDS-1 | √ | [40] | ||||
| Actinobacteria | √ | √ | L. helveticus OFS 1515 and L. fermentum DR9,L. casei LC122 | √ | [40,41,42] | |
| [39] | [37,40,41,42] | L. delbrueckiisubsp. Bulgaricus 2038, L. acidophilus DDS-1, Lactobacillus paracasei KW3110. L. plantarum TWK10 | √ | [37,38,39] | ||
| Genera: | ||||||
| Bacteroides species diversity | √ | √ | L. plantarum CCFM10, L. paracasei, L. paracasei KW3110 | √ | [36,37] | |
| [39,41] | [36,37] | L. helveticusOFS 1515 and L. fermentum DR9, L. plantarum TWK10 | √ | [39,41] | ||
| Clostridium | √ | √ | L. paracasei KW3110, plantarum CCFM10, L. paracasei, L. casei LC122 | √ | [36,42] | |
| [36,37] | [42] | L. paracasei | √ | [37] | ||
| Lactobacillus | √ | √ | L. plantarum CCFM10, L. acidophilus DDS-1, L. helveticusOFS 1515 and L. fermentum DR9, L. casei LC122 | √ | √ | [36,41,42] |
| [36,37,39] | [41,42] | |||||
| L. paracasei KW3110, L. plantarum TWK10 | [37,39] | |||||
| Facultative anaerobes: | ||||||
| Streptococci, Staphylococci, Enterococci, Enterobacteria | √ | √ | L. plantarum CCFM10, L. casei LC122 | √ | [26,42] | |
| [37,39] | [36,42] | |||||
| L. paracasei KW3110, L. plantarum TWK10 | √ | [37,39] | ||||
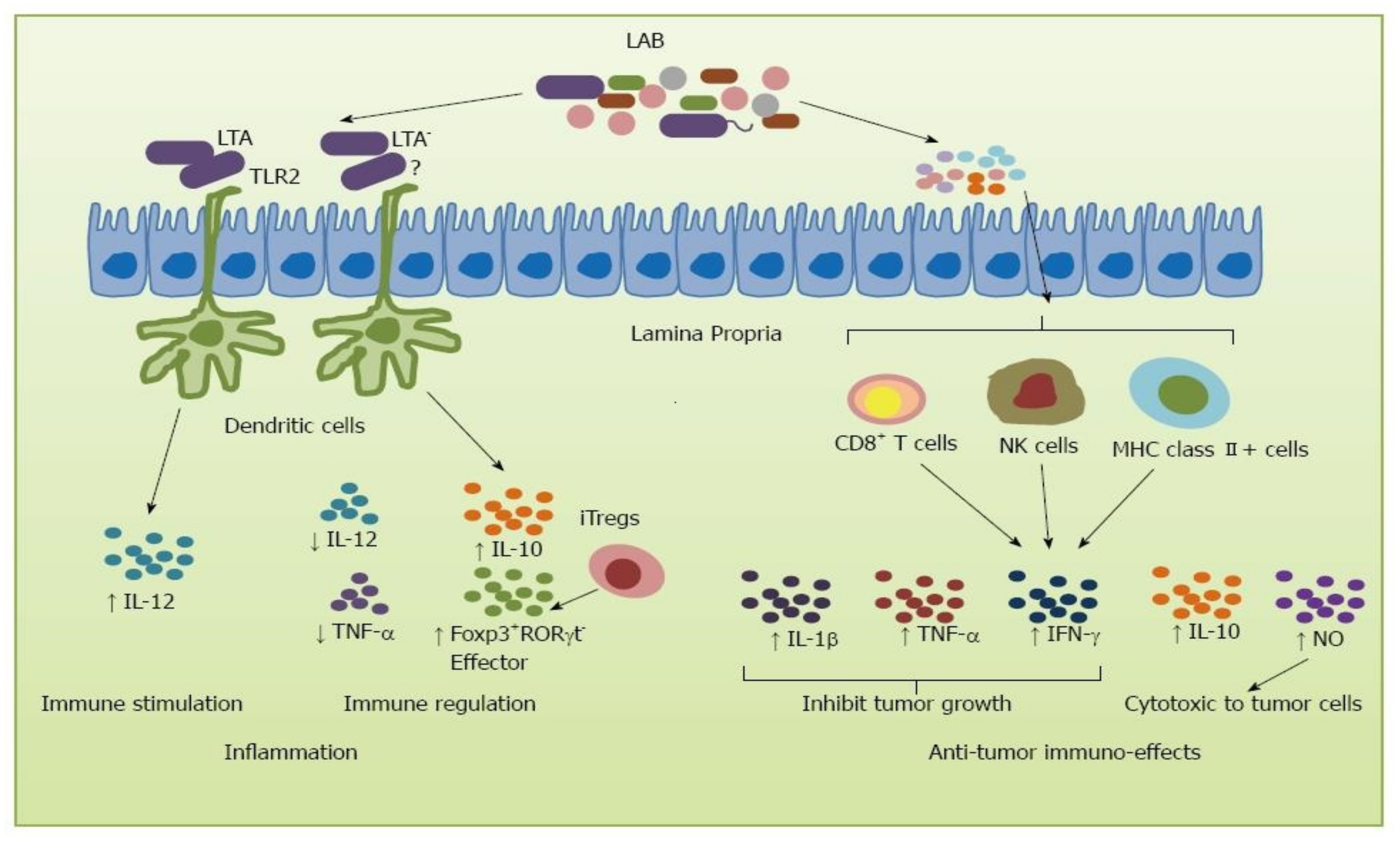
3. Ageing-Related Decline in Immune System and the Role of Lactobacillus
4. Ageing-Related Oxidative Stress and the Role of Lactobacillus
5. Age-Related Gene Suppression and the Role of Lactobacillus
| Strain | Organ | Gene | Up-Regulated | Down-Regulated | Ref: |
|---|---|---|---|---|---|
| L. mucosae LMU1001 | Intestinal tract | MT1 | Yes | [99] | |
| MT2 | Yes | ||||
| GPX1 | Yes | ||||
| GPX2 | Yes | ||||
| SOD | Yes | ||||
| L. plantarum CCFM10 | Liver | Peroxiredoxin | Yes | [36] | |
| Glutathione peroxidase | Yes | ||||
| Glutathione reductase | Yes | ||||
| Thioredoxin reductase | Yes | ||||
| L. acidophilus LaVK2 | Liver | PPAR-a | Yes | [100] | |
| Klotho | Yes | ||||
| SMP-30 | |||||
| Kidney | PPAR-a | Yes | |||
| Klotho | Yes | ||||
| SMP-30 | Yes | ||||
| L.plantarum K68 | Liver | TLR4 | Yes | [101] | |
| Foxp3 | Yes | ||||
| SOCS3 | Yes | ||||
| L.plantarum AR501 | Liver | GST | Yes | [85] | |
| GCLc | Yes | ||||
| GCLm | Yes | ||||
| NQO1 | Yes | ||||
| L.plantarum CQPC11 | Liver | nNOS, eNOS, Cu/Zn-SOD, Mn-SOD, CAT, HO-1, Nrf2, γ-GCS, NQO1 | Yes | [102] | |
| iNOS | Yes | ||||
| Spleen | nNOS, eNOS, Cu/Zn-SOD, Mn-SOD, CAT, HO-1, Nrf2, γ-GCS, NQO1 | Yes | |||
| iNOS | Yes |
6. Brain Ageing and the Role of Lactobacillus
7. Skin Ageing and the Role of Lactobacillus
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Gilbert, H.T.J.; Swift, J. The consequences of ageing, progeroid syndromes and cellular senescence on mechanotransduction and the nucleus. Exp. Cell Res. 2019, 378, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Phillip, J.M.; Aifuwa, I.; Walston, J.; Wirtz, D. The Mechanobiology of Aging. Annu. Rev. Biomed. Eng. 2015, 17, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjun, V.; Swift, J. Therapeutic Manipulation of Ageing: Repurposing Old Dogs and Discovering New Tricks. EBioMedicine 2016, 14, 24–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finamore, A.; Roselli, M.; Donini, L.M.; Brasili, E.; Rami, R.; Carnevali, P.; Mistura, L.; Pinto, A.; Giusti, A.; Mengheri, E. Supplementation with Bifidobacterium longum Bar33 and Lactobacillus helveticus Bar13 mixture improves immunity in elderly humans (over 75 years) and aged mice. Nutrition 2019, 63–64, 184–192. [Google Scholar] [CrossRef]
- Hor, Y.Y.; Ooi, C.-H.; Khoo, B.-Y.; Choi, S.-B.; Seeni, A.; Shamsuddin, S.; Oon, C.E.; Ong, K.-L.; Jeong, W.-S.; Liong, M.-T. Lactobacillus strains alleviated aging symptoms and aging-induced metabolic disorders in aged rats. J. Med. Food 2019, 22, 1–13. [Google Scholar] [CrossRef]
- Cheng, L.-H.; Cheng, S.-H.; Wu, C.-C.; Huang, C.-L.; Wen, P.-J.; Chang, M.-Y.; Tsai, Y.-C. Lactobacillus paracasei PS23 dietary supplementation alleviates muscle aging via ghrelin stimulation in d-galactose-induced aging mice. J. Funct. Foods 2021, 85, 104651. [Google Scholar] [CrossRef]
