Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health
Abstract
1. Introduction
2. Phytoestrogens—General Data
2.1. Isoflavones
2.2. Prenylflavonoids
2.3. Coumestans
2.4. Lignans
2.5. Stilbenes
3. Bioavailability and Metabolism of Dietary Phytoestrogens
3.1. Isoflavones
3.2. Prenylflavonoids
3.3. Coumestans
3.4. Lignans
3.5. Stilbenes
4. The Relationship between Dietary Phytoestrogens and Gut Microbiota: Impact on Human Health
4.1. The Content of Phytoestrogens in Food
4.2. Reciprocal Modulation between Dietary Phytoestrogens and Gut Microbiota
5. Epigenetic Modulator Capacity of Dietary Phytoestrogens
5.1. Isoflavones
5.2. Prenylflavonoids
5.3. Coumestans
5.4. Lignans
5.5. Stilbenes
6. Future Perspective and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Paul, B.; Barnes, S.; Demark-Wahnefried, W.; Morrow, C.; Salvador, C.; Skibola, C.; Tollefsbol, T.O. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clin. Epigenetics 2015, 7, 112. [Google Scholar] [CrossRef]
- Forslund, L.C.; Andersson, H.C. Phytoestrogens in Foods on the Nordic Market: A Literature Review on Occurrence and Levels, 2017th ed.; Nordisk Ministerråd: Copenhagen, Denmark, 2017; p. 179. [Google Scholar]
- Frankenfeld, C.L. Dairy consumption is a significant correlate of urinary equol concentration in a representative sample of US adults. Am. J. Clin. Nutr. 2011, 93, 1109–1116. [Google Scholar] [CrossRef]
- Krizova, L.; Dadakova, K.; Kasparovska, J.; Kasparovsky, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into Polyphenol and Gut Microbiota Crosstalk: Are Their Metabolites the Key to Understand Protective Effects against Metabolic Disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Knaze, V.; Lujan-Barroso, L.; Kuhnle, G.G.; Mulligan, A.A.; Touillaud, M.; Slimani, N.; Romieu, I.; Powell, N.; Tumino, R.; et al. Dietary intakes and food sources of phytoestrogens in the European Prospective Investigation into Cancer and Nutrition (EPIC) 24-hour dietary recall cohort. Eur. J. Clin. Nutr. 2012, 66, 932–941. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hemon, B.; Moskal, A.; Overvad, K.; Tjonneland, A.; Kyro, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Bukovsky, I.; Rembert, E.; Yonas, W.; Alwarith, J.; Barnard, N.D.; Kahleova, H. The Effects of Vegetarian and Vegan Diets on Gut Microbiota. Front. Nutr. 2019, 6, 47. [Google Scholar] [CrossRef]
- Peiroten, A.; Bravo, D.; Landete, J.M. Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit. Rev. Food Sci. Nutr. 2020, 60, 1922–1937. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hameed, A.S.; Rawat, P.S.; Meng, X.; Liu, W. Biotransformation of dietary phytoestrogens by gut microbes: A review on bidirectional interaction between phytoestrogen metabolism and gut microbiota. Biotechnol. Adv. 2020, 43, 107576. [Google Scholar] [CrossRef]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.; Kulling, S.E. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Rafii, F. The role of colonic bacteria in the metabolism of the natural isoflavone daidzin to equol. Metabolites 2015, 5, 56–73. [Google Scholar] [CrossRef]
- Possemiers, S.; Bolca, S.; Eeckhaut, E.; Depypere, H.; Verstraete, W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: Producer phenotyping and relation with intestinal community. FEMS Microbiol. Ecol. 2007, 61, 372–383. [Google Scholar] [CrossRef]
- Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules 2020, 25, 5709. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef]
- Huser, S.; Guth, S.; Joost, H.G.; Soukup, S.T.; Kohrle, J.; Kreienbrock, L.; Diel, P.; Lachenmeier, D.W.; Eisenbrand, G.; Vollmer, G.; et al. Effects of isoflavones on breast tissue and the thyroid hormone system in humans: A comprehensive safety evaluation. Arch. Toxicol. 2018, 92, 2703–2748. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Wozniak, K.; Aprotosoaie, A.C.; Trifan, A. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. Nutr. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Risk assessment for peri- and post-menopausal women taking food supplements containing isolated isoflavones. EFSA J. 2015, 13, 4246. [CrossRef]
- Koonrungsesomboon, N.; Khatsri, R.; Wongchompoo, P.; Teekachunhatean, S. The impact of genetic polymorphisms on CYP1A2 activity in humans: A systematic review and meta-analysis. Pharm. J. 2018, 18, 760–768. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef]
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The mammalian epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Memedula, S.; Belmont, A.S. Sequential recruitment of HAT and SWI/SNF components to condensed chromatin by VP16. Curr. Biol. 2003, 13, 241–246. [Google Scholar] [CrossRef]
- Vanden Berghe, W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: Lifelong remodeling of our epigenomes. Pharmacol. Res. 2012, 65, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Pop, S.; Enciu, A.M.; Tarcomnicu, I.; Gille, E.; Tanase, C. Phytochemicals in cancer prevention: Modulating epigenetic alterations of DNA methylation. Phytochem. Rev. 2019, 18, 1005–1024. [Google Scholar] [CrossRef]
- Vuorelaa, P.; Leinonenb, M.; Saikkuc, P.; Tammelaa, P.; Rauhad, J.P.; Wennberge, T.; Vuorela, H. Natural products in the process of finding new drug candidates. Curr. Med. Chem. 2004, 11, 1375–1389. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Brandt, K.; Mølgaard, J.P. Organic agriculture: Does it enhance or reduce the nutritional value of plant foods? J. Sci. Food Agric. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Murkies, A.L.; Wilcox, G.; Davis, S.R. Clinical review 92: Phytoestrogens. J. Clin. Endocrinol. Metab. 1998, 83, 297–303. [Google Scholar] [CrossRef]
- Miksicek, R.J. Estrogenic flavonoids: Structural requirements for biological activity. Proc. Soc. Exp. Biol. Med. 1995, 208, 44–50. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.U.; Boucher, B.A.; Liu, Z.; Cotterchio, M.; Kreiger, N. Phytoestrogen content of foods consumed in Canada, including isoflavones, lignans, and coumestan. Nutr. Cancer 2006, 54, 184–201. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A. Phytoestrogens. Annu. Rev. Plant Biol. 2004, 55, 225–261. [Google Scholar] [CrossRef] [PubMed]
- Tolleson, W.H.; Doerge, D.R.; Churchwell, M.I.; Marques, M.M.; Roberts, D.W. Metabolism of biochanin A and formononetin by human liver microsomes in vitro. J. Agric. Food Chem. 2002, 50, 4783–4790. [Google Scholar] [CrossRef]
- Nikolić, I.L.; Savić-Gajić, I.M.; Tačić, A.D.; Savić, I.M. Classification and biological Activity of phytoestrogens: A review. Adv. Technol. 2017, 6, 96–106. [Google Scholar]
- Bustamante-Rangel, M.; Delgado-Zamarreno, M.M.; Perez-Martin, L.; Rodriguez-Gonzalo, E.; Dominguez-Alvarez, J. Analysis of Isoflavones in Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 391–411. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kazlauskaite, J.A.; Kopustinskiene, D.M. Pleiotropic Effects of Isoflavones in Inflammation and Chronic Degenerative Diseases. Int. J. Mol. Sci. 2021, 22, 5656. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, K.; Sasaki, K.; Tsurumaru, Y. Prenylation of aromatic compounds, a key diversification of plant secondary metabolites. Phytochemistry 2009, 70, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, P.; Possemiers, S.; Campbell, D.; Gill, C.; Rowland, I. A comparison of the anticancer properties of isoxanthohumol and 8-prenylnaringenin using in vitro models of colon cancer. Biofactors 2013, 39, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yu, J.; Zhan, J.; Yang, L.; Guo, L.; Xu, Y. Pharmacokinetics, Tissue Distribution, and Metabolism Study of Icariin in Rat. Biomed. Res. Int. 2017, 2017, 4684962. [Google Scholar] [CrossRef]
- Tamir, S.; Eizenberg, M.; Somjen, D.; Stern, N.; Shelach, R.; Kaye, A.; Vaya, J. Estrogenic and antiproliferative properties of glabridin from licorice in human breast cancer cells. Cancer Res. 2000, 60, 5704–5709. [Google Scholar]
- Tronina, T.; Poplonski, J.; Bartmanska, A. Flavonoids as Phytoestrogenic Components of Hops and Beer. Molecules 2020, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xiang, D.; Chen, X.; Huo, H. Role of Characteristic Components of Humulus lupulus in Promoting Human Health. J. Agric. Food Chem. 2019, 67, 8291–8302. [Google Scholar] [CrossRef]
- van de Schans, M.G.; Ritschel, T.; Bovee, T.F.; Sanders, M.G.; de Waard, P.; Gruppen, H.; Vincken, J.P. Involvement of a Hydrophobic Pocket and Helix 11 in Determining the Modes of Action of Prenylated Flavonoids and Isoflavonoids in the Human Estrogen Receptor. Chembiochem 2015, 16, 2668–2677. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef]
- Nehybova, T.; Smarda, J.; Benes, P. Plant coumestans: Recent advances and future perspectives in cancer therapy. Anticancer Agents Med. Chem. 2014, 14, 1351–1362. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Simons, R.; Vincken, J.P.; Bohin, M.C.; Kuijpers, T.F.; Verbruggen, M.A.; Gruppen, H. Identification of prenylated pterocarpans and other isoflavonoids in Rhizopus spp. elicited soya bean seedlings by electrospray ionisation mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 25, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.; Januário, A.H.; Lourenço, M.V.; Pereira, A.M.S.; Pereira, P.S. Neutralizing effects of Brazilian plants against snake venoms. Drugs Future 2004, 29, 1105. [Google Scholar] [CrossRef]
