Chemical and Biological Profile and Allergenicity of Thymus baicalensis Plant of Mongolian Origin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Extraction of Phenolic and Flavonoid Compounds
2.3. Total Phenolic and Flavonoid Content
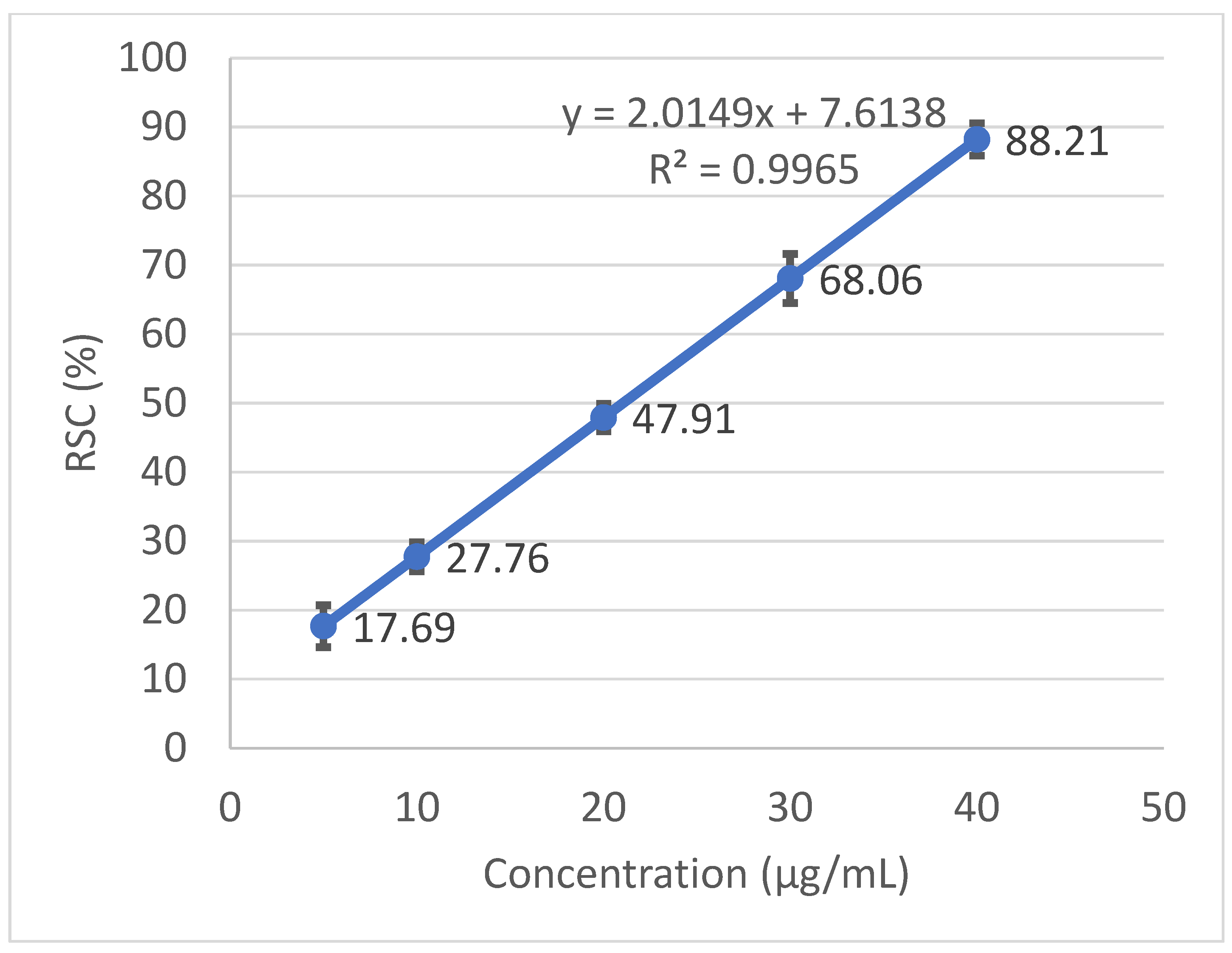
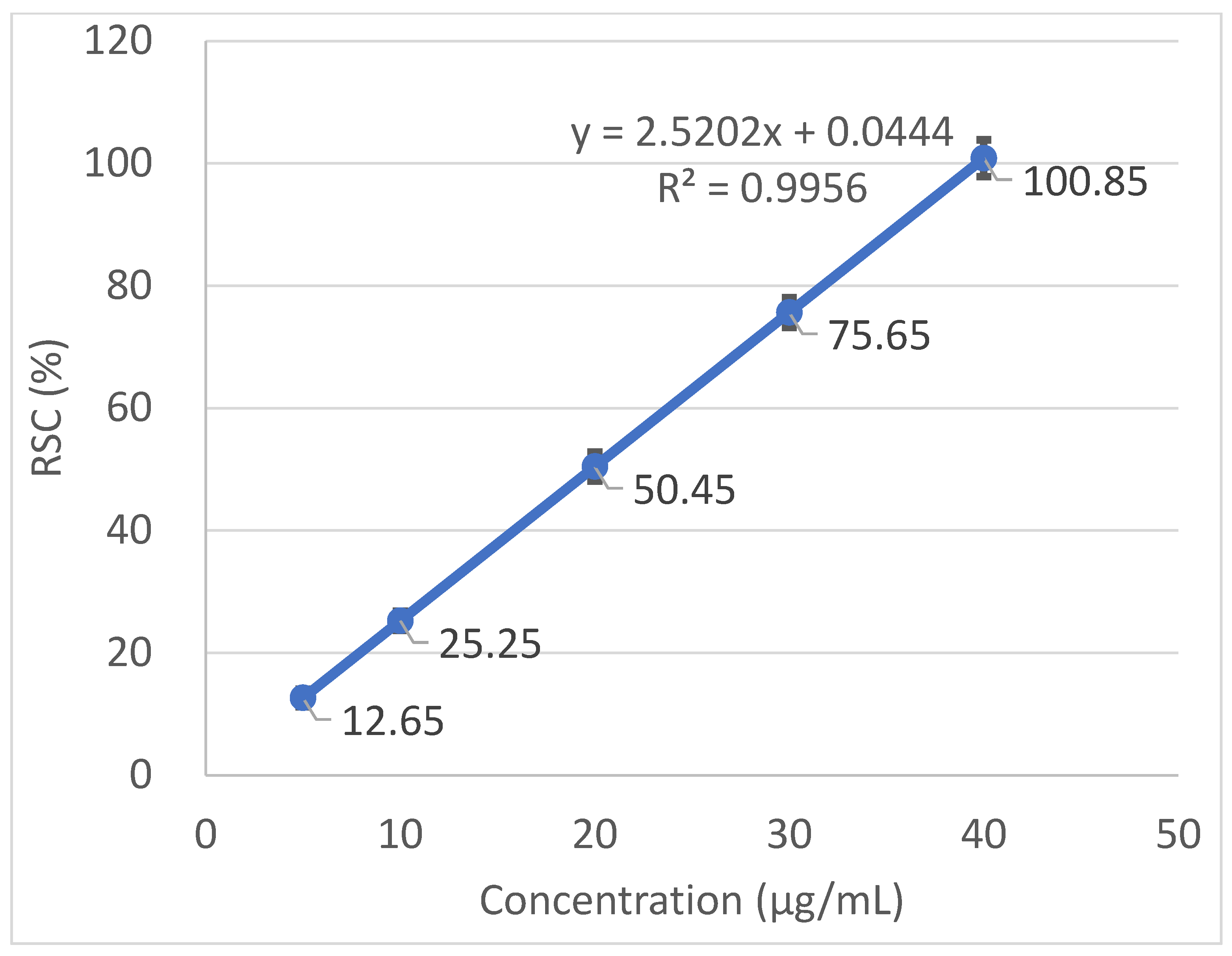
2.4. Free Radical Scavenging Activity and Calculation of the IC50
- A—average absorbance of the sample;
- A0—average absorbance of a control (DPPH).