- Setbo, E.; Campbell, K.; O’Cuiv, P.; Hubbard, R. Utility of Probiotics for Maintenance or Improvement of Health Status in Older People—A Scoping Review. J. Nutr. Health Aging 2019, 23, 364–372. [Google Scholar] [CrossRef]
- Costello, E.K.; Stagaman, K.; Dethlefsen, L.; Bohannan, B.J.M.; Relman, D.A. The application of ecological theory toward an understanding of the human microbiome. Science 2012, 336, 1255–1262. [Google Scholar] [CrossRef]
- Fanaro, S.; Chierici, R.; Guerrini, P.; Vigi, V. Intestinal microflora in early infancy: Composition and development. Acta Paediatr. 2003, 91, 48–55. [Google Scholar] [CrossRef]
- Berg, R.D. The indigenous gastrointestinal microflora. Trends Microbiol. 1996, 4, 430–435. [Google Scholar] [CrossRef]
- Penders, J.; Stobberingh, E.E.; van den Brandt, P.A.; Thijs, C. The role of the intestinal microbiota in the development of atopic disorders. Allergy 2007, 62, 1223–1236. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Smidt, H.; De Vos, W.M. Diversity of the human gastrointestinal tract microbiota revisited. Environ. Microbiol. 2007, 9, 2125–2136. [Google Scholar] [CrossRef]
- Hsiao, W.W.L.; Metz, C.; Singh, D.P.; Roth, J. The Microbes of the Intestine: An Introduction to Their Metabolic and Signaling Capabilities. Endocrinol. Metab. Clin. N. Am. 2008, 37, 857–871. [Google Scholar] [CrossRef]
- Frank, D.N.; Amand, A.L.S.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef]
- Yamashiro, Y. Gut Microbiota in Health and Disease. Ann. Nutr. Metab. 2018, 71, 242–246. [Google Scholar] [CrossRef]
- Bischoff, S.C. Microbiota and aging. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 26–30. [Google Scholar] [CrossRef]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef]
- Pérez Martínez, G.; Bäuerl, C.; Collado, M.C. Understanding gut microbiota in elderly’s health will enable intervention through probiotics. Benef. Microbes 2014, 5, 235–246. [Google Scholar] [CrossRef]
- Rondanelli, M.; Giacosa, A.; Faliva, M.A.; Perna, S.; Allieri, F.; Castellazzi, A.M. Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases 2015, 3, 156–162. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Li, B.; Evivie, S.E.; Lu, J.; Jiao, Y.; Wang, C.; Li, Z.; Liu, F.; Huo, G. Lactobacillus helveticus KLDS1.8701 alleviates d-galactose-induced aging by regulating Nrf-2 and gut microbiota in mice. Food Funct. 2018, 9, 6586–6598. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Chai, W.; Sun, C.; Zhang, T.; Zhao, C.; Yuan, Y.; Wang, X.; Liu, H.; Ye, H. Lactobacillus paracasei Jlus66 extenuate oxidative stress and inflammation via regulation of intestinal flora in rats with non alcoholic fatty liver disease. Food Sci. Nutr. 2019, 7, 2636–2646. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.J.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef]
- Xin, J.; Zeng, D.; Wang, H.; Ni, X.; Yi, D.; Pan, K.; Jing, B. Preventing non-alcoholic fatty liver disease through Lactobacillus johnsonii BS15 by attenuating inflammation and mitochondrial injury and improving gut environment in obese mice. Appl. Microbiol. Biotechnol. 2014, 98, 6817–6829. [Google Scholar] [CrossRef]
- Park, D.-Y.; Ahn, Y.-T.; Park, S.-H.; Huh, C.-S.; Yoo, S.-R.; Yu, R.; Sung, M.-K.; McGregor, R.A.; Choi, M.-S. Supplementation of Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 in Diet-Induced Obese Mice Is Associated with Gut Microbial Changes and Reduction in Obesity. PLoS ONE 2013, 8, e59470. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics may delay the progression of nonalcoholic fatty liver disease by restoring the gut microbiota structure and improving intestinal endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.E.; Guarner, F.; Guerrant, R.; Holt, P.R.; Quigley, E.M.M.; Sartor, R.B.; Sherman, P.M.; Mayer, E.A. An update on the use and investigation of probiotics in health and disease. Gut 2013, 62, 787–796. [Google Scholar] [CrossRef]