- Chung, I.M.; Rajakumar, G.; Lee, J.H.; Kim, S.H.; Thiruvengadam, M. Ethnopharmacological uses, phytochemistry, biological activities, and biotechnological applications of Eclipta prostrata. Appl. Microbiol. Biotechnol. 2017, 101, 5247–5257. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.Y.; Seo, D.B.; Shin, H.J.; Lee, S.J. Effect of Aspergillus oryzae-challenged germination on soybean isoflavone content and antioxidant activity. J. Agric. Food Chem. 2012, 60, 2807–2814. [Google Scholar] [CrossRef]
- Pan, J.-Y.; Chen, S.-L.; Yang, M.-H.; Wu, J.; Sinkkonen, J.; Zou, K. An update on lignans: Natural products and synthesis. Nat. Prod. Rep. 2009, 26. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.K.; Chapple, C. The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef]
- Satake, H.; Koyama, T.; Bahabadi, S.E.; Matsumoto, E.; Ono, E.; Murata, J. Essences in metabolic engineering of lignan biosynthesis. Metabolites 2015, 5, 270–290. [Google Scholar] [CrossRef] [PubMed]
- Satake, H.; Ono, E.; Murata, J. Recent advances in the metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. J. Agric. Food Chem. 2013, 61, 11721–11729. [Google Scholar] [CrossRef]
- Hayashi, K.; Narutaki, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol. Pharm. Bull. 2010, 33, 1199–1205. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Toledo, E.; Delgado-Rodriguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef]
- Riviere, C.; Pawlus, A.D.; Merillon, J.M. Natural stilbenoids: Distribution in the plant kingdom and chemotaxonomic interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Romero-Perez, A.I.; Ibern-Gomez, M.; Lamuela-Raventos, R.M.; de La Torre-Boronat, M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999, 47, 1533–1536. [Google Scholar] [CrossRef] [PubMed]
- Vinas, P.; Martinez-Castillo, N.; Campillo, N.; Hernandez-Cordoba, M. Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography-mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods. J. Chromatogr. A 2011, 1218, 639–646. [Google Scholar] [CrossRef]
- Tang, Y.L.; Chan, S.W. A review of the pharmacological effects of piceatannol on cardiovascular diseases. Phytother Res. 2014, 28, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef]
- Basu, P.; Maier, C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed. Pharmacother. 2018, 107, 1648–1666. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Mapesa, J.O. Phenolics in Human Nutrition: Importance of the Intestinal Microbiome for Isoflavone and Lignan Bioavailability. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2433–2463. [Google Scholar]
- Barnes, S.; Prasain, J.; D’Alessandro, T.; Arabshahi, A.; Botting, N.; Lila, M.A.; Jackson, G.; Janle, E.M.; Weaver, C.M. The metabolism and analysis of isoflavones and other dietary polyphenols in foods and biological systems. Food Funct. 2011, 2, 235–244. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006, 8, E101–E111. [Google Scholar] [CrossRef] [PubMed]
- Smit, S.; Szymanska, E.; Kunz, I.; Gomez Roldan, V.; van Tilborg, M.W.; Weber, P.; Prudence, K.; van der Kloet, F.M.; van Duynhoven, J.P.; Smilde, A.K.; et al. Nutrikinetic modeling reveals order of genistein phase II metabolites appearance in human plasma. Mol. Nutr. Food Res. 2014, 58, 2111–2121. [Google Scholar] [CrossRef]
- Rowland, I.; Faughnan, M.; Hoey, L.; Wahala, K.; Williamson, G.; Cassidy, A. Bioavailability of phyto-oestrogens. Br. J. Nutr. 2003, 89 (Suppl. 1), S45–S58. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Zubik, L.; Meydani, M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am. J. Clin. Nutr. 2003, 77, 1459–1465. [Google Scholar] [CrossRef]
- Franke, A.A.; Lai, J.F.; Halm, B.M. Absorption, distribution, metabolism, and excretion of isoflavonoids after soy intake. Arch. Biochem. Biophys. 2014, 559, 24–28. [Google Scholar] [CrossRef]
- Mayo, B.; Vazquez, L.; Florez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. O-desmethylangolensin: The importance of equol’s lesser known cousin to human health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Matthies, A.; Blaut, M.; Braune, A. Isolation of a Human Intestinal Bacterium Capable of Daidzein and Genistein Conversion. Appl. Environ. Microbiol. 2009, 75, 1740–1744. [Google Scholar] [CrossRef]
- Yang, Z.; Zhu, W.; Gao, S.; Yin, T.; Jiang, W.; Hu, M. Breast cancer resistance protein (ABCG2) determines distribution of genistein phase II metabolites: Reevaluation of the roles of ABCG2 in the disposition of genistein. Drug Metab. Dispos. 2012, 40, 1883–1893. [Google Scholar] [CrossRef]
- Liu, F.; Pei, S.; Li, W.; Wang, X.; Liang, C.; Yang, R.; Zhang, Z.; Yao, X.; Fang, D.; Xie, S.; et al. Characterization of Formononetin Sulfonation in SULT1A3 Overexpressing HKE293 Cells: Involvement of Multidrug Resistance-Associated Protein 4 in Excretion of Sulfate. Front. Pharmacol. 2020, 11, 614756. [Google Scholar] [CrossRef]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Riaz, A.; Sadiqa, A.; Zara, R.; Zafar, S.; et al. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Matthies, A.; Loh, G.; Blaut, M.; Braune, A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J. Nutr. 2012, 142, 40–46. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355S–1362S. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Iino, C.; Shimoyama, T.; Iino, K.; Yokoyama, Y.; Chinda, D.; Sakuraba, H.; Fukuda, S.; Nakaji, S. Daidzein Intake Is Associated with Equol Producing Status through an Increase in the Intestinal Bacteria Responsible for Equol Production. Nutrients 2019, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Decroos, K.; Vanhemmens, S.; Cattoir, S.; Boon, N.; Verstraete, W. Isolation and characterisation of an equol-producing mixed microbial culture from a human faecal sample and its activity under gastrointestinal conditions. Arch. Microbiol. 2005, 183, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Hori, S.; Nakagawa, H. Lactobacillus rhamnosus JCM 2771: Impact on metabolism of isoflavonoids in the fecal flora from a male equol producer. Curr. Microbiol. 2011, 62, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Casey, K.; Bowey, E.A.; Duffy, R.; Davies, M.; Rowland, I.R.; Lloyd, A.S.; Murray, A.; Thompson, R.; Clarke, D.B. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am. J. Clin. Nutr. 2004, 80, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Morton, M.S.; Arisaka, O.; Miyake, N.; Morgan, L.D.; Evans, B.A. Phytoestrogen concentrations in serum from Japanese men and women over forty years of age. J. Nutr. 2002, 132, 3168–3171. [Google Scholar] [CrossRef]
- Nikolic, D.; Li, Y.; Chadwick, L.R.; van Breemen, R.B. In vitro studies of intestinal permeability and hepatic and intestinal metabolism of 8-prenylnaringenin, a potent phytoestrogen from hops (Humulus lupulus L.). Pharm. Res. 2006, 23, 864–872. [Google Scholar] [CrossRef]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef]
- Possemiers, S.; Rabot, S.; Espin, J.C.; Bruneau, A.; Philippe, C.; Gonzalez-Sarrias, A.; Heyerick, A.; Tomas-Barberan, F.A.; De Keukeleire, D.; Verstraete, W. Eubacterium limosum activates isoxanthohumol from hops (Humulus lupulus L.) into the potent phytoestrogen 8-prenylnaringenin in vitro and in rat intestine. J. Nutr. 2008, 138, 1310–1316. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef]
- Licciardi, P.V.; Wong, S.S.; Tang, M.L.; Karagiannis, T.C. Epigenome targeting by probiotic metabolites. Gut Pathog. 2010, 2, 24. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Kim, M.; Han, J. Icariin Metabolism by Human Intestinal Microflora. Molecules 2016, 21, 1158. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Wong, S.P.; Yong, E.L. Sensitive and rapid method to quantify icaritin and desmethylicaritin in human serum using gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 857, 47–52. [Google Scholar] [CrossRef]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and Its Metabolites as Potential Protective Phytochemicals against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Kent, U.M.; Aviram, M.; Rosenblat, M.; Hollenberg, P.F. The licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab. Dispos. 2002, 30, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chen, X.; Liang, J.; Yu, X.Q.; Xu, A.L.; Chan, E.; Wei, D.; Huang, M.; Wen, J.Y.; Yu, X.Y.; et al. Role of P-glycoprotein in the intestinal absorption of glabridin, an active flavonoid from the root of Glycyrrhiza glabra. Drug Metab. Dispos. 2007, 35, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.; Nakagawa, K.; Kitano, M.; Ikematsu, H.; Nakamura, K.; Yokota, S.; Tominaga, Y.; Arai, N.; Mae, T. Clinical safety of licorice flavonoid oil (LFO) and pharmacokinetics of glabridin in healthy humans. J. Am. Coll. Nutr. 2007, 26, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mallis, L.M.; Sarkahian, A.B.; Harris, H.A.; Zhang, M.Y.; McConnell, O.J. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 796, 71–86. [Google Scholar] [CrossRef]
- Landete, J.M.; Arques, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Munoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- Li, L.; Huang, X.J.; Peng, J.L.; Zheng, M.Y.; Zhong, D.F.; Zhang, C.F.; Chen, X.Y. Wedelolactone metabolism in rats through regioselective glucuronidation catalyzed by uridine diphosphate-glucuronosyltransferases 1As (UGT1As). Phytomedicine 2016, 23, 340–349. [Google Scholar] [CrossRef]
- Jaskulski, S.; Jung, A.Y.; Behrens, S.; Johnson, T.; Kaaks, R.; Thone, K.; Flesch-Janys, D.; Sookthai, D.; Chang-Claude, J. Circulating enterolactone concentrations and prognosis of postmenopausal breast cancer: Assessment of mediation by inflammatory markers. Int. J. Cancer 2018, 143, 2698–2708. [Google Scholar] [CrossRef]