2.5. GC-MS Determination of Volatile Oil Components
2.6. LC-MS Determination of Phenolic Compounds
2.7. Extraction of Lipids
2.8. GC-MS Determination of Lipids
2.9. Antimicrobial Activity
2.10. Extraction of Food Allergens
2.11. Allergenic Protein Content Determination
3. Results
3.1. Spectrophotometric Determination of Total Phenolic and Flavonoid Content
3.2. Antioxidant Activity
3.3. GC-MS Determination of Volatile Oil Composition
3.4. LC-MS Determination of Polyphenolic Compounds
3.5. LC-MS Determination of Lipids
3.6. Antimicrobial Properties
3.7. Ingestive Allergenicity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TFC | total flavonoid content |
| TPC | total phenolic content |
| GAE | gallic acid |
| QE | quercitin |
| DPPH | 2,2-difenylo-1-pikrylohydrazyl |
| IC50 | half-maximal inhibitory concentration |
| GC-MS | gas chromatography-mass spectroscopy |
| LC-MS | liquid chromatography-mass spectroscopy |
| MIC | minimum inhibitory concentration |
| TSFA | total saturated fatty acid |
| TMFA | total monounsaturated fatty acid |
| TPFA | total polyunsaturated fatty acid |
References
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schafer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Talovskaya, E.B. Thymus baicalensis (Lamiaceae) Morphological Transformation Under Different Environmental Conditions. Contemp. Probl. Ecol. 2015, 8, 607–613. [Google Scholar] [CrossRef]
- Chirikova, N.K.; Olennikov, D.N. Phenolic Compounds from Siberian Species Thymus baicalensis and T. sibiricus. Chem. Nat. Compd. 2018, 54, 572–576. [Google Scholar] [CrossRef]
- Sarfaraz, D.; Rahimmalek, M.; Saeidi, G. Polyphenolic and Molecular Variation in Thymus Species Using HPLC and SRAP Analyses. Sci. Rep. 2021, 11, 5019. [Google Scholar] [CrossRef] [PubMed]
- Figas, A.; Tomaszewska-Sowa, M.; Kobierski, M.; Sawilska, A.K.; Klimkowska, K. Hazard of Contamination with Heavy Metals in Thymus serpyllum L. Herbs from Rural Areas. Agriculture 2021, 11, 375. [Google Scholar] [CrossRef]
- Ouknin, M.; Abderrahmane, R.; Arjouni, M.; Majidi, L. Mineral Composition, Multivariate Analysis of Some Oligo-Elements and Heavy Metals in Some Species of Genus Thymus. J. Mater. Environ. Sci. 2018, 9, 980–985. [Google Scholar]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocarlan, N.; Spinu, M. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Chen, Q.; Zhang, Z.; He, H.; Ma, X.; Liu, Z.; Ge, B.; Huang, F. Novel Enteromorpha Prolifera Based Carbon Dots: Probing the Radical Scavenging of Natural Phenolic Compounds. Colloids Surf. B Biointerfaces 2019, 174, 161–167. [Google Scholar] [CrossRef]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary Polyphenols in Metabolic and Neurodegenerative Diseases: Molecular Targets in Autophagy and Biological Effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and Negative Effects of Polyphenol Incorporation in Baked Foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Ivasenko, S.; Zhumabekova, A.; Ludwiczuk, A.; Skalicka–Wozniak, K.; Marchenko, A.; Ishmuratova, M.; Poleszak, E.; Korona-Glowniak, I.; Akhmetova, S.; Karilkhan, I.; et al. Antimicrobial Activity and Polyphenol Profiles of Hydroalcoholic Extracts of Thymus rasitatus Klokov and Thymus eremita Klokov. Maced J. Med. Sci. 2021, 9, 313–317. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Said, Z.; Garrouj, D.; Pagán, R.; Chabi, M.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of Harvest Time on Yield, Chemical Composition, Antimicrobial and Antioxidant Activities of Thymus vulgaris and Mentha pulegium Essential Oils. Eur J. Med. Plants 2015, 8, 69–77. [Google Scholar]