- Bron, P.A.; Van Baarlen, P.; Kleerebezem, M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 2012, 10, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; De Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Kankainen, M.; Paulin, L.; Tynkkynen, S.; Von Ossowski, I.; Reunanen, J.; Partanen, P.; Satokari, R.; Vesterlund, S.; Hendrickx, A.P.A.; Lebeer, S.; et al. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc. Natl. Acad. Sci. USA 2009, 106, 17193–17198. [Google Scholar] [CrossRef]
- Lebeer, S.; Verhoeven, T.L.A.; Claes, I.J.J.; De Hertogh, G.; Vermeire, S.; Buyse, J.; Van Immerseel, F.; Vanderleyden, J.; De Keersmaecker, S.C.J. FISH analysis of Lactobacillus biofilms in the gastrointestinal tract of different hosts. Lett. Appl. Microbiol. 2011, 52, 220–226. [Google Scholar] [CrossRef]
- Lahti, L.; Salonen, A.; Kekkonen, R.A.; Salojärvi, J.; Jalanka-Tuovinen, J.; Palva, A.; Orešič, M.; de Vos, W.M. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. PeerJ 2013, 1, e32. [Google Scholar] [CrossRef]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.-M.; Botelho, C.; et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. mBio 2015, 6, e00231-15. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, F.; Yan, S.; Zhai, Q.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose-induced aging mice. Food Funct. 2018, 9, 917–924. [Google Scholar] [CrossRef]
- Morita, Y.; Jounai, K.; Sakamoto, A.; Tomita, Y.; Sugihara, Y.; Suzuki, H.; Ohshio, K.; Otake, M.; Fujiwara, D.; Kanauchi, O.; et al. Long-term intake of Lactobacillus paracasei KW3110 prevents age-related chronic inflammation and retinal cell loss in physiologically aged mice. Aging 2018, 10, 2723–2740. [Google Scholar] [CrossRef]
- Usui, Y.; Kimura, Y.; Satoh, T.; Takemura, N.; Ouchi, Y.; Ohmiya, H.; Kobayashi, K.; Suzuki, H.; Koyama, S.; Hagiwara, S.; et al. Effects of long-term intake of a yogurt fermented with Lactobacillus delbrueckii subsp. bulgaricus 2038 and Streptococcus thermophilus 1131 on mice. Int. Immunol. 2018, 30, 319–331. [Google Scholar] [CrossRef]
- Lee, C.-C.; Liao, Y.-C.; Lee, M.-C.; Lin, K.-J.; Hsu, H.-Y.; Chiou, S.-Y.; Young, S.-L.; Lin, J.-S.; Huang, C.-C.; Watanabe, K. Lactobacillus plantarum TWK10 Attenuates Aging-Associated Muscle Weakness, Bone Loss, and Cognitive Impairment by Modulating the Gut Microbiome in Mice. Front. Nutr. 2021, 8, 753. [Google Scholar] [CrossRef]
- Vemuri, R.; Shinde, T.; Gundamaraju, R.; Gondalia, S.V.; Karpe, A.V.; Beale, D.J.; Martoni, C.J.; Eri, R. Lactobacillus acidophilus DDS-1 modulates the gut microbiota and improves metabolic profiles in aging mice. Nutrients 2018, 10, 1255. [Google Scholar] [CrossRef]
- Hor, Y.-Y.; Lew, L.-C.; Jaafar, M.H.; Lau, A.S.-Y.; Ong, J.-S.; Kato, T.; Nakanishi, Y.; Azzam, G.; Azlan, A.; Ohno, H.; et al. Lactobacillus sp. improved microbiota and metabolite profiles of aging rats. Pharmacol. Res. 2019, 146, 104312. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, X.; Zheng, L.; Wang, Z.; Wu, L.; Jiang, J.; Yang, T.; Ma, L.; Fu, Z. Lactobacillus and Bifidobacterium Improves Physiological Function and Cognitive Ability in Aged Mice by the Regulation of Gut Microbiota. Mol. Nutr. Food Res. 2019, 63, e1900603. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Chen, H.; Wei, H.; Wan, C. Potential of Lactobacillus plantarum ZDY2013 and Bifidobacterium bifidum WBIN03 in relieving colitis by gut microbiota, immune, and anti-oxidative stress. Can. J. Microbiol. 2018, 64, 327–337. [Google Scholar] [CrossRef]
- Chen, D.; Yang, Z.; Chen, X.; Huang, Y.; Yin, B.; Guo, F.; Zhao, H.; Zhao, T.; Qu, H.; Huang, J.; et al. The effect of Lactobacillus rhamnosus hsryfm 1301 on the intestinal microbiota of a hyperlipidemic rat model. BMC Complement. Altern. Med. 2014, 14, 386. [Google Scholar] [CrossRef]
- Effros, R.B. Roy Walford and the immunologic theory of aging. Immun. Ageing 2005, 2, 7. [Google Scholar] [CrossRef]