- Liu, Z.; Fei, Y.J.; Cao, X.H.; Xu, D.; Tang, W.J.; Yang, K.; Xu, W.X.; Tang, J.H. Lignans intake and enterolactone concentration and prognosis of breast cancer: A systematic review and meta-analysis. J. Cancer 2021, 12, 2787–2796. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estevez, A.M.; Bastias, J.M.; Munoz, O. Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.) determined by single-batch in vitro simulation of the digestive process. J. Sci. Food Agric. 2014, 94, 1729–1738. [Google Scholar] [CrossRef]
- Clavel, T.; Henderson, G.; Engst, W.; Dore, J.; Blaut, M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol. 2006, 55, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Woting, A.; Clavel, T.; Loh, G.; Blaut, M. Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbiol. Ecol. 2010, 72, 507–514. [Google Scholar] [CrossRef]
- Jin, J.S.; Hattori, M. Further studies on a human intestinal bacterium Ruminococcus sp. END-1 for transformation of plant lignans to mammalian lignans. J. Agric. Food Chem. 2009, 57, 7537–7542. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wahala, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Quartieri, A.; Garcia-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomas-Barberan, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. Anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef]
- De Silva, S.F.; Alcorn, J. Flaxseed Lignans as Important Dietary Polyphenols for Cancer Prevention and Treatment: Chemistry, Pharmacokinetics, and Molecular Targets. Pharmaceuticals 2019, 12, 68. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Thompson, L.U. Prolonged administration of secoisolariciresinol diglycoside increases lignan excretion and alters lignan tissue distribution in adult male and female rats. Br. J. Nutr. 2010, 104, 833–841. [Google Scholar] [CrossRef]
- Bowers, L.W.; Lineberger, C.G.; Ford, N.A.; Rossi, E.L.; Punjala, A.; Camp, K.K.; Kimler, B.K.; Fabian, C.J.; Hursting, S.D. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFkappaB signaling, and inhibits mammary tumor growth. Breast Cancer Res. Treat. 2019, 173, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Ingles, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Delmas, D.; Aires, V.; Limagne, E.; Dutartre, P.; Mazue, F.; Ghiringhelli, F.; Latruffe, N. Transport, stability, and biological activity of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 48–59. [Google Scholar] [CrossRef]
- Henry, C.; Vitrac, X.; Decendit, A.; Ennamany, R.; Krisa, S.; Merillon, J.M. Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J. Agric. Food Chem. 2005, 53, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Gonzalvez, M.; Larrosa, M.; Yanez-Gascon, M.J.; Garcia-Almagro, F.J.; Ruiz-Ros, J.A.; Tomas-Barberan, F.A.; Garcia-Conesa, M.T.; Espin, J.C. Resveratrol in primary and secondary prevention of cardiovascular disease: A dietary and clinical perspective. Ann. N. Y. Acad. Sci. 2013, 1290, 37–51. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.H.; Ku, C.F.; Zhang, H.J.; Tsang, S.W. Design, synthesis and evaluation of novel dihydrostilbene derivatives as potential anti-melanogenic skin-protecting agents. Eur. J. Med. Chem. 2018, 143, 1254–1260. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef]
- Niles, R.M.; Cook, C.P.; Meadows, G.G.; Fu, Y.M.; McLaughlin, J.L.; Rankin, G.O. Resveratrol is rapidly metabolized in athymic (nu/nu) mice and does not inhibit human melanoma xenograft tumor growth. J. Nutr. 2006, 136, 2542–2546. [Google Scholar] [CrossRef]
- Li, F.; Sun, Y.; Song, M.; Wu, X.; Xiao, H. Gastrointestinal biotransformation of resveratrol in mice. FASEB J. 2016, 30. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ho, P.C.; Lin, H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.W.; Garcia, A.M.; Meyskens, F.L., Jr. Differences in the glucuronidation of resveratrol and pterostilbene: Altered enzyme specificity and potential gender differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Wyns, C.; Possemiers, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; Verstraete, W.; Heyerick, A. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17beta-estradiol equivalents. J. Nutr. 2009, 139, 2293–2300. [Google Scholar] [CrossRef]
- van der Velpen, V.; Hollman, P.C.; van Nielen, M.; Schouten, E.G.; Mensink, M.; Van’t Veer, P.; Geelen, A. Large inter-individual variation in isoflavone plasma concentration limits use of isoflavone intake data for risk assessment. Eur. J. Clin. Nutr. 2014, 68, 1141–1147. [Google Scholar] [CrossRef]
- Milder, I.E.; Arts, I.C.; van de Putte, B.; Venema, D.P.; Hollman, P.C. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of beverages, nuts, seeds, and oils. J. Agric. Food Chem. 2008, 56, 7311–7315. [Google Scholar] [CrossRef]
- Kuhnle, G.G.; Dell’aquila, C.; Aspinall, S.M.; Runswick, S.A.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen content of cereals and cereal-based foods consumed in the UK. Nutr. Cancer 2009, 61, 302–309. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Runswick, S.A.; Bingham, S.A. Variability of phytoestrogen content in foods from different sources. Food Chem. 2009, 113, 1184–1187. [Google Scholar] [CrossRef]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Cady, N.; Peterson, S.R.; Freedman, S.N.; Mangalam, A.K. Beyond Metabolism: The Complex Interplay Between Dietary Phytoestrogens, Gut Bacteria, and Cells of Nervous and Immune Systems. Front. Neurol. 2020, 11, 150. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.V. Human genetics: Dr Watson’s base pairs. Nature 2008, 452, 819–820. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gong, X.; Huang, L.; Chen, P.; Wang, T.; Zhou, W.; Luo, K.; Wang, J. MiR-199a-5p regulates sirtuin1 and PI3K in the rat hippocampus with intrauterine growth restriction. Sci. Rep. 2018, 8, 13813. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Rudenko, O.; Egerod, K.L.; Husted, A.S.; Kovatcheva-Datchary, P.; Akrami, R.; Kristensen, M.; Schwartz, T.W.; Backhed, F. Microbial fermentation of flaxseed fibers modulates the transcriptome of GPR41-expressing enteroendocrine cells and protects mice against diet-induced obesity. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E453–E463. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Chaplin, A.; Carpene, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef]
- Yang, C.; Deng, Q.; Xu, J.; Wang, X.; Hu, C.; Tang, H.; Huang, F. Sinapic acid and resveratrol alleviate oxidative stress with modulation of gut microbiota in high-fat diet-fed rats. Food Res. Int. 2019, 116, 1202–1211. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drozdz, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Hinnebusch, B.F.; Meng, S.; Wu, J.T.; Archer, S.Y.; Hodin, R.A. The effects of short-chain fatty acids on human colon cancer cell phenotype are associated with histone hyperacetylation. J. Nutr. 2002, 132, 1012–1017. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Ercolini, D.; Fogliano, V. Food Design to Feed the Human Gut Microbiota. J. Agric. Food Chem. 2018, 66, 3754–3758. [Google Scholar] [CrossRef] [PubMed]
- Bialesova, L.; Novotna, A.; Macejova, D.; Brtko, J.; Dvorak, Z. Agonistic effect of selected isoflavones on arylhydrocarbon receptor in a novel AZ-AhR transgenic gene reporter human cell line. Gen. Physiol. Biophys. 2015, 34, 331–334. [Google Scholar] [CrossRef][Green Version]
- Goya-Jorge, E.; Jorge Rodriguez, M.E.; Veitia, M.S.; Giner, R.M. Plant Occurring Flavonoids as Modulators of the Aryl Hydrocarbon Receptor. Molecules 2021, 26, 2315. [Google Scholar] [CrossRef]
- Mohammadi-Bardbori, A.; Bengtsson, J.; Rannug, U.; Rannug, A.; Wincent, E. Quercetin, resveratrol, and curcumin are indirect activators of the aryl hydrocarbon receptor (AHR). Chem. Res. Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Ju, Y.H.; Sheng, S.; Williams, L.D.; Doerge, D.R.; Katzenellenbogen, B.S.; Helferich, W.G.; Katzenellenbogen, J.A. Equol, a natural estrogenic metabolite from soy isoflavones: Convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004, 12, 1559–1567. [Google Scholar] [CrossRef]
- Lim, W.; Jeong, W.; Song, G. Coumestrol suppresses proliferation of ES2 human epithelial ovarian cancer cells. J. Endocrinol. 2016, 228, 149–160. [Google Scholar] [CrossRef]
- Kim, J.E.; Lee, S.Y.; Jang, M.; Choi, H.K.; Kim, J.H.; Chen, H.; Lim, T.G.; Dong, Z.; Lee, K.W. Coumestrol Epigenetically Suppresses Cancer Cell Proliferation: Coumestrol Is a Natural Haspin Kinase Inhibitor. Int. J. Mol. Sci. 2017, 18, 2228. [Google Scholar] [CrossRef]
- Nehybova, T.; Smarda, J.; Daniel, L.; Brezovsky, J.; Benes, P. Wedelolactone induces growth of breast cancer cells by stimulation of estrogen receptor signalling. J. Steroid Biochem. Mol. Biol. 2015, 152, 76–83. [Google Scholar] [CrossRef]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) Extract and 6-Prenylnaringenin Induce P450 1A1 Catalyzed Estrogen 2-Hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef]
- Bolton, J.L.; Dunlap, T.L.; Hajirahimkhan, A.; Mbachu, O.; Chen, S.-N.; Chadwick, L.; Nikolic, D.; van Breemen, R.B.; Pauli, G.F.; Dietz, B.M. The Multiple Biological Targets of Hops and Bioactive Compounds. Chem. Res. Toxicol. 2019, 32, 222–233. [Google Scholar] [CrossRef]
- Mu, J.; Zhu, D.; Shen, Z.; Ning, S.; Liu, Y.; Chen, J.; Li, Y.; Li, Z. The repressive effect of miR-148a on Wnt/beta-catenin signaling involved in Glabridin-induced anti-angiogenesis in human breast cancer cells. BMC Cancer 2017, 17, 307. [Google Scholar] [CrossRef]