- Rabzhaeva, A. Component composition of the essential oil of Thymus baicalensis (lamiaceae) at different seasonal stage. Rastitel’nye Resursy 2012, 48, 234–237. [Google Scholar]
- Tabti, L.; El Amine Dib, M.; Gaouar Benyelles, N.; Djabou, N.; Bouayad Alam, S.; Paolini, J.; Costa, J.; Muselli, A. Fatty-Acid Composition and Antifungal Activity of Extracts of Thymus capitatus. J. Herbs Spices Med. Plants 2015, 21, 203–210. [Google Scholar] [CrossRef]
- Zaïri, A.; Nouir, S.; Zarrouk, A.; Haddad, H.; Khélifa, A.; Achour, L.; Tangy, F.; Chaouachi, M.; Trabelsi, M. Chemical Composition, Fatty Acids Profile and Biological Properties of Thymus capitatus (L.) Hoffmanns, Essential Oil. Sci. Rep. 2019, 9, 20134. [Google Scholar] [CrossRef]
- Kalsoom, R.; Haider, M.; Chohan, S. Phytochemical Analysis and Antifungal Activity of Some Medicinal Plants Against Alternaria Specie Isolated from Onion. J. Anim. Plant Sci. 2020, 30, 454–460. [Google Scholar]
- Sójka, M.; Macierzyński, J.; Zaweracz, W.; Buczek, M. Transfer and Mass Balance of Ellagitannins, Anthocyanins, Flavan-3-ols, and Flavonols during the Processing of Red Raspberries (Rubus idaeus L.) to Juice. J. Agric. Food Chem. 2016, 64, 5549–5563. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard, M07-A10; CLSI: Wayne, PA, USA, 2015; Volume 35. [Google Scholar]
- Gedikoğlu, A.; Sökmen, M.; Çivit, A. Evaluation of Thymus vulgaris and Thymbra spicata Essential Oils and Plant Extracts for Chemical Composition, Antioxidant, and Antimicrobial Properties. Food Sci. Nutr. 2019, 7, 1704–1714. [Google Scholar] [CrossRef] [Green Version]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Cardoso, S.M. Health-Promoting Effects of Thymus Phenolic-Rich Extracts: Antioxidant, Anti-inflammatory and Antitumoral Properties. Antioxidants 2020, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, O.; Hazzit, M.; Mouhouche, F.; Baaliouamer, A. Influence of Extraction Duration on the Chemical Composition and Biological Activities of Essential Oil of Thymus pallescens de Noé. Arab. J. Sci. Eng. 2015, 40, 1855–1865. [Google Scholar] [CrossRef]
- Jarić, S.; Mitrović, M.; Pavlović, P. Review of Ethnobotanical, Phytochemical, and Pharmacological Study of Thymus serpyllum L. eCAM 2015, 2015, 101978. [Google Scholar]
- Nikolić, B.; Matović, M.; Mladenović, K.; Todosijević, M.; Stanković, J.; Đorđević, I.; Marin, P.D.; Tešević, V. Volatiles of Thymus serpyllum Obtained by Three Different Methods. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Fierascu, R.C.; Fierascu, I.; Baroi, A.M.; Ortan, A. Selected Aspects Related to Medicinal and Aromatic Plants as Alternative Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 1521. [Google Scholar] [CrossRef]
- Kiliç, Ö.; Özdemir, F.A. Essential Oil Composition of Two Thymus kotschyanus Boiss. Varietes from Elazığ (Turkey). Prog. Nutr. 2017, 19, 85–89. [Google Scholar]