- McElhaney, J.E. Overcoming the challenges of immunosenescence in the prevention of acute respiratory illness in older people. Conn. Med. 2003, 67, 469–474. [Google Scholar]
- Uciechowski, P.; Kahmann, L.; Plümäkers, B.; Malavolta, M.; Mocchegiani, E.; Dedoussis, G.; Herbein, G.; Jajte, J.; Fulop, T.; Rink, L. TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp. Gerontol. 2008, 43, 493–498. [Google Scholar] [CrossRef]
- Sharma, R.; Kapila, R.; Dass, G.; Kapila, S. Improvement in Th1/Th2 immune homeostasis, antioxidative status and resistance to pathogenic E. coli on consumption of probiotic Lactobacillus rhamnosus fermented milk in aging mice. Age 2014, 36, 9686. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; Macpherson, A.J. Interactions between the microbiota and the immune system. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Özçam, M.; Tocmo, R.; Oh, J.-H.; Afrazi, A.; Mezrich, J.D.; Roos, S.; Claesen, J.; van Pijkeren, J.-P. Gut symbionts Lactobacillus reuteri R2lc and 2010 encode a polyketide synthase cluster that activates the mammalian aryl hydrocarbon receptor. Appl. Environ. Microbiol. 2019, 85, e01661-18. [Google Scholar] [CrossRef]
- Tejada-Simon, M.V.; Lee, J.H.; Ustunol, Z.; Pestka, J.J. Ingestion of yogurt containing Lactobacillus acidophilus and Bifidobacterium to potentiate immunoglobulin A responses to cholera toxin in mice. J. Dairy Sci. 1999, 82, 649–660. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Lee, H.-J.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Lactobacillus plantarum C29 alleviates NF-κB activation and Th17/Treg imbalance in mice with TNBS-induced colitis. Food Agric. Immunol. 2018, 29, 577–589. [Google Scholar] [CrossRef]
- Roessler, A.; Friedrich, U.; Vogelsang, H.; Bauer, A.; Kaatz, M.; Hipler, U.C.; Schmidt, I.; Jahreis, G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin. Exp. Allergy 2008, 38, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, S.; Jung, S.K.; Kim, D.; Oh, K.B.; Yang, H.; Lee, H.C.; Jin, J.Y.; Sun, L.H.; Lee, S.; Byun, S.J. Oral administration of Lactobacillus reuteri expressing a 3D8 single-chain variable fragment (scFv) enhances chicken growth and conserves immune homeostasis. 3Biotech 2019, 9, 282. [Google Scholar] [CrossRef]
- Cross, M.L.; Ganner, A.; Teilab, D.; Fray, L.M. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol. Med Microbiol. 2004, 42, 173–180. [Google Scholar] [CrossRef]
- Hart, A.L.; Lammers, K.; Brigidi, P.; Vitali, B.; Rizzello, F.; Gionchetti, P.; Campieri, M.; Kamm, M.A.; Knight, S.C.; Stagg, A.J. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004, 53, 1602–1609. [Google Scholar] [CrossRef]
- Zhu, Z.; Ye, J.; Ma, Y.; Hua, P.; Huang, Y.; Fu, X.; Li, D.; Yuan, M.; Xia, Z. Function of T regulatory type 1 cells is down-regulated and is associated with the clinical presentation of coronary artery disease. Hum. Immunol. 2018, 79, 564–570. [Google Scholar] [CrossRef]
- Velez, E.M.M.; Galdeano, C.M.; Carmuega, E.; Weill, R.; Bonet, M.E.B.; Perdigón, G. Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice. Br. J. Nutr. 2015, 114, 566–576. [Google Scholar] [CrossRef]
- Anatriello, E.; Cunha, M.; Nogueira, J.; Carvalho, J.L.; Sá, A.K.; Miranda, M.; Castro-Faria-Neto, H.; Keller, A.C.; Aimbire, F. Oral feeding of Lactobacillus bulgaricus N45.10 inhibits the lung inflammation and airway remodeling in murine allergic asthma: Relevance to the Th1/Th2 cytokines and STAT6/T-bet. Cell. Immunol. 2019, 341, 103928. [Google Scholar] [CrossRef]
- Sheih, Y.-H.; Chiang, B.-L.; Wang, L.-H.; Liao, C.-K.; Gill, H.S. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J. Am. Coll. Nutr. 2001, 20, 149–156. [Google Scholar] [CrossRef]
- Jang, Y.J.; Kim, W.-K.; Han, D.H.; Lee, K.; Ko, G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes 2019, 10, 696–711. [Google Scholar] [CrossRef]
- Perdigón, G.; Fuller, R.; Raya, R. Lactic acid bacteria and their effect on the immune system. Curr. Issues Intest. Microbiol. 2001, 2, 27–42. [Google Scholar]