- Jiang, F.; Li, Y.; Mu, J.; Hu, C.; Zhou, M.; Wang, X.; Si, L.; Ning, S.; Li, Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol. Carcinog. 2016, 55, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tong, B.; Yang, Y.; Luo, J.; Yuan, X.; Wei, Z.; Yue, M.; Xia, Y.; Dai, Y. Arctigenin functions as a selective agonist of estrogen receptor β to restrict mTORC1 activation and consequent Th17 differentiation. Oncotarget 2016, 7, 83893–83906. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.J.; Kuo, P.L.; Hsu, Y.C.; Huang, Y.F.; Tsai, E.M.; Hsu, Y.L. Arctigenin, a dietary phytoestrogen, induces apoptosis of estrogen receptor-negative breast cancer cells through the ROS/p38 MAPK pathway and epigenetic regulation. Free Radic Biol. Med. 2014, 67, 159–170. [Google Scholar] [CrossRef]
- Ahmad, A.; Zhu, Y.; Kawaguchi, K.; Kiyama, R. Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS ONE 2017, 12, e0171390. [Google Scholar] [CrossRef]
- Kiyama, R. Biological effects induced by estrogenic activity of lignans. Trends Food Sci. Technol. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Pan, C.; Hu, Y.; Li, J.; Wang, Z.; Huang, J.; Zhang, S.; Ding, L. Estrogen Receptor-α36 Is Involved in Pterostilbene-Induced Apoptosis and Anti-Proliferation in In Vitro and In Vivo Breast Cancer. PLoS ONE 2014, 9, e104459. [Google Scholar] [CrossRef]
- Mak, K.K.; Wu, A.T.; Lee, W.H.; Chang, T.C.; Chiou, J.F.; Wang, L.S.; Wu, C.H.; Huang, C.Y.; Shieh, Y.S.; Chao, T.Y.; et al. Pterostilbene, a bioactive component of blueberries, suppresses the generation of breast cancer stem cells within tumor microenvironment and metastasis via modulating NF-kappaB/microRNA 448 circuit. Mol. Nutr. Food Res. 2013, 57, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Eichholzer, M.; Richard, A.; Nicastro, H.L.; Platz, E.A.; Linseisen, J.; Rohrmann, S. Urinary lignans and inflammatory markers in the US National Health and Nutrition Examination Survey (NHANES) 1999–2004 and 2005–2008. Cancer Causes Control 2014, 25, 395–403. [Google Scholar] [CrossRef]
- Beetch, M.; Harandi-Zadeh, S.; Shen, K.; Lubecka, K.; Kitts, D.D.; O’Hagan, H.M.; Stefanska, B. Dietary antioxidants remodel DNA methylation patterns in chronic disease. Br. J. Pharmacol. 2020, 177, 1382–1408. [Google Scholar] [CrossRef]
- Carlos-Reyes, A.; Lopez-Gonzalez, J.S.; Meneses-Flores, M.; Gallardo-Rincon, D.; Ruiz-Garcia, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernandez de la Cruz, O.N.; Lopez-Camarillo, C. Dietary Compounds as Epigenetic Modulating Agents in Cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef]
- Rando, O.J. Combinatorial complexity in chromatin structure and function: Revisiting the histone code. Curr. Opin. Genet. Dev. 2012, 22, 148–155. [Google Scholar] [CrossRef]
- Strahl, B.D.; Allis, C.D. The language of covalent histone modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef]
- Revollo, J.R.; Li, X. The ways and means that fine tune Sirt1 activity. Trends Biochem. Sci. 2013, 38, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Redondo, P.; Vaquero, A. The diversity of histone versus nonhistone sirtuin substrates. Genes Cancer 2013, 4, 148–163. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Villar-Garea, A.; Boix-Chornet, M.; Espada, J.; Schotta, G.; Bonaldi, T.; Haydon, C.; Ropero, S.; Petrie, K.; et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005, 37, 391–400. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pop, S.; Enciu, A.M.; Necula, L.G.; Tanase, C. Long non-coding RNAs in brain tumours: Focus on recent epigenetic findings in glioma. J. Cell Mol. Med. 2018, 22, 4597–4610. [Google Scholar] [CrossRef]
- Beckedorff, F.C.; Amaral, M.S.; Deocesano-Pereira, C.; Verjovski-Almeida, S. Long non-coding RNAs and their implications in cancer epigenetics. Biosci. Rep. 2013, 33, e00061. [Google Scholar] [CrossRef]
- Shankar, E.; Kanwal, R.; Candamo, M.; Gupta, S. Dietary phytochemicals as epigenetic modifiers in cancer: Promise and challenges. Semin. Cancer Biol. 2016, 40–41, 82–99. [Google Scholar] [CrossRef]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular Mechanisms of Action of Genistein in Cancer: Recent Advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Adjakly, M.; Bosviel, R.; Rabiau, N.; Boiteux, J.P.; Bignon, Y.J.; Guy, L.; Bernard-Gallon, D. DNA methylation and soy phytoestrogens: Quantitative study in DU-145 and PC-3 human prostate cancer cell lines. Epigenomics 2011, 3, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic regulation of multiple tumor-related genes leads to suppression of breast tumorigenesis by dietary genistein. PLoS ONE 2013, 8, e54369. [Google Scholar] [CrossRef]
- Xie, Q.; Bai, Q.; Zou, L.Y.; Zhang, Q.Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.D.; Mi, M.T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Donovan, M.G.; Papoutsis, A.J.; Doetschman, T.C.; Selmin, O.I. Genistein Prevents BRCA1 CpG Methylation and Proliferation in Human Breast Cancer Cells with Activated Aromatic Hydrocarbon Receptor. Curr. Dev. Nutr. 2017, 1, e000562. [Google Scholar] [CrossRef]
- Bosviel, R.; Dumollard, E.; Dechelotte, P.; Bignon, Y.J.; Bernard-Gallon, D. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? OMICS 2012, 16, 235–244. [Google Scholar] [CrossRef]
- Bosviel, R.; Durif, J.; Dechelotte, P.; Bignon, Y.J.; Bernard-Gallon, D. Epigenetic modulation of BRCA1 and BRCA2 gene expression by equol in breast cancer cell lines. Br. J. Nutr. 2012, 108, 1187–1193. [Google Scholar] [CrossRef]
- Fang, M.Z.; Chen, D.; Sun, Y.; Jin, Z.; Christman, J.K.; Yang, C.S. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin. Cancer Res. 2005, 11, 7033–7041. [Google Scholar] [CrossRef]
- Sundaram, M.K.; Unni, S.; Somvanshi, P.; Bhardwaj, T.; Mandal, R.K.; Hussain, A.; Haque, S. Genistein Modulates Signaling Pathways and Targets Several Epigenetic Markers in HeLa Cells. Genes 2019, 10, 955. [Google Scholar] [CrossRef]
- Dagdemir, A.; Durif, J.; Ngollo, M.; Bignon, Y.J.; Bernard-Gallon, D. Histone lysine trimethylation or acetylation can be modulated by phytoestrogen, estrogen or anti-HDAC in breast cancer cell lines. Epigenomics 2013, 5, 51–63. [Google Scholar] [CrossRef]
- Li, Y.; Meeran, S.M.; Patel, S.N.; Chen, H.; Hardy, T.M.; Tollefsbol, T.O. Epigenetic reactivation of estrogen receptor-alpha (ERalpha) by genistein enhances hormonal therapy sensitivity in ERalpha-negative breast cancer. Mol. Cancer 2013, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- de la Parra, C.; Castillo-Pichardo, L.; Cruz-Collazo, A.; Cubano, L.; Redis, R.; Calin, G.A.; Dharmawardhane, S. Soy Isoflavone Genistein-Mediated Downregulation of miR-155 Contributes to the Anticancer Effects of Genistein. Nutr. Cancer 2016, 68, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S.M.; O’Neill, K.M.; McKenna, M.M.; Walsh, C.P.; McKenna, D.J. Regulation of miR-200c and miR-141 by Methylation in Prostate Cancer. Prostate 2016, 76, 1146–1159. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Hinoda, Y.; Shahryari, V.; Deng, G.; Tanaka, Y.; Tabatabai, Z.L.; Dahiya, R. Genistein downregulates onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation and histone modification in prostate cancer cells. Br. J. Cancer 2014, 110, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ueno, K.; Nakajima, K.; Tabatabai, Z.L.; Hinoda, Y.; Ishii, N.; Dahiya, R. Genistein downregulates onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells. Br. J. Cancer 2013, 108, 2070–2078. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Han, Q.; Wang, X.; Zhao, D.; Liu, J. Identification and biological evaluation of natural product Biochanin A. Bioorg. Chem. 2020, 97, 103674. [Google Scholar] [CrossRef]
- Wang, A.L.; Li, Y.; Zhao, Q.; Fan, L.Q. Formononetin inhibits colon carcinoma cell growth and invasion by microRNA149mediated EphB3 downregulation and inhibition of PI3K/AKT and STAT3 signaling pathways. Mol. Med. Rep. 2018, 17, 7721–7729. [Google Scholar] [CrossRef]
- Chen, J.; Ge, B.; Wang, Y.; Ye, Y.; Zeng, S.; Huang, Z. Biochanin A promotes proliferation that involves a feedback loop of microRNA-375 and estrogen receptor alpha in breast cancer cells. Cell Physiol. Biochem. 2015, 35, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Wang, Y.; Ye, Y.; Huang, Z. Differential ability of formononetin to stimulate proliferation of endothelial cells and breast cancer cells via a feedback loop involving MicroRNA-375, RASD1, and ERalpha. Mol. Carcinog. 2018, 57, 817–830. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Li, Z.; Yan, H.; Qin, J.; Li, T. Formononetin inhibits human bladder cancer cell proliferation and invasiveness via regulation of miR-21 and PTEN. Food Funct. 2017, 8, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Y.; Zheng, J.M.; Yan, X.L.; Chen, H.M.; Chen, J.K.; Huang, H.Q. Formononetin sensitizes glioma cells to doxorubicin through preventing EMT via inhibition of histone deacetylase 5. Int. J. Clin. Exp. Pathol. 2015, 8, 6434–6441. [Google Scholar]
- Oza, M.J.; Kulkarni, Y.A. Formononetin attenuates kidney damage in type 2 diabetic rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef]
- Wu, J.; Xu, W.; Ma, L.; Sheng, J.; Ye, M.; Chen, H.; Zhang, Y.; Wang, B.; Liao, M.; Meng, T.; et al. Formononetin relieves the facilitating effect of lncRNA AFAP1-AS1-miR-195/miR-545 axis on progression and chemo-resistance of triple-negative breast cancer. Aging 2021, 13, 18191–18222. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, L.; Yu, M.; Liu, X.; Ma, W.; Huang, L.; Li, X.; Ye, X. S-equol inhibits proliferation and promotes apoptosis of human breast cancer MCF-7cells via regulating miR-10a-5p and PI3K/AKT pathway. Arch. Biochem. Biophys. 2019, 672, 108064. [Google Scholar] [CrossRef]
- Imai-Sumida, M.; Dasgupta, P.; Kulkarni, P.; Shiina, M.; Hashimoto, Y.; Shahryari, V.; Majid, S.; Tanaka, Y.; Dahiya, R.; Yamamura, S. Genistein Represses HOTAIR/Chromatin Remodeling Pathways to Suppress Kidney Cancer. Cell Physiol. Biochem. 2020, 54, 53–70. [Google Scholar] [CrossRef] [PubMed]