- Mseddi, K.; Alimi, F.; Noumi, E.; Veettil, V.N.; Deshpande, S.; Adnan, M.; Hamdi, A.; Elkahoui, S.; Alghamdi, A.; Kadri, A.; et al. Thymus musilii Velen. as a Promising Source of Potent Bioactive Compounds with its Pharmacological Properties: In Vitro and In Silico Analysis. Arab. J. Chem. 2020, 13, 6782–6801. [Google Scholar] [CrossRef]
- Mohammadi, S.; Tabrizi, L.; Shokrpour, M.; Hadian, J.; Schulz, H.; Riewe, D. Morphological and Phytochemical Screening of Some Thymus Ecotypes (Thymus spp.) Native to Iran in Order to Select Elite Genotypes. J. Appl. Bot. Food Qual. 2020, 93, 186–196. [Google Scholar]
- Khorasany, S.; Azizi, M.; Barzegar, M.; Hamidi Esfahani, Z. A Study on the Chemical Composition and Antifungal Activity of Essential Oil from Thymus caramanicus, Thymus daenensis and Ziziphora clinopodiaides. Acta Sci. Agric. 2018, 2, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Dizajeyekan, Y.; Razban Haghighi, A.; Rangavaran, R. A first report of a new chemotype of Thymus migricus (Klokov & Desj-Shost) from East Azarbaijan province of Iran. J. Agric. Sci. 2016, 61, 163–169. [Google Scholar]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus proximus Serg. Essential Oil and Its Major Constituents. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Khanahmadi, F.; Rahimmalek, M.; Sabzalian, M.R.; Tohidi, B. Variation of Essential Oil Content and Composition, Phenolics, and Yield Related Traits Using Different Pollination Systems in Populations of Thymus Species. Chem Biodivers. 2021, 18, e2100097. [Google Scholar] [CrossRef]
- Mahboubi, M.; Heidarytabar, R.; Mahdizadeh, E.; Hosseini, H. Antimicrobial Activity and Chemical Composition of Thymus Species and Zataria multiflora Essential Oils. Agric. Nat. Resour. 2017, 51, 395–401. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Darwish, N.H.E.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In Vitro Antifungal and Topical Anti-Inflammatory Properties of Essential Oil from Wild-Growing Thymus vulgaris (Lamiaceae) Used for Medicinal Purposes in Algeria: A New Source of Carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Güvenir, M.; Hanoğlu, A.; Yiğit Hanoğlu, D.; Süer, K.; Baser, K.H.C.; Yavuz, D.; Sanlidag, T. Antimicrobial Activity of the Essential oil of Thymus capitatus against Helicobacter pylori. Acta Pol. Pharm. 2020, 77, 155–160. [Google Scholar] [CrossRef]
- Rodrigues, V.; Cabral, C.; Évora, L.; Ferreira, I.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Chemical Composition, Anti-inflammatory Activity and Cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. Essential Oil and Its Main Compounds. Arab. J. Chem. 2019, 12, 3236–3243. [Google Scholar]
- Simeoni, M.C.; Pellegrini, M.; Sergi, M.; Pittia, P.; Ricci, A.; Compagnone, D. Analysis of Polyphenols in the Lamiaceae Family by Matrix Solid-Phase Dispersion Extraction Followed by Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination. ACS Omega 2018, 3, 17610–17616. [Google Scholar] [CrossRef]
- Sonmezdag, A.S.; Kelebek, H.; Selli, S. Characterization of Aroma-Active and Phenolic Profiles of Wild Thyme (Thymus serpyllum) by GC-MS-Olfactometry and LC-ESI-MS/MS. JFST 2016, 53, 1957–1965. [Google Scholar] [CrossRef] [Green Version]