- Villena, J.; Oliveira, M.L.S.; Ferreira, P.C.D.; Salva, S.; Alvarez, S. Lactic acid bacteria in the prevention of pneumococcal respiratory infection: Future opportunities and challenges. Int. Immunopharmacol. 2011, 11, 1633–1645. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Núñez, I.N.; de Moreno de LeBlanc, A.; Carmuega, E.; Weill, R.; Perdigón, G. Impact of a probiotic fermented milk in the gut ecosystem and in the systemic immunity using a non-severe protein-energy-malnutrition model in mice. BMC Gastroenterol. 2011, 11, 64. [Google Scholar] [CrossRef]
- De Moreno de LeBlanc, A.; Castillo, N.A.; Perdigon, G. Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection. Int. J. Food Microbiol. 2010, 138, 223–231. [Google Scholar] [CrossRef]
- Villena, J.; Medina, M.; Raya, R.; Alvarez, S. Oral immunization with recombinant Lactococcus lactis confers protection against respiratory pneumococcal infection. Can. J. Microbiol. 2008, 54, 845–853. [Google Scholar] [CrossRef]
- Yasui, H.; Kiyoshima, J.; Hori, T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin. Vaccine Immunol. 2004, 11, 675–679. [Google Scholar] [CrossRef]
- Salva, S.; Villena, J.; Alvarez, S. Immunomodulatory activity of Lactobacillus rhamnosus strains isolated from goat milk: Impact on intestinal and respiratory infections. Int. J. Food Microbiol. 2010, 141, 82–89. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Covasa, M. Emerging roles of lactic acid bacteria in protection against colorectal cancer. World J. Gastroenterol. 2014, 20, 7878–7886. [Google Scholar] [CrossRef]
- Sanz, A.; Stefanatos, R.K.A. The Mitochondrial Free Radical Theory of Aging: A Critical View. Curr. Aging Sci. 2010, 1, 10–21. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yi, R.; Zhou, X.; Mu, J.; Long, X.; Pan, Y.; Song, J.-L.; Park, K.-Y. Preventive effect of Lactobacillus plantarum KSFY02 isolated from naturally fermented yogurt from Xinjiang, China, on D-galactose–induced oxidative aging in mice. J. Dairy Sci. 2019, 102, 5899–5912. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Du, P.; Smith, E.E.; Wang, S.; Jiao, Y.; Guo, L.; Huo, G.; Liu, F. In vitro and in vivo evaluation of an exopolysaccharide produced by Lactobacillus helveticus KLDS1.8701 for the alleviative effect on oxidative stress. Food Funct. 2019, 10, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef]
- Ding, W.; Wang, L.; Zhang, J.; Ke, W.; Zhou, J.; Zhu, J.; Guo, X.; Long, R. Characterization of antioxidant properties of lactic acid bacteria isolated from spontaneously fermented yak milk in the Tibetan Plateau. J. Funct. Foods 2017, 35, 481–488. [Google Scholar] [CrossRef]
- Sun, J.; Hu, X.-L.; Le, G.-W.; Shi, Y.-H. Lactobacilli prevent hydroxy radical production and inhibit Escherichia coli and Enterococcus growth in system mimicking colon fermentation. Lett. Appl. Microbiol. 2010, 50, 264–269. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; del Carmen, S.; Miyoshi, A.; Azevedo, V.; Sesma, F.; Langella, P.; Bermúdez-Humarán, L.G.; Watterlot, L.; Perdigon, G.; de Moreno de LeBlanc, A. Use of superoxide dismutase and catalase producing lactic acid bacteria in TNBS induced Crohn’s disease in mice. J. Biotechnol. 2011, 151, 287–293. [Google Scholar] [CrossRef]
- Daniel, C.; Poiret, S.; Goudercourt, D.; Dennin, V.; Leyer, G.; Pot, B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl. Environ. Microbiol. 2006, 72, 5799–5805. [Google Scholar] [CrossRef]
- Ahire, J.J.; Mokashe, N.U.; Patil, H.J.; Chaudhari, B.L. Antioxidative potential of folate producing probiotic Lactobacillus helveticus CD6. J. Food Sci. Technol. 2013, 50, 26–34. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Astiazarán-García, H.; Estrada-Montoya, M.C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Modulatory Effect of the Intracellular Content of Lactobacillus casei CRL 431 Against the Aflatoxin B1-Induced Oxidative Stress in Rats. Probiotics Antimicrob. Proteins 2019, 11, 470–477. [Google Scholar] [CrossRef]