- King-Batoon, A.; Leszczynska, J.M.; Klein, C.B. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ. Mol. Mutagen. 2008, 49, 36–45. [Google Scholar] [CrossRef]
- Jha, A.K.; Nikbakht, M.; Parashar, G.; Shrivastava, A.; Capalash, N.; Kaur, J. Reversal of hypermethylation and reactivation of the RARbeta2 gene by natural compounds in cervical cancer cell lines. Folia Biol. 2010, 56, 195–200. [Google Scholar]
- Phillip, C.J.; Giardina, C.K.; Bilir, B.; Cutler, D.J.; Lai, Y.H.; Kucuk, O.; Moreno, C.S. Genistein cooperates with the histone deacetylase inhibitor vorinostat to induce cell death in prostate cancer cells. BMC Cancer 2012, 12, 145. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Hidaka, H.; Majid, S.; Saini, S.; Arora, S.; Deng, G.; Shahryari, V.; Chang, I.; et al. Genistein up-regulates tumor suppressor microRNA-574-3p in prostate cancer. PLoS ONE 2013, 8, e58929. [Google Scholar] [CrossRef]
- Chiyomaru, T.; Yamamura, S.; Fukuhara, S.; Yoshino, H.; Kinoshita, T.; Majid, S.; Saini, S.; Chang, I.; Tanaka, Y.; Enokida, H.; et al. Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS ONE 2013, 8, e70372. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Zhu, B. Genistein inhibits the proliferation of human multiple myeloma cells through suppression of nuclear factor-kappaB and upregulation of microRNA-29b. Mol. Med. Rep. 2016, 13, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Vardi, A.; Bosviel, R.; Rabiau, N.; Adjakly, M.; Satih, S.; Dechelotte, P.; Boiteux, J.P.; Fontana, L.; Bignon, Y.J.; Guy, L.; et al. Soy phytoestrogens modify DNA methylation of GSTP1, RASSF1A, EPH2 and BRCA1 promoter in prostate cancer cells. Vivo 2010, 24, 393–400. [Google Scholar]
- Karsli-Ceppioglu, S.; Ngollo, M.; Adjakly, M.; Dagdemir, A.; Judes, G.; Lebert, A.; Boiteux, J.P.; Penault, L.F.; Bignon, Y.J.; Guy, L.; et al. Genome-wide DNA methylation modified by soy phytoestrogens: Role for epigenetic therapeutics in prostate cancer? OMICS 2015, 19, 209–219. [Google Scholar] [CrossRef]
- Greathouse, K.L.; Bredfeldt, T.; Everitt, J.I.; Lin, K.; Berry, T.; Kannan, K.; Mittelstadt, M.L.; Ho, S.M.; Walker, C.L. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol. Cancer Res. 2012, 10, 546–557. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Sieli, P.T.; Warzak, D.A.; Ellersieck, M.R.; Pennington, K.A.; Roberts, R.M. Maternal exposure to bisphenol A and genistein has minimal effect on A(vy)/a offspring coat color but favors birth of agouti over nonagouti mice. Proc. Nat. Acad. Sci. USA 2013, 110, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; Coort, S.; Ruijters, E.J.; Godschalk, R.W.; van Schooten, F.J.; Barjesteh van Waalwijk van Doorn-Khosrovani, S. Epigenetics: Prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011, 25, 797–807. [Google Scholar] [CrossRef]
- Howard, T.D.; Ho, S.M.; Zhang, L.; Chen, J.; Cui, W.; Slager, R.; Gray, S.; Hawkins, G.A.; Medvedovic, M.; Wagner, J.D. Epigenetic changes with dietary soy in cynomolgus monkeys. PLoS ONE 2011, 6, e26791. [Google Scholar] [CrossRef]
- Zhuang, K.; Jiang, X.; Liu, R.; Ye, C.; Wang, Y.; Wang, Y.; Quan, S.; Huang, H. Formononetin Activates the Nrf2/ARE Signaling Pathway Via Sirt1 to Improve Diabetic Renal Fibrosis. Front. Pharmacol. 2020, 11, 616378. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Treatment in Type 2 Diabetic Rats Reduces Insulin Resistance and Hyperglycemia. Front. Pharmacol. 2018, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Ameliorates Diabetic Neuropathy by Increasing Expression of SIRT1 and NGF. Chem. Biodivers 2020, 17, e2000162. [Google Scholar] [CrossRef] [PubMed]
- Lyn-Cook, B.D.; Blann, E.; Payne, P.W.; Bo, J.; Sheehan, D.; Medlock, K. Methylation profile and amplification of proto-oncogenes in rat pancreas induced with phytoestrogens. Proc. Soc. Exp. Biol. Med. 1995, 208, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Hitzman, R.T.; Dunlap, T.L.; Howell, C.E.; Chen, S.N.; Vollmer, G.; Pauli, G.F.; Bolton, J.L.; Dietz, B.M. 6-Prenylnaringenin from Hops Disrupts ERalpha-Mediated Downregulation of CYP1A1 to Facilitate Estrogen Detoxification. Chem. Res. Toxicol. 2020, 33, 2793–2803. [Google Scholar] [CrossRef]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Bocker, A.; Leischner, C.; Riepl, H.; et al. 6- and 8-Prenylnaringenin, Novel Natural Histone Deacetylase Inhibitors Found in Hops, Exert Antitumor Activity on Melanoma Cells. Cell. Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef]
- Jiang, F.; Mu, J.; Wang, X.; Ye, X.; Si, L.; Ning, S.; Li, Z.; Li, Y. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS ONE 2014, 9, e96698. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.B.; Wang, J.W.; Xiao, H.; Zhang, Q.S.; Kan, W.S.; Mo, F.B.; Hu, S.; Ye, S.N. Icariin may benefit the mesenchymal stem cells of patients with steroid-associated osteonecrosis by ABCB1-promoter demethylation: A preliminary study. Osteoporos. Int. 2015, 26, 187–197. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, T.; Wu, J.; Kalionis, B.; Zhang, C.; Yuan, D.; Huang, J.; Cai, W.; Fang, H.; Xia, S. Icariin intervenes in cardiac inflammaging through upregulation of SIRT6 enzyme activity and inhibition of the NF-kappa B pathway. Biomed. Res. Int. 2015, 2015, 895976. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-kappaB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, X.; Tong, Y.; Feng, X.; Wang, Y.; Wang, C.; Jiang, Y. Icariin modulates the sirtuin/NFkappaB pathway and exerts antiaging effects in human lung fibroblasts. Mol. Med. Rep. 2020, 22, 3833–3839. [Google Scholar] [CrossRef]
- Li, J.; Jiang, K.; Zhao, F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol. Rep. 2015, 33, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.; Zhang, J.; Chen, L.; Duan, S.; Tang, J.; Xu, W.; Li, A. Icariin, a flavonoid with anti-cancer effects, alleviated paclitaxel-induced neuropathic pain in a SIRT1-dependent manner. Mol. Pain. 2018, 14, 1744806918768970. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Cole, A.; McLaughlin, S.; Du, W. Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition. Cell Cycle 2018, 17, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Liu, Y.; Wu, H.; Zhao, M.; Tan, Y.; Li, D.; Long, H.; Dai, Y.; Yung, S.; Chan, T.M.; et al. The role of icaritin in regulating Foxp3/IL17a balance in systemic lupus erythematosus and its effects on the treatment of MRL/lpr mice. Clin. Immunol. 2016, 162, 74–83. [Google Scholar] [CrossRef]
- Seo, D.B.; Jeong, H.W.; Lee, S.J.; Lee, S.J. Coumestrol induces mitochondrial biogenesis by activating Sirt1 in cultured skeletal muscle cells. J. Agric. Food Chem. 2014, 62, 4298–4305. [Google Scholar] [CrossRef] [PubMed]
- Romanchikova, N.; Trapencieris, P. Wedelolactone Targets EZH2-mediated Histone H3K27 Methylation in Mantle Cell Lymphoma. Anticancer Res. 2019, 39, 4179–4184. [Google Scholar] [CrossRef]
- Chen, H.; Gao, S.; Li, J.; Liu, D.; Sheng, C.; Yao, C.; Jiang, W.; Wu, J.; Chen, S.; Huang, W. Wedelolactone disrupts the interaction of EZH2-EED complex and inhibits PRC2-dependent cancer. Oncotarget 2015, 6, 13049–13059. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, L.; Che, F.; Lu, Y.; Xie, Z.; Wang, H. Arctigenin attenuates ischemic stroke via SIRT1-dependent inhibition of NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 2017, 493, 821–826. [Google Scholar] [CrossRef]
- Song, J.; Li, N.; Xia, Y.; Gao, Z.; Zou, S.F.; Yan, Y.H.; Li, S.H.; Wang, Y.; Meng, Y.K.; Yang, J.X.; et al. Arctigenin Confers Neuroprotection Against Mechanical Trauma Injury in Human Neuroblastoma SH-SY5Y Cells by Regulating miRNA-16 and miRNA-199a Expression to Alleviate Inflammation. J. Mol. Neurosci. 2016, 60, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Zhou, Y.; Chen, T.; Lei, J.C.; Jiang, X.J. AMPK/SIRT1 Pathway is Involved in Arctigenin-Mediated Protective Effects against Myocardial Ischemia-Reperfusion Injury. Front. Pharmacol. 2020, 11, 616813. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Solorzano, W.; Diaz, T.; Magyar, C.E.; Henning, S.M.; Vadgama, J.V. Arctigenin inhibits prostate tumor cell growth in vitro and in vivo. Clin. Nutr. Exp. 2017, 13, 1–11. [Google Scholar] [CrossRef]
- Cha, H.J.; Lee, G.T.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Lee, N.K.; Kim, S.Y.; Lee, B.M.; An, I.S.; Hahn, H.J.; et al. Photoprotective effect of arctiin against ultraviolet B-induced damage in HaCaT keratinocytes is mediated by microRNA expression changes. Mol. Med. Rep. 2014, 10, 1363–1370. [Google Scholar] [CrossRef]
- Lee, G.T.; Cha, H.J.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Ahn, K.J.; An, I.S.; An, S.; Bae, S. Arctiin induces an UVB protective effect in human dermal fibroblast cells through microRNA expression changes. Int. J. Mol. Med. 2014, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y. Flaxseed secoisolariciresinol diglucoside and enterolactone down-regulated epigenetic modification associated gene expression in murine adipocytes. J. Funct. Foods 2016, 23, 523–531. [Google Scholar] [CrossRef]
- Taibi, A.; Lin, Z.; Tsao, R.; Thompson, L.U.; Comelli, E.M. Effects of Flaxseed and Its Components on Mammary Gland MiRNome: Identification of Potential Biomarkers to Prevent Breast Cancer Development. Nutrients 2019, 11, 2656. [Google Scholar] [CrossRef] [PubMed]