- Raudone, L.; Zymone, K.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V.; Janulis, V. Phenological Changes in Triterpenic and Phenolic Composition of Thymus L. Species. Ind. Crops Prod. 2017, 109, 445–451. [Google Scholar] [CrossRef]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous Characterization and Quantification of Phenolic Compounds in Thymus x citriodorus Using a Validated HPLC–UV and ESI–MS Combined Method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Cacan, E.; Kokten, K.; Kilic, O. Leaf fatty acid composition of some Lamiaceae taxa from Turkey. Prog. Nutr. 2018, 20, 231–236. [Google Scholar]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Ahmad, A.; Van Vuuren, S.; Viljoen, A. Unravelling the Complex Antimicrobial Interactions of Essential Oils—The Case of Thymus vulgaris (Thyme). Molecules 2014, 19, 2896–2910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Džamić, A.M.; Nikolić, B.J.; Giweli, A.A.; Mitić-Ćulafić, D.S.; Soković, M.D.; Ristić, M.S.; Knežević-Vukčević, J.B.; Marin, P.D. Libyan Thymus Capitatus Essential Oil: Antioxidant, Antimicrobial, Cytotoxic and Colon Pathogen Adhesion-Inhibition Properties. J. Appl. Microbiol. 2015, 119, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, T.; Winther, L.; Till, S.J.; Panzner, P.; Knulst, A.; Valovirta, E. Birch Pollen Allergy in Europe. Allergy 2019, 74, 1237–1248. [Google Scholar] [CrossRef] [Green Version]
- Aninowski, M.; Leszczyńska, J. The Determination of Potentially Allergenicity of Selected Herbs. Biotech. Food Sci. 2019, 83, 3–11. [Google Scholar]
| Disease | Way of Preparing Herbs |
|---|---|
| Respiratory tract Pharyngeal diseases Periodontal infections | Hot water extract |
| Antiseptic (cleaner or sanitizer) | Oil extract |
| Stress and depression symptoms Poor general health | Burning |
| Balancing progesterone hormone | Oil extract |
| Alcohol addiction treatment | Water extract |
| Compound | Total, % | Compound | Total, % |
|---|---|---|---|
| β-Thujene | 0.49 | Borneol | 5.65 |
| α-Pinene | 3.07 | Terpinen-4-ol | 17.12 |
| Camphene | 2.18 | α-Terpineol | 7.06 |
| Sabinene | 1.74 | Piperitol, cis | 0.23 |
| β-Pinene | 1.63 | Piperitol, trans | 0.15 |
| 2,3-Dehydro-1,8-cineole | 0.09 | Bornyl acetate | 0.24 |
| β-Myrcene | 26.15 | Thymol | 0.16 |
| α-Phellandrene | 0.08 | Carvacrol | 0.08 |
| α-Terpinene | 3.37 | Car-3-ene | 0.12 |
| β-Cymene | 1.08 | β-Bourbonene | 0.19 |
| 1,8-Cineole | 4.43 | Caryophylle | 3.14 |
| Limonene | 0.78 | Humulene | 0.16 |
| (E)-beta-ocimene | 0.03 | Germacrene D | 1.87 |
| γ-Terpinene | 6.98 | Bicyclogermacrene | 0.57 |
| Sabinene hydrate, cis | 2.15 | α-Farnesene | 0.10 |
| Terpinolene | 1.32 | δ-Cadinene | 0.10 |
| Linalool | 1.97 | Spathulenol | 0.23 |
| (Z)-p-menth-2-en-1-ol | 0.72 | Caryophyllene oxide | 0.62 |
| Camphor | 0.28 | ||
| Total identified | 96.33 |
| No. | Identification | Rt | UV Max | [M-H] m/z | Fragmentation Ions | Total, mg kg−1 |
|---|---|---|---|---|---|---|
| 1 | Quinic acid | 4.120 | 240 | 191.02 | 111.01, 87.01, 85.03 | 23.7 |
| 2 | Chlorogenic acid | 18.83 | 250/325 | 335.09 | 191.06 | 44.6 |
| 3 | Caffeic acid | 20.64 | 322 | 179.03 | 135.04 | 78.8 |
| 4 | Apigenin C-hexoside-C-hexoside | 21.54 | 271/342 | 593.15 | 473.11 | 228.1 |
| 5 | Rosmarinic acid derivative | 22.73 | 282 | 377.09 | 359.08, 197.05, 161.02 | 136.1 |
| 6 | 5-O-feruloyquinic acid | 24.36 | 325 | 367.10 | 191.06, 173.05 | 29.3 |