- Wang, A.N.; Yi, X.W.; Yu, H.F.; Dong, B.; Qiao, S.Y. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J. Appl. Microbiol. 2009, 107, 1140–1148. [Google Scholar] [CrossRef]
- Xu, C.; Qiao, L.; Ma, L.; Guo, Y.; Dou, X.; Yan, S.; Zhang, B.; Roman, A. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate intestinal epithelial barrier dysfunction caused by oxidative stress via nrf2 signaling-mediated mitochondrial pathway. Int. J. Nanomed. 2019, 14, 4491–4502. [Google Scholar] [CrossRef]
- Songisepp, E.; Kullisaar, T.; Hütt, P.; Elias, P.; Brilene, T.; Zilmer, M.; Mikelsaar, M. A new probiotic cheese with antioxidative and antimicrobial activity. J. Dairy Sci. 2004, 87, 2017–2023. [Google Scholar] [CrossRef]
- Lin, X.; Xia, Y.; Wang, G.; Xiong, Z.; Zhang, H.; Lai, F.; Ai, L. Lactobacillus plantarum AR501 Alleviates the Oxidative Stress of D-Galactose-Induced Aging Mice Liver by Upregulation of Nrf2-Mediated Antioxidant Enzyme Expression. J. Food Sci. 2018, 83, 1990–1998. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Wang, Y.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef]
- Jones, R.M.; Neish, A.S. Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 2017, 105, 41–47. [Google Scholar] [CrossRef]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–284. [Google Scholar] [CrossRef]
- Kim, D.H.; Feinbaum, R.; Alloing, G.; Emerson, F.E.; Garsin, D.A.; Inoue, H.; Tanaka-Hino, M.; Hisamoto, N.; Matsumoto, K.; Tan, M.-W.; et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 2002, 297, 623–626. [Google Scholar] [CrossRef]
- Zugasti, O.; Ewbank, J.J. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat. Immunol. 2009, 10, 249–256. [Google Scholar] [CrossRef]
- So, S.; Tokumaru, T.; Miyahara, K.; Ohshima, Y. Control of lifespan by food bacteria, nutrient limitation and pathogenicity of food in C. elegans. Mech. Ageing Dev. 2011, 132, 210–212. [Google Scholar] [CrossRef]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; et al. Anti-Inflammatory Lactobacillus rhamnosus CNCM I-3690 Strain Protects against Oxidative Stress and Increases Lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e52493. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236. [Google Scholar] [CrossRef]
- Van der Hoeven, R.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar] [CrossRef]
- Inoue, H.; Hisamoto, N.; An, J.H.; Oliveira, R.P.; Nishida, E.; Blackwell, T.K.; Matsumoto, K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005, 19, 2278–2283. [Google Scholar] [CrossRef]
- Papp, D.; Csermely, P.; Sőti, C. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 2012, 8, e1002673. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Malyar, R.M.; Li, H.; Enayatullah, H.; Hou, L.; Farid, R.A.; Liu, D.; Bhat, J.A.; Miao, J.; Gan, F.; Huang, K.; et al. Zinc-enriched probiotics enhanced growth performance, antioxidant status, immune function, gene expression, and morphological characteristics of Wistar rats raised under high ambient temperature. 3Biotech 2019, 9, 291. [Google Scholar] [CrossRef]
- Yu, X.; Li, S.; Yang, D.; Qiu, L.; Wu, Y.; Wang, D.; Shah, N.P.; Xu, F.; Wei, H. A novel strain of Lactobacillus mucosae isolated from a Gaotian villager improves in vitro and in vivo antioxidant as well as biological properties in d-galactose-induced aging mice. J. Dairy Sci. 2016, 99, 903–914. [Google Scholar] [CrossRef]
- Kaushal, D.; Kansal, V.K. Probiotic Dahi containing Lactobacillus acidophilus and Bifidobacterium bifidum alleviates age-inflicted oxidative stress and improves expression of biomarkers of ageing in mice. Mol. Biol. Rep. 2012, 39, 1791–1799. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Su, Y.-W.; Ong, W.-K.; Cheng, T.-H.; Tsai, Y.-C. Oral administration of Lactobacillus plantarum K68 ameliorates DSS-induced ulcerative colitis in BALB/c mice via the anti-inflammatory and immunomodulatory activities. Int. Immunopharmacol. 2011, 11, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, J.; Zhou, X.; Yi, R.; Mu, J.; Long, X.; Pan, Y.; Zhao, X.; Liu, W. Lactobacillus plantarum CQPC11 isolated from Sichuan pickled cabbages antagonizes D-galactose-induced oxidation and aging in mice. Molecules 2018, 23, 3026. [Google Scholar] [CrossRef] [PubMed]