- Christofidou-Solomidou, M.; Pietrofesa, R.; Arguiri, E.; McAlexander, M.A.; Witwer, K.W. Dietary flaxseed modulates the miRNA profile in irradiated and non-irradiated murine lungs: A novel mechanism of tissue radioprotection by flaxseed. Cancer Biol. Ther. 2014, 15, 930–937. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Kuo, C.H.; Kuo, H.F.; Chen, Y.S.; Wang, S.L.; Chao, D.; Lee, M.S.; Hung, C.H. Sesamin suppresses macrophage-derived chemokine expression in human monocytes via epigenetic regulation. Food Funct. 2014, 5, 2494–2500. [Google Scholar] [CrossRef]
- McCann, M.J.; Rowland, I.R.; Roy, N.C. The anti-proliferative effects of enterolactone in prostate cancer cells: Evidence for the role of DNA licencing genes, mi-R106b cluster expression, and PTEN dosage. Nutrients 2014, 6, 4839–4855. [Google Scholar] [CrossRef]
- Spallotta, F.; Cencioni, C.; Straino, S.; Nanni, S.; Rosati, J.; Artuso, S.; Manni, I.; Colussi, C.; Piaggio, G.; Martelli, F.; et al. A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. J. Biol. Chem. 2013, 288, 11004–11012. [Google Scholar] [CrossRef]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zhang, P.; Herrmann, A.; Yang, C.; Xin, H.; Wang, Z.; Hoon, D.S.; Forman, S.J.; Jove, R.; Riggs, A.D.; et al. Acetylated STAT3 is crucial for methylation of tumor-suppressor gene promoters and inhibition by resveratrol results in demethylation. Proc. Nat. Acad. Sci. USA 2012, 109, 7765–7769. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Shayan, P.; Popper, B.; Goel, A.; Shakibaei, M. Sirt1 Is Required for Resveratrol-Mediated Chemopreventive Effects in Colorectal Cancer Cells. Nutrients 2016, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, G.; Yao, H.; Sundar, I.K.; Caito, S.; Rahman, I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010, 393, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19, 2118. [Google Scholar] [CrossRef]
- Beetch, M.; Lubecka, K.; Kristofzski, H.; Suderman, M.; Stefanska, B. Subtle Alterations in DNA Methylation Patterns in Normal Cells in Response to Dietary Stilbenoids. Mol. Nutr. Food Res. 2018, 62, e1800193. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, B.; Salame, P.; Bednarek, A.; Fabianowska-Majewska, K. Comparative effects of retinoic acid, vitamin D and resveratrol alone and in combination with adenosine analogues on methylation and expression of phosphatase and tensin homologue tumour suppressor gene in breast cancer cells. Br. J. Nutr. 2012, 107, 781–790. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent gamma-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Kala, R.; Tollefsbol, T.O. A Novel Combinatorial Epigenetic Therapy Using Resveratrol and Pterostilbene for Restoring Estrogen Receptor-alpha (ERalpha) Expression in ERalpha-Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0155057. [Google Scholar] [CrossRef]
- Papoutsis, A.J.; Lamore, S.D.; Wondrak, G.T.; Selmin, O.I.; Romagnolo, D.F. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 2010, 140, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, B.; Ghosh, K.; Kanade, S.R. Resveratrol modulates epigenetic regulators of promoter histone methylation and acetylation that restores BRCA1, p53, p21(CIP1) in human breast cancer cell lines. Biofactors 2019, 45, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, E.; Dai, J.; Liu, B.; Han, Z.; Wu, J.; Zhang, S.; Peng, B.; Zhang, Y.; Jiang, Y. A novel long noncoding RNA AK001796 acts as an oncogene and is involved in cell growth inhibition by resveratrol in lung cancer. Toxicol. Appl. Pharmacol. 2015, 285, 79–88. [Google Scholar] [CrossRef]
- Roy, S.K.; Chen, Q.; Fu, J.; Shankar, S.; Srivastava, R.K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE 2011, 6, e25166. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Adair, B.; Alder, H.; Limagne, E.; Taccioli, C.; Ferracin, M.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol decreases the levels of miR-155 by upregulating miR-663, a microRNA targeting JunB and JunD. Carcinogenesis 2010, 31, 1561–1566. [Google Scholar] [CrossRef]
- Fudhaili, A.; Yoon, N.A.; Kang, S.; Ryu, J.; Jeong, J.Y.; Lee, D.H.; Kang, S.S. Resveratrol epigenetically regulates the expression of zinc finger protein 36 in nonsmall cell lung cancer cell lines. Oncol. Rep. 2019, 41, 1377–1386. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Zang, W.; Wang, X.; Liu, Z.; Li, W.; Jia, J. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS ONE 2013, 8, e70627. [Google Scholar] [CrossRef]
- Geng, W.; Guo, X.; Zhang, L.; Ma, Y.; Wang, L.; Liu, Z.; Ji, H.; Xiong, Y. Resveratrol inhibits proliferation, migration and invasion of multiple myeloma cells via NEAT1-mediated Wnt/beta-catenin signaling pathway. Biomed. Pharmacother. 2018, 107, 484–494. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Torres, E.; Hernandez-Oliveras, A.; Meneses-Morales, I.; Rodriguez, G.; Fuentes-Garcia, G.; Zarain-Herzberg, A. Resveratrol up-regulates ATP2A3 gene expression in breast cancer cell lines through epigenetic mechanisms. Int. J. Biochem. Cell Biol. 2019, 113, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Hicks, C.; Levenson, A.S. Resveratrol and prostate cancer: Promising role for microRNAs. Mol. Nutr. Food Res. 2011, 55, 1219–1229. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Rimando, A.M.; Zhang, X.; Levenson, A.S. Resveratrol and pterostilbene epigenetically restore PTEN expression by targeting oncomiRs of the miR-17 family in prostate cancer. Oncotarget 2015, 6, 27214–27226. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Li, K.; Tzivion, G.; Levenson, A.S. Resveratrol regulates PTEN/Akt pathway through inhibition of MTA1/HDAC unit of the NuRD complex in prostate cancer. Biochim. Biophys. Acta 2015, 1853, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J.; Alder, H.; Volinia, S.; Delmas, D.; Latruffe, N.; Croce, C.M. Resveratrol modulates the levels of microRNAs targeting genes encoding tumor-suppressors and effectors of TGFbeta signaling pathway in SW480 cells. Biochem. Pharmacol. 2010, 80, 2057–2065. [Google Scholar] [CrossRef]
- Papoutsis, A.J.; Selmin, O.I.; Borg, J.L.; Romagnolo, D.F. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: Preventive effects of resveratrol. Mol. Carcinog. 2015, 54, 261–269. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA effects of resveratrol on mammary tumors vs. normal tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Tome-Carneiro, J.; Larrosa, M.; Yanez-Gascon, M.J.; Davalos, A.; Gil-Zamorano, J.; Gonzalvez, M.; Garcia-Almagro, F.J.; Ruiz Ros, J.A.; Tomas-Barberan, F.A.; Espin, J.C.; et al. One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- Liu, W.H.; Chang, L.S. Suppression of Akt/Foxp3-mediated miR-183 expression blocks Sp1-mediated ADAM17 expression and TNFalpha-mediated NFkappaB activation in piceatannol-treated human leukemia U937 cells. Biochem. Pharmacol. 2012, 84, 670–680. [Google Scholar] [CrossRef]
- Kawakami, S.; Kinoshita, Y.; Maruki-Uchida, H.; Yanae, K.; Sai, M.; Ito, T. Piceatannol and its metabolite, isorhapontigenin, induce SIRT1 expression in THP-1 human monocytic cell line. Nutrients 2014, 6, 4794–4804. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Z.; Gao, T. Piceatannol induced apoptosis through up-regulation of microRNA-181a in melanoma cells. Biol. Res. 2017, 50, 36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jia, R.; Wang, C.; Hu, T.; Wang, F. Piceatannol promotes apoptosis via up-regulation of microRNA-129 expression in colorectal cancer cell lines. Biochem. Biophys. Res. Commun. 2014, 452, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Piao, Z.H.; Jin, L.; Kim, J.H.; Kim, G.R.; Ryu, Y.; Lin, M.Q.; Kim, H.S.; Kee, H.J.; Jeong, M.H. Piceatannol Attenuates Renal Fibrosis Induced by Unilateral Ureteral Obstruction via Downregulation of Histone Deacetylase 4/5 or p38-MAPK Signaling. PLoS ONE 2016, 11, e0167340. [Google Scholar] [CrossRef]
- Lubecka, K.; Kurzava, L.; Flower, K.; Buvala, H.; Zhang, H.; Teegarden, D.; Camarillo, I.; Suderman, M.; Kuang, S.; Andrisani, O.; et al. Stilbenoids remodel the DNA methylation patterns in breast cancer cells and inhibit oncogenic NOTCH signaling through epigenetic regulation of MAML2 transcriptional activity. Carcinogenesis 2016, 37, 656–668. [Google Scholar] [CrossRef]
- Greco, E.A.; Lenzi, A.; Migliaccio, S.; Gessani, S. Epigenetic Modifications Induced by Nutrients in Early Life Phases: Gender Differences in Metabolic Alteration in Adulthood. Front. Genet. 2019, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu. Rev. Public Health 2018, 39, 309–333. [Google Scholar] [CrossRef]
- Kundakovic, M.; Gudsnuk, K.; Franks, B.; Madrid, J.; Miller, R.L.; Perera, F.P.; Champagne, F.A. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc. Nat. Acad. Sci. USA 2013, 110, 9956–9961. [Google Scholar] [CrossRef]
- Jadhav, R.R.; Santucci-Pereira, J.; Wang, Y.V.; Liu, J.; Nguyen, T.D.; Wang, J.; Jenkins, S.; Russo, J.; Huang, T.H.; Jin, V.X.; et al. DNA Methylation Targets Influenced by Bisphenol A and/or Genistein Are Associated with Survival Outcomes in Breast Cancer Patients. Genes 2017, 8, 144. [Google Scholar] [CrossRef]
- Guo, X.; Cai, Q.; Bao, P.; Wu, J.; Wen, W.; Ye, F.; Zheng, W.; Zheng, Y.; Shu, X.O. Long-term soy consumption and tumor tissue MicroRNA and gene expression in triple-negative breast cancer. Cancer 2016, 122, 2544–2551. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Huang, Y.; Huang, X.; Xu, L.; Lv, Z. Genistein demethylates the promoter of CHD5 and inhibits neuroblastoma growth in vivo. Int. J. Mol. Med. 2012, 30, 1081–1086. [Google Scholar] [CrossRef]