| 7 | Quercetin-7-O-glucuronide | 25.47 | 344 | 477.07 | 301.04 | 586.1 |
| 8 | Luteolin-7-O-glucuronide | 29.13 | 283/335 | 461.07 | 285.04, 113.02 | 10,112.1 |
| 9 | Apigenin-7-O-glucuronide | 33.26 | 269/342 | 445.08 | 269.05, 175.02, 113.02, | 3634.4 |
| 10 | Rosmarinic acid | 34.30 | 253/329 | 359.08 | 197.05, 161.02 | 1840.4 |
| Total polyphenols: 16,713.6 | ||||||
| Compound | Total, % |
|---|---|
| Caproic acid (C6:0) | 0.89 |
| Myristic acid (C14:0) | 1.44 |
| Pentadecanoic acid (C15:0) | 0.61 |
| Palmitic acid (C16:0) | 31.05 |
| Stearic acid (C18:0) | 2.45 |
| Arachidic acid (C20:0) | 3.49 |
| Behenic acid (C22:0) | 1.30 |
| Lignoceric acid (C24:0) | 3.09 |
| Nonanedioic acid, dimethyl- | 3.97 |
| Benzene-1,2-dicarboxylic acid, butyl- | 1.07 |
| Heptadecanoic acid, ethyl- | 2.37 |
| 2-methyloctacosane | 1.20 |
| Pentacosane | 1.51 |
| Hexacosane | 11.54 |
| Heptacosane | 1.51 |
| Octacosane | 1.98 |
| Nonacosane | 9.57 |
| Arachidic acid | 3.49 |
| 1,4-dihydroxy-p-menth-2-ene | 1.68 |
| Borneol | 1.82 |
| Ursolic acid | 4.24 |
| Oleanolic acid | 9.72 |
| Gram+ | MIC, %(v/v) | Gram- | MIC, %(v/v) | Fungi | MIC, %(v/v) |
|---|---|---|---|---|---|
| M. flavus | 4.162 | P. fluorescens | 4.162 | S. cerevisiae | 0.260 |
| S. aureus | 16.667 | P. aeruginosa | 8.325 | C. vini | 2.081 |
| B. subtilis | 33.333 | E. coli | 8.325 | A. niger | 8.325 |
| S. epidermidis | 33.333 | E. aerogenes | 16.667 | P. expansum | 8.325 |
| Species | Thymol (%) | Carvacrol (%) | p-Cymene (%) | γ-Terpinene (%) | Reference |
|---|---|---|---|---|---|
| T. kotschyanus | 26.3–31.2 | 19.5–24.3 | 11.2–17.6 | 5.3–8.4 | [27] |
| T. musilii | 67.7 | 3.4 | 4.6 | 2.6 | [28] |
| T. daenensis | 47.08–82.01 | 0.77–24.39 | 2.76–5.37 | 1.06–4.07 | [29] |
| T. caramanicus | 4.14 | 65.52 | 13.21 | 4.44 | [30] |
| T. migricus | 1.41 | 0.29 | - | - | [31] |
| T. proximus | 0.05 | 8.47 | 44.26 | 33.17 | [32] |
| T. trautvetteri | 63.3–71.2 | 5.35–12.3 | 2.16–3.18 | 0.37–1.09 | [33] |
| T. fedtschenkoi | 50.61 | 6.58 | 7.69 | 3.16 | [34] |
| T. vulgaris | 3.99 | 56.79 | 12.8 | 11.17 | [35] |
| T. capitatus | 47.2–57.1 | 5.7–8.5 | 12.3–15.1 | 4.9–10.0 | [36] |
| T. zygis | 19.5 | 16.3 | 22.0 | 7.4 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narangerel, T.; Sójka, M.; Bonikowski, R.; Jastrząbek, K.; Sroczyński, W.; Plucińska, A.; Kunicka-Styczyńska, A.; Śmigielski, K.; Majak, I.; Bartos, A.; et al. Chemical and Biological Profile and Allergenicity of Thymus baicalensis Plant of Mongolian Origin. Antioxidants 2021, 10, 1905. https://doi.org/10.3390/antiox10121905
Narangerel T, Sójka M, Bonikowski R, Jastrząbek K, Sroczyński W, Plucińska A, Kunicka-Styczyńska A, Śmigielski K, Majak I, Bartos A, et al. Chemical and Biological Profile and Allergenicity of Thymus baicalensis Plant of Mongolian Origin. Antioxidants. 2021; 10(12):1905. https://doi.org/10.3390/antiox10121905
Chicago/Turabian StyleNarangerel, Tuya, Michał Sójka, Radosław Bonikowski, Konrad Jastrząbek, Witold Sroczyński, Aleksandra Plucińska, Alina Kunicka-Styczyńska, Krzysztof Śmigielski, Iwona Majak, Adrian Bartos, and et al. 2021. "Chemical and Biological Profile and Allergenicity of Thymus baicalensis Plant of Mongolian Origin" Antioxidants 10, no. 12: 1905. https://doi.org/10.3390/antiox10121905