- Wolitzky-Taylor, K.B.; Castriotta, N.; Lenze, E.J.; Stanley, M.A.; Craske, M.G. Anxiety disorders in older adults: A comprehensive review. Depress. Anxiety 2010, 27, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Goyal, A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Chen, L.-H.; Chen, Y.-H.; Cheng, K.-C.; Chien, T.-Y.; Chan, C.-H.; Tsao, S.-P.; Huang, H.-Y. Antiobesity effect of Lactobacillus reuteri 263 associated with energy metabolism remodeling of white adipose tissue in high-energy-diet-fed rats. J. Nutr. Biochem. 2018, 54, 87–94. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liu, W.-H.; Wu, C.-C.; Juan, Y.-C.; Wu, Y.-C.; Tsai, H.-P.; Wang, S.; Tsai, Y.-C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; dos Santos Pedroso, G.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats Through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef]
- Bäckman, L.; Nyberg, L.; Lindenberger, U.; Li, S.-C.; Farde, L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci. Biobehav. Rev. 2006, 30, 791–807. [Google Scholar] [CrossRef]
- Luo, J.; Wang, T.; Liang, S.; Hu, X.; Li, W.; Jin, F. Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 2014, 57, 327–335. [Google Scholar] [CrossRef]
- Savignac, H.M.; Tramullas, M.; Kiely, B.; Dinan, T.G.; Cryan, J.F. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015, 287, 59–72. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Takeda, T.; Hosokawa, M.; Takeshita, S.; Irino, M.; Higuchi, K.; Matsushita, T.; Tomita, Y.; Yasuhira, K.; Hamamoto, H.; Shimizu, K.; et al. A new murine model of accelerated senescence. Mech. Ageing Dev. 1981, 17, 183–194. [Google Scholar] [CrossRef]
- Pérez-Cáceres, D.; Ciudad-Roberts, A.; Rodrigo, M.T.; Pubill, D.; Camins, A.; Camarasa, J.; Escubedo, E.; Pallàs, M. Depression-like behavior is dependent on age in male SAMP8 mice. Biogerontology 2013, 14, 165–176. [Google Scholar] [CrossRef]
- Rhea, E.M.; Banks, W.A. The SAMP8 mouse for investigating memory and the role of insulin in the brain. Exp. Gerontol. 2017, 94, 64–68. [Google Scholar] [CrossRef]
- Zaydi, A.I.; Lew, L.-C.; Hor, Y.-Y.; Jaafar, M.H.; Chuah, L.-O.; Yap, K.-P.; Azlan, A.; Azzam, G.; Liong, M.-T. Lactobacillus plantarum DR7 improved brain health in aging rats via the serotonin, inflammatory and apoptosis pathways. Benef. Microbes 2020, 11, 753–766. [Google Scholar] [CrossRef]
- Chong, H.X.; Yusoff, N.A.A.; Hor, Y.Y.; Lew, L.C.; Jaafar, M.H.; Choi, S.B.; Yusoff, M.S.B.; Wahid, N.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 355–373. [Google Scholar] [CrossRef]
- De Palma, G.; Collins, S.M.; Bercik, P. The microbiota-gut-brain axis in functional gastrointestinal disorders. Gut Microbes 2014, 5, 419–429. [Google Scholar] [CrossRef]
- Distrutti, E.; O’Reilly, J.-A.; McDonald, C.; Cipriani, S.; Renga, B.; Lynch, M.A.; Fiorucci, S. Modulation of intestinal microbiota by the probiotic VSL#3 resets brain gene expression and ameliorates the age-related deficit in LTP. PLoS ONE 2014, 9, e106503. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Liu, C.-F.; Tseng, K.-C.; Mau, J.-L.; Pan, T.-M. Immunomodulatory effects of dead Lactobacillus on murine splenocytes and macrophages. Food Agric. Immunol. 2012, 23, 183–202. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Chen, L.-H.; Wang, M.-F.; Hsu, C.-C.; Chan, C.-H.; Li, J.-X.; Huang, H.-Y. Lactobacillus paracasei PS23 delays progression of age-related cognitive decline in senescence accelerated mouse prone 8 (SAMP8) mice. Nutrients 2018, 10, 894. [Google Scholar] [CrossRef]
- Cho, S. The Role of Functional Foods in Cutaneous Anti-aging. J. Lifestyle Med. 2014, 4, 8–16. [Google Scholar] [CrossRef]
- Vierkötter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermato-Endocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef]