- van Duursen, M.B.M. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Hine, C.; Tian, X.; Van Meter, M.; Au, M.; Vaidya, A.; Seluanov, A.; Gorbunova, V. SIRT6 promotes DNA repair under stress by activating PARP1. Science 2011, 332, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Xu, W.; Kong, D. Icariin inhibits cell proliferation, migration and invasion by down-regulation of microRNA-625-3p in thyroid cancer cells. Biomed. Pharmacother. 2019, 109, 2456–2463. [Google Scholar] [CrossRef]
- Tanigawara, Y. Role of P-glycoprotein in drug disposition. Ther. Drug Monit. 2000, 22, 137–140. [Google Scholar] [CrossRef]
- Lim, W.; Jeong, M.; Bazer, F.W.; Song, G. Coumestrol Inhibits Proliferation and Migration of Prostate Cancer Cells by Regulating AKT, ERK1/2, and JNK MAPK Cell Signaling Cascades. J. Cell. Physiol. 2017, 232, 862–871. [Google Scholar] [CrossRef]
- Zafar, A.; Singh, S.; Naseem, I. Cytotoxic activity of soy phytoestrogen coumestrol against human breast cancer MCF-7 cells: Insights into the molecular mechanism. Food Chem. Toxicol. 2017, 99, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Deplus, R.; Blanchon, L.; Rajavelu, A.; Boukaba, A.; Defrance, M.; Luciani, J.; Rothe, F.; Dedeurwaerder, S.; Denis, H.; Brinkman, A.B.; et al. Regulation of DNA methylation patterns by CK2-mediated phosphorylation of Dnmt3a. Cell Rep. 2014, 8, 743–753. [Google Scholar] [CrossRef]
- Huertas, D.; Soler, M.; Moreto, J.; Villanueva, A.; Martinez, A.; Vidal, A.; Charlton, M.; Moffat, D.; Patel, S.; McDermott, J.; et al. Antitumor activity of a small-molecule inhibitor of the histone kinase Haspin. Oncogene 2012, 31, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Brauer, C.; Thu, K.L.; Mason, J.M.; Blaser, H.; Bray, M.R.; Mak, T.W. Targeting Mitosis in Cancer: Emerging Strategies. Mol. Cell 2015, 60, 524–536. [Google Scholar] [CrossRef]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by EZH2: From biological insights to therapeutic potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef]
- Mehlich, D.; Garbicz, F.; Włodarski, P.K. The emerging roles of the polycistronic miR-106b∼25 cluster in cancer—A comprehensive review. Biomed. Pharmacother. 2018, 107, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef]
- Hyun, K.; Jeon, J.; Park, K.; Kim, J. Writing, erasing and reading histone lysine methylations. Exp. Mol. Med. 2017, 49, e324. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, B.; Karlic, H.; Varga, F.; Fabianowska-Majewska, K.; Haslberger, A. Epigenetic mechanisms in anti-cancer actions of bioactive food components—the implications in cancer prevention. Br. J. Pharmacol. 2012, 167, 279–297. [Google Scholar] [CrossRef]
- Beetch, M.; Stefanska, B. DNA Methylation in Anti-cancer Effects of Dietary Catechols and Stilbenoids: An Overview of Underlying Mechanisms. In Handbook of Nutrition, Diet, and Epigenetics; Springer: Cham, Switzerland, 2019; pp. 1819–1844. [Google Scholar]
- Wehde, B.L.; Radler, P.D.; Shrestha, H.; Johnson, S.J.; Triplett, A.A.; Wagner, K.U. Janus Kinase 1 Plays a Critical Role in Mammary Cancer Progression. Cell Rep. 2018, 25, 2192–2207 e2195. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Xu, X.J.; Nelson, L.; Cacicedo, J.M.; Saha, A.K.; Lan, F.; Ido, Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010, 298, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, Z. Activation of SIRT1 by resveratrol requires lamin A. Aging 2013, 5, 94–95. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Hubbard, B.P. Lifespan and healthspan extension by resveratrol. Biochim. Biophys. Acta 2015, 1852, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Michaille, J.J.; Piurowski, V.; Rigot, B.; Kelani, H.; Fortman, E.C.; Tili, E. MiR-663, a MicroRNA Linked with Inflammation and Cancer That Is under the Influence of Resveratrol. Medicines 2018, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Tili, E.; Michaille, J.J. Resveratrol, MicroRNAs, Inflammation, and Cancer. J. Nucleic Acids 2011, 2011, 102431. [Google Scholar] [CrossRef]
- Venkatadri, R.; Muni, T.; Iyer, A.K.; Yakisich, J.S.; Azad, N. Role of apoptosis-related miRNAs in resveratrol-induced breast cancer cell death. Cell Death Dis. 2016, 7, e2104. [Google Scholar] [CrossRef]
- Martel, J.; Ojcius, D.M.; Ko, Y.F.; Ke, P.Y.; Wu, C.Y.; Peng, H.H.; Young, J.D. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef] [PubMed]
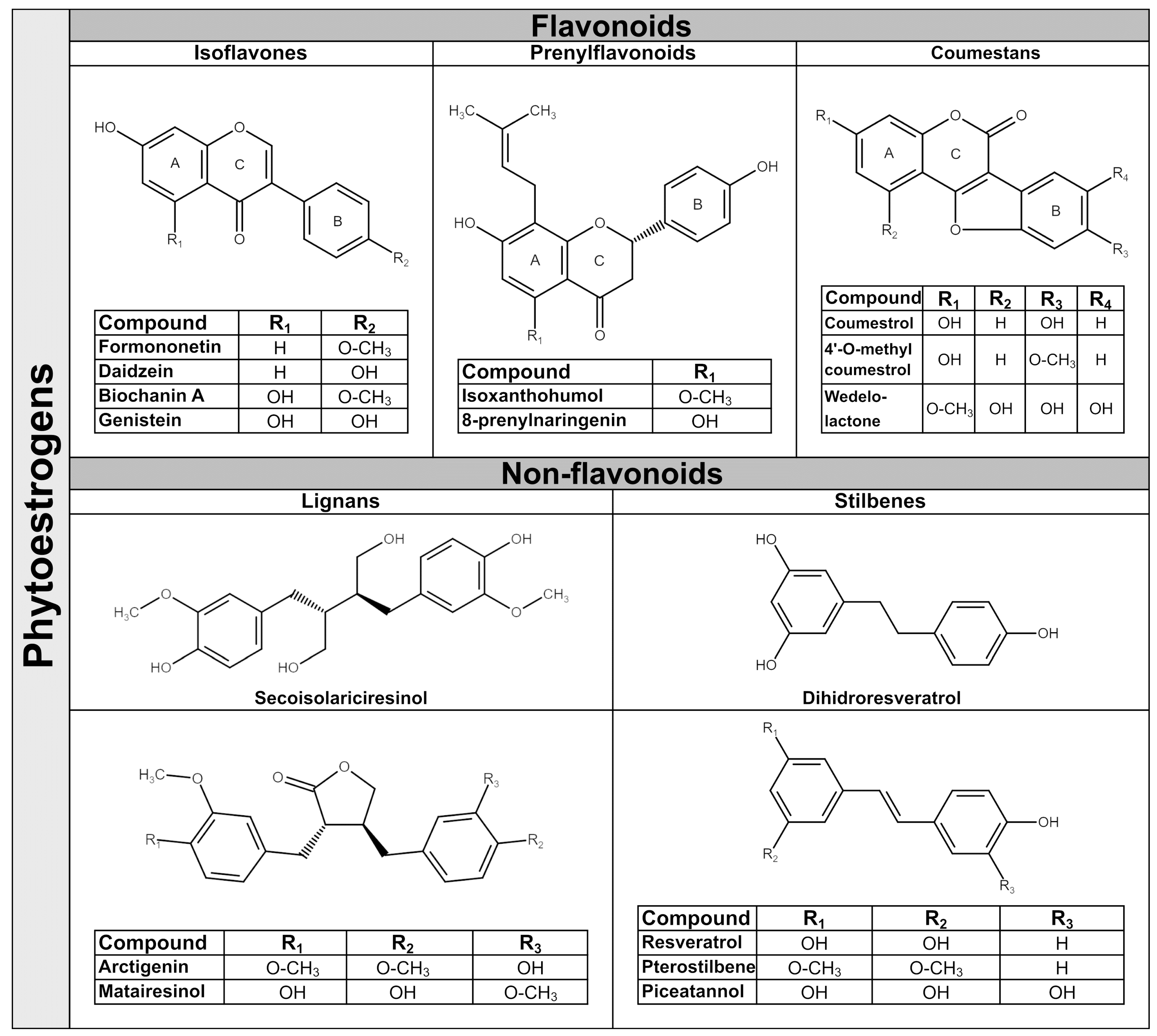
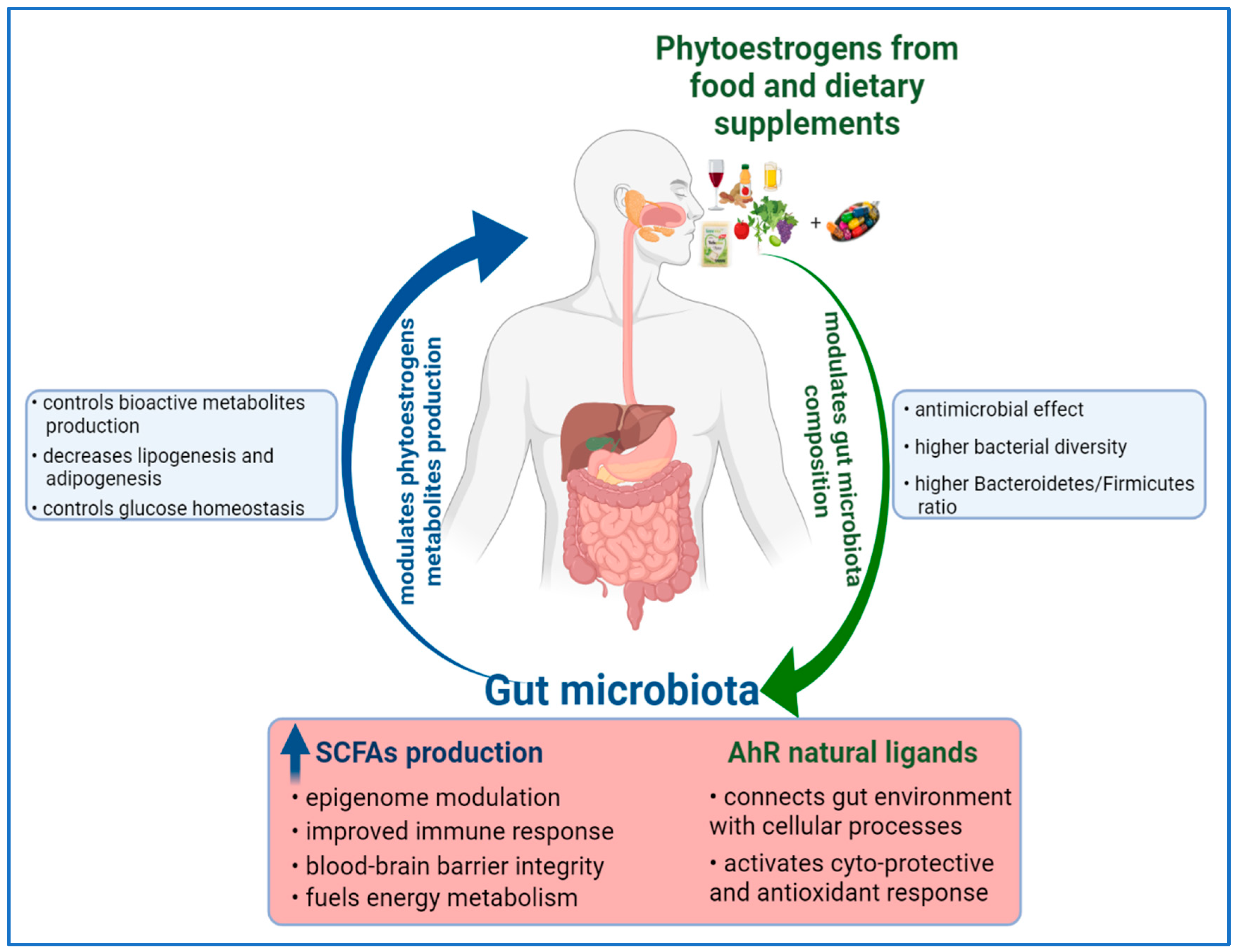
| Source | Isoflavones | Coumestans | Prenylflavonoids | Lignans | Stilbenes | Total | References |
|---|---|---|---|---|---|---|---|
| I. Soy and processed soy products | |||||||
| whole soybean | 5.47–159.61 | 0.0015–0.225 | N/D | 0.154–0.270 | N/D | 5.625–160.11 | [4,65] |
| soybean sprout | 0.674–14.05 | 0.1–0.34 | N/D | 0.12–0.15 | N/D | 0.894–14.54 | [4,65] |
| roasted soybean | 148.5–246.95 | 0.02–0.03 | N/D | 0.09 | N/D | 148.61–247.07 | [4,65] |
| tofu (incl. fermented) | 22.7–48.51 | 0.0007–0.12 | N/D | 0.09–0.16 | N/D | 22.79–48.79 | [4,63,134] |
| edamame (fresh soybeans) | 44.99–48.95 | N/D | N/D | 0.3 | N/D | 45.29–49.25 | [4,65,134] |
| tempe (fermented soybeans) | 60.61–147.74 | 0.0006 | N/D | 0.01–0.02 | N/D | 60.62–147.76 | [4,65,134] |
| miso paste (fermented soybeans) | 41.15–63.09 | 0.00024–0.04 | N/D | 0.02–0.03 | N/D | 41.17–63.16 | [4,134] |
| II. Seeds, bread and cereal | |||||||
| flax seeds | 0.07–0.321 | 0.03–0.047 | N/D | 0.013–301.13 | N/D | 0.113–301.497 | [4,134,135] |
| sesame seeds | N/D | 0.0004 | N/D | 39.348–74.95 | N/D | 39.35–74.95 | [134] |
| granola | 0.02–93.9 | N/ D | N/D | 0.21–0.764 | N/D | 0.23–94.664 | [134,136] |
| sunflower seeds | N/D | 0.002–0.01 | N/D | 0.891–2.10 | N/D | 0.893–2.11 | [63,134] |
| pumpkin seeds | 0.017–0.018 | N/D | N/D | 0.52 | N/D | 0.537–0.538 | [135] |
| flax and/or soy-containing bread | 0.297–14.67 | 0.01–0.09 | N/D | 0.01–1.379 | N/D | 0.317–16.139 | [134,136] |
| III. Nuts | |||||||
| almond | 0.01–0.044 | 0.02 | N/D | 0.112–0.92 | N/D | 0.142–0.984 | [63,65] |
| Brazil nuts | 0.105–0.109 | 0.01–0.02 | N/D | 0.77–0.782 | N/D | 0.885–0.911 | [63,135] |
| cashew | 0.01–0.023 | 0.0004 | N/D | 0.17–56.33 | N/D | 0.180–56.353 | [4,134,135] |
| peanut | 0.02–0.57 | 0.0001–0.002 | N/D | 0.026–6.803 | 0.071–0.178 | 0.117–7.553 | [135] |
| pistachios | 0.033–3.63 | 0.007–0.01 | N/D | 0.029–0.19 | 0.009–0.167 | 0.078–3.997 | [135] |
| walnut | 0.03–7.9 | 0.0006 | N/D | 0.086–0.13 | N/D | 0.117–8.031 | [63,135] |
| IV. Vegetables | |||||||
| alfalfa sprout | 0.39–5.507 | 0.0025–0.105 | 0.045 | 0.02–0.0448 | N/D | 0.458–5.702 | [4] |
| broccoli | 0.044–0.134 | N/D | N/D | 0.787–130.01 | N/D | 0.831–130.14 | [4,134] |
| Brussel sprout | N/D | 0.04 | N/D | 50.36–72.36 | N/D | 50.40–72.40 | [136,137] |
| cabbage | 0.0031 | N/D | N/D | 0.03–78.31 | N/D | 0.033–78.31 | [4,65] |
| carrot | 0.0052–0.0066 | 0.001–0.0014 | N/D | 0.006–7.66 | N/D | 0.012–7.67 | [65,137] |
| cauliflower | 2.3–6.7 | 0.8–1.8 | N/D | 25.2–145.1 | N/D | 28.30–153.60 | [65] |
| green bean | 0.04–0.718 | N/D | N/D | 0.065–23.07 | N/D | 0.105–23.79 | [63,65] |
| sauerkraut | N/D | N/D | N/D | 18.3–31.6 | N/D | 18.3–31.6 | [65] |
| V. Fruits | |||||||
| apple | 0.0009–0.067 | 0.0011 | N/D | 0.0012–0.1 | 0.0395–0.0956 | 0.0427–0.2637 | [134] |
| apricot | 0.0070 | N/D | N/D | 0.22–42.97 | N/D | 0.227–42.977 | [4,65] |
| dried apricot | 0.0390 | 0.0042 | N/D | 0.4 | N/D | 0.443 | [4] |
| coconut | 0.02–44.107 | N/D | N/D | 0.023–0.032 | N/D | 0.043–44.139 | [135] |
| grape | 0.0350 | N/D | N/D | 0.029–5.4 | 0.345 | 0.409–5.780 | [4] |
| orange | 0.002–0.0024 | 0.013–0.053 | N/D | 0.078–2.71 | N/D | 0.093–2.765 | [65] |
| pear | 0.0007–0.027 | N/D | N/D | 0.038–18.96 | 0.0276–0.0502 | 0.066–19.037 | [4,65] |
| plum | 0.007–0.015 | N/D | N/D | 0.005–0.589 | 0.019–0.048 | 0.031–0.652 | [65,134] |
| dried plums | 0.0042 | 0.0018 | N/D | 0.1775 | N/D | 0.184 | [4] |
| VI. Beverages | |||||||
| beer | 0.0015–0.13 | N/D | 0.044–5.189 | 0.01–0.63 | 0.01–1.479 | 0.066–7.428 | [65,134] |
| cocoa | N/D | N/D | N/D | 0.03–0.3 | 0.14–0.23 | 0.170–0.530 | [63] |
| coffee | 0.04–0.051 | 0.03 | N/D | 0.01–6.41 | N/D | 0.08–6.491 | [134,135] |
| tea leaves/infusion | 0.007–0.05 | 0.03 | N/D | 0.022–3.12 | N/D | 0.059–3.2 | [65] |
| red wine | 0.001–0.02 | 0.0001 | 0.85 | 0.134–1.2 | 0.78–4.35 | 1.765–6.420 | [65,134] |
| white wine | 0.001–0.024 | 0.0001 | 0.23 | 0.008–0.4 | 1.05 | 1.289–1.704 | [65,134] |
| Phytoestrogens | Relative Binding Affinity to ERs | Estrogenic Effects | References | |
|---|---|---|---|---|
| Isoflavones | Daidzein | -Preferential affinity to ERβ in comparison with ERα (RBA 0.5 v 0.1); -Lowest binding affinity of all isoflavones. | -Inhibits ovarian cancer cells’ proliferation, migration, and induces cell-cycle arrest as selective ERβ agonist; -Chemoprevention in endometrial cancer. | [6,7] |
| Genistein | -Higher affinity to ERβ in comparison with ERα (RBA 31–87 v 1–4). | -Regulated expression of target endogenous genes (CYP17, PR, ER-α, and ER-β). -Modulates ER levels in the liver, testis and lung; -Inhibits aromatase expression in breast cancer tissue; -Enhances osteoblastic differentiation and maturation by activation of ER; -Effects on reproductive and nonreproductive organs of mice models; -Preventive effect on breast and prostate cancers; -Decreases ovarian cancer risk; | [6,7,70] | |
| S-equol | -Preferentially binding to ERβ (RBA 10× higher for ERβ than ERα); -Higher binding affinity than its precursor daidzein. | -Binds dihydrotestosterone and inhibits in vivo prostate cancer growth; -Potent agent for menopause-related symptom relief. | [19,160] | |
| Comestans | Coumestrol | -High binding capacity, similar to estradiol (RBA for ERβ: 77–140 and for ERα: 12–20). | -Inhibits aromatase and 3α-hydroxysteroid dehydrogenase activities; -Anticancer for ovarian, breast, lung, cervical and prostate, cancers through ER signaling pathways; -decreased endometrial cancer risk; | [161,162] |