- Quan, T.; Fisher, G.J. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: A mini-review. Gerontology 2015, 61, 427–434. [Google Scholar] [CrossRef]
- Han, S.; Shin, J.; Lim, S.; Ahn, H.Y.; Kim, B.; Cho, Y. Dietary effect of Lactobacillus plantarum CJLP55 isolated from kimchi on skin pH and its related biomarker levels in adult subjects. J. Nutr. Health 2019, 52, 149–156. [Google Scholar] [CrossRef]
- Guéniche, A.; Philippe, D.; Bastien, P.; Blum, S.; Buyukpamukcu, E.; Castiel-Higounenc, I. Probiotics for photoprotection. Derm. Endocrinol. 2009, 1, 275–279. [Google Scholar] [CrossRef]
- Peguet-Navarro, J.; Dezutter-Dambuyant, C.; Buetler, T.; Leclaire, J.; Smola, H.; Blum, S.; Bastien, P.; Breton, L.; Gueniche, A. Supplementation with oral probiotic bacteria protects human cutaneous immune homeostasis after UV exposure-double blind, randomized, placebo controlled clinical trial. Eur. J. Dermatol. 2008, 18, 504–511. [Google Scholar] [CrossRef]
- Bouilly-Gauthier, D.; Jeannes, C.; Maubert, Y.; Duteil, L.; Queille-Roussel, C.; Piccardi, N.; Montastier, C.; Manissier, P.; Piérard, G.; Ortonne, J.-P. Clinical evidence of benefits of a dietary supplement containing probiotic and carotenoids on ultraviolet-induced skin damage. Br. J. Dermatol. 2010, 163, 536–543. [Google Scholar] [CrossRef]
- Foolad, N.; Brezinski, E.A.; Chase, E.P.; Armstrong, A.W. Effect of nutrient supplementation on atopic dermatitis in children: A systematic review of probiotics, prebiotics, formula, and fatty acids. JAMA Dermatol. 2013, 149, 350–355. [Google Scholar] [CrossRef]
- Ta, V.; Laubach, S. Probiotic Administration in Early Life, Atopy, and Asthma: A Meta-Analysis of Clinical Trials. Pediatrics 2014, 134, S141. [Google Scholar] [CrossRef]
- Satoh, T.; Murata, M.; Iwabuchi, N.; Odamaki, T.; Wakabayashi, H.; Yamauchi, K.; Abe, F.; Xiao, J.Z. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Benef. Microbes 2015, 6, 497–504. [Google Scholar] [CrossRef]
- Ra, J.; Lee, D.E.; Kim, S.H.; Jeong, J.-W.; Ku, H.K.; Kim, T.-Y.; Choi, I.-D.; Jeung, W.; Sim, J.-H.; Ahn, Y.-T. Effect of oral administration of Lactobacillus plantarum HY7714 on epidermal hydration in ultraviolet B-irradiated hairless mice. J. Microbiol. Biotechnol. 2014, 24, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.E.; Huh, C.-S.; Ra, J.; Choi, I.-D.; Jeong, J.-W.; Kim, S.-H.; Ryu, J.H.; Seo, Y.K.; Koh, J.S.; Lee, J.-H.; et al. Clinical evidence of effects of Lactobacillus plantarum HY7714 on skin aging: A randomized, double blind, placebo-controlled study. J. Microbiol. Biotechnol. 2015, 25, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lim, J.; Bang, I.; Kim, I.; Kim, D.; Kang, S.C. Fermentation product with new equol-producing Lactobacillus paracasei as a probiotic-like product candidate for prevention of skin and intestinal disorder. J. Sci. Food Agric. 2019, 99, 4200–4210. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishaq, M.; Khan, A.; Bacha, A.S.; Shah, T.; Hanif, A.; Ahmad, A.A.; Ke, W.; Li, F.; Ud Din, A.; Ding, Z.; et al. Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review. Antioxidants 2021, 10, 1930. https://doi.org/10.3390/antiox10121930
Ishaq M, Khan A, Bacha AS, Shah T, Hanif A, Ahmad AA, Ke W, Li F, Ud Din A, Ding Z, et al. Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review. Antioxidants. 2021; 10(12):1930. https://doi.org/10.3390/antiox10121930
Chicago/Turabian StyleIshaq, Muhammad, Ashiq Khan, Ali Sher Bacha, Tariq Shah, Anum Hanif, Anum Ali Ahmad, Wencan Ke, Fuhou Li, Ahmad Ud Din, Zitong Ding, and et al. 2021. "Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review" Antioxidants 10, no. 12: 1930. https://doi.org/10.3390/antiox10121930
APA StyleIshaq, M., Khan, A., Bacha, A. S., Shah, T., Hanif, A., Ahmad, A. A., Ke, W., Li, F., Ud Din, A., Ding, Z., & Guo, X. (2021). Microbiota Targeted Interventions of Probiotic Lactobacillus as an Anti-Ageing Approach: A Review. Antioxidants, 10(12), 1930. https://doi.org/10.3390/antiox10121930