| Wedelolactone | -Acts as an agonist to both ERs. | -Activates expression of ER-regulated genes in ER-positive breast cancer cells; -Stimulates transactivation by AP-1 on ER signaling pathway. | [163] | |
| Prenylflavonoids | 6-PN | -Very weak binding capacity to ERα and ERβ (less than 1/100 of 8PN RBA to ERβ; -Weak aromatase inhibitor; | -Alters ERα-AhR crosstalk under estrogenic conditions; -Preferentially induce non-genotoxic estrogen metabolism | [164,165] |
| 8-PN | -Binds tighter to ERα compared to ERβ; -Acting as a pure estrogen with activity similar to estrone; -Potent aromatase inhibitor; | -Reduces the raised skin temperatures in a menopausal rat model; -Reduces hot flashes in postmenopausal women; -Increases uterine weight and height of luminal epithelial cells; -Weak osteoprotective effects; | [164,165] | |
| Glabridin | -Binds to ERβ with the same affinity as genistein; -Weak binding affinity to ERα. | -Induced dose-dependent increase in estrogenic activity and cell proliferation in Ishikawa cells; -Exerts estrogenic activity via the ER-α-SRC-1-co-activator complex; -Acts through the ER to induce beneficial effects of estrogen in bone and cardiovascular tissues. | [166,167] | |
| Lignans | Arctigenin | -Moderate binding capacity for ERβ; -Weak binding affinity to ERα. | -Inhibits the activation of mTORC1 pathway by targeting ERβ; -Promotes ERβ activation in T lymphocytes | [168,169] |
| Enterolactone | -Weak binding affinity to ERα and ERβ; | -Induces ERα/β transcriptional activation; -Activates estrogen-responsive genes, through direct binding to the ligand-binding domains of ERα; -Increases the expression of endogenous estrogen-responsive genes in mouse uterus. -Reduces the risk of hormone-dependent breast, uterus and prostate cancers. | [170,171] | |
| Stilbenes | Resveratrol | -Binds with similar affinity to ER-α and ER-β; -Weak estrogenic binding ability in comparison with estradiol (~7000 times less powerful). | -Inhibits endometrial aromatase and COX-2 expression; -Suppresses vascular smooth muscle proliferation and promotes re-endothelialization after aorta injury; -Mediates its neuroprotective effects via ER activation; -Interferes with intestinal and hepatic metabolism of estrogens | [121,123] |
| Pterostilbenes | -Binds with approximatively similar affinity to ER-α and ER-β. | -Inhibits colon cancer tumors growth in mice models through an ER-β-mediated mechanism; -Induces apoptosis in breast cancer cells and necrosis in xenograft tumors by targeting ER-α36; -Enhances the sex hormone secretion, further improving follicle development. | [172,173] | |
| RBA, Relative Binding Affinity. | ||||
| Dietary Phytoestrogen | Concentration | Cell Line/In Vivo Models | Epigenetic Changes | Targeted Gene | Biological Activity | References |
|---|---|---|---|---|---|---|
| I. Isoflavones | ||||||
| Genistein | In vitro 0.5–1 μM | MCF-7 | ↓DNMT1, ↓CpG methylation at BRCA1, ESR1 promoter | ↑BRCA1, p53, CYP1A1, ↓cyclin D1 | -Anti-proiferative and chemopreventive effect in breast cancer cells with activated AhR. | [190] |
| 3.125 μM | MDA-MB-468, | ↓CpG methylation at GSTP1 promoter | ↑GSTP1 | -Preventive effect, activates phaseI enzyme in TNBC cells. | [211] | |
| 5 μM | MDA-MB-435, Hs578T | ↓miR-155 | ↑FOXO3, PTEN, casein kinase, p27, ↓β-catenin | -Inhibits cells viability and induces apoptosis in TNBC cells. | [197] | |
| 18.5 μM | MCF-7 MDA-MB-231 | ↓Global DNA methylation, ↓CpG methylation BRCA1, BRCA2, MeCP2 promoters; ↓H3K9me3, H3K4me3, H3K27me3; ↑H4K8ac, H3K4ac at promoters of EZH2,BRCA1, ERα, ERβ, SRC3, p300 | ↑BRCA1, ↑BRCA2; ↓EZH2, ↑p300, ↑SRC3 | -Inhibition of breast cancer cells’ proliferation. | [191,195] | |
| 10–20 μM | UACC-3199, KYSE 510, SiHa; DU145, LNCaP, PC-3, ARCaP-E, ARCaP-M | ↓DNMT1, ↓CpG methylation of BRCA1, ESR1 promoters; ↓CpG methylation at RARβ2, p16, MGMT, ↓DNMTs; ↓DNA methylation at RARβ 2; ↑H3K9ac at promoters of APC, SOX7, SFRP1, SFRP2, DKK, WIF1, ↑HAT1 | ↑BRCA1, ER α, p53 CYP1A1, ↓cyclin D1 ↑RARβ2, p16, MGMT ↑RARβ2; ↑SOX7, SFRP1, BRCA1, BARD1, RAD23B, XRCC2, ↓BIRC7, SLUG, HES1, TGFBIII | -Anti-proiferative and chemopreventive effect in breast cancer cells with activated AhR; -Inhibition of esophageal squamous cell carcinoma growth; -Induces apoptosis in cervix squamous cells carcinoma; -Reduces proliferation and induces apoptosis in prostate cancer cells | [190,193,212,213] | |
| 25 μM | MDA-MB-157, MDA-MB-231; A-498; 786-O; Caki-2; PC3, DU145, RWPE-1; 786-O, ACHN; PC-3, DU145 | ↑Ac-H3, ↓DNMT1, HDAC1; ↓miR-1260b; ↓CpG methylation at SFRP1 ↓H3K9me2, H2K9me3, H3K27me3 at SFRP1, Smad4 genes; ↓lnc HOTAIR, ↓EZH2, ↑miR-574-3p, ↑mir-34a | ↑Erα; ↑SFRP1, Dkk2, Smad4; ↓ARID1A, EED SMARCB1, SNAIL, ↑ZO-1; ↓RAC1, ↓EGFR, ↓EP300 ↓MMP9, VEGF | -Inhibitits proliferation, invasion of TNBC, renal carcinoma and prostate cancer cells; -Enhances tamoxifen induced anticancer effect in TNBC cells; -Promotes apoptosis in renal carcinoma and prostate cancer cells; -Inhibits cell proliferation, migration and invasion in vitro and in vivo in prostate cancer; | [196,199,200,210,214,215] | |
| 5–40 μM | SH, SHR | ↑Global H3ac, ↑H3K4me3, ↓H3K27me3, H3K9me3 at p16, p21 promoters, ↑HMTs activities | ↑p21, p16, ↓BMI1, c-MYC | -Inhibits growth of breast cancer cells, but no effect on normal cells; -Preventive effect on breast tumorigenesis in vivo. | [188] | |
| 20–40 μM | U266 | ↑miR-29b | ↓NF-κB | -Inhibits proliferation and induces apoptosis in multiple myeloma. | [216] | |
| 40 µM | DU-145, PC-3 LNCaP | ↓CpG methylation at BRCA1, GSTP1, EPHB2, RASSF1A promoters; altered methylation pattern of MAD1L1, TRAF7, KDM4B, hTERT genes; ↑miR-200c | ↑ BRCA1, GSTP1, ↑EPHB2; Potential target genes SOX2, ZEB1 | -Inhibits prostate cancer cells’ proliferation, clonogenic potential and induces apoptosis. | [187,217,218] | |
| 50 µM | HeLa | ↓DNA methylation at TP53, PTEN, CDH1, DAPK1, FHIT, RUNX3, SOCS1 promoters, ↓DNMTs, HDACs, HMTs | ↑TP53, PTEN, CDH1, DAPK1, FHIT, RUNX3, SOCS1 | -Anti-proliferative effect on cervical tumor cells. | [194] | |
| 60–100 μM | MCF-7, MDA-MB-231 | ↓Global DNA methylation; ↓DNAmethylation at TSG promoters; ↓DNMT1 | ↑ATM, ↑APC,↑PTEN | -Reduced cellular viability and anti-proliferative effect on breast cancer cells; | [189] | |
| In vivo 2 mg/day 50 mg /kg 250 mg/kg 270 mg/kg | Neuroblastoma xenografts, Eker rats, Avy female mice, tumor xenograft mice, 123/SvJ:C57BL/6J mice | ↓DNTM3B, ↓CHD5 promoter methylation; ↓EZH2, ↓H3K27me3; ↑CpG methylation at Avy IAP gene; ↓DNMT1, HDAC1; ↓HDACs activity; ↑DNA methylation in repetitive elements; ↓HDAC6 | ↑CHD5; ↑PI3K/AKT pathways in uterus; ↓ectopic Agouti expression; ↑ERα, ↑PCNA in breast tissues; ↓p21, cyclin D1, PCNA, IGF2 expression in adult mice | -Decrease of tumor size and frequency. -Increases hypersensivity of ER-responsive genes in neonatal uteri and adult myometrium; -Reduces obesity offsprings, phenotypes changes; -Re-sensitizing ERα-negative breast cancer to therapy; -Prenatal exposure leads to long-term epigenetics changes. | [196,219,220,221,222] | |
| Soy based diet | Cynomolgus monkeys | ↓DNA methylation at promoter of HOXA5, HOXA11, HOXB1, ABCG5 | ↑HOXA5, HOXA11, HOXB1, ABCG5 | -Decrease in fasting insulin and HOMA index values. | [223] | |
| Daidzein | 20–50 μM | KYSE 510 | ↓DNA methylation at RARβ2 promoter; ↓DNMTs activity | ↑RARβ2 | -Dose-dependent inhibition of cells growth. | [193] |
| 78.5 μM | MCF-7, MDA-MB 231 | ↓Global DNA methylation, ↓CpG methylation at BRCA1, BRCA2 promoters, ↓MeCP2; ↓H3K9, H3K27, H3K4 (me3) ↑H4K8ac, H3K4ac at promoters of EZH2, BRCA1, ERα, ERβ, SRC3, p300 | ↑BRCA1, BRCA2; ↓EZH2, ↑p300, SRC3 | -Inhibition of ER(-) and ER(+) breast cancer cells’ proliferation. | [191,195] | |
| 110 µM | DU-145, PC-3 LNCaP | ↓CpG methylation at BRCA1, GSTP1, EPHB2 promoters; altered methylation pattern of MAD1L1, TRAF7, KDM4B, hTERT | ↑BRCA1, GSTP1, EPHB2 | -Inhibits prostate cancer cells’ proliferation and induces apoptosis. | [187,218] | |
| Biochanin A | 2–6 μM | T47-D, MCF-7 | ↑miR-375 | ↑ERα, ↑Bcl -2 | -Promotes proliferation of breast cancer cells. | [203] |
| 2.95 μM | MGC-803 | ↓LSD1, ↑H3K4me1/2, H3K9 me1/2 | ↓MAO-A/B, Bcl-2, ↑Bax | -Suppresses colony formation and migration and induces apoptosis in gastric cancer cells. | [201] | |
| 20–50 μM | KYSE 510 | ↓CpG methylation at RARβ promoter, ↓DNMTs activity | ↑RARβ | -Inhibits the growth of eosphageal squamous cells. | [193] | |
| Formononetin | In vitro 2–6 μM | HUVEC | ↑miR-375 | ↑ERα, Bcl-2, ↓RASD1 | -Promotes cell proliferation and inhibits apoptosis. | [204] |
| 10–20 μM | GMCs | ↑SIRT1 | ↑Nrf2/ARE ↓Fibronectin, ICAM-1 | -Inhibits hyperglycemia-induced ROS overproduction in glomerular mesangial cells. | [224] | |
| 40 μM | BT-549, MDA-MB-231 | ↓lncRNA AFAP1-AS1 ↑miR-545, miR-195 | ↓CDK4, Raf-1 | -Inhibits proliferation, migration and invasion of TNBC cells. | [208] | |
| 20–100 μM | SW1116, HCT116 | ↑miR-149 | ↓EphB3, cyclin D1, MMP2/9, ↓PI3K/AKT ↓STAT3 | -Inhibits colon carcinoma cell proliferation and invasion. | [202] | |
| 50–200 μM | T24 | ↓miR-21 | ↑PTEN, ↓p-Akt | -Inhibits proliferation, induces apoptosis and decreases invasiveness of bladder cancer. | [205] | |
| 100 µM | U87MG | ↓HDAC5 | ↓Vimentin ↑E-cadherin | -Enhances the cytotoxicity of doxorubicin in glioma cells. | [206] | |
| In vivo 4–8 mg/kg/day | Ovariectomized rats | ↑miR-375 | ↑ERα, ↑Bcl-2, ↓RASD1 | -Lower risk of postmenopausal breast cancer development. | [204] | |
| 20–40 mg/kg | Diabetic type II rats | ↑SIRT1 in pancreatic tissues and sciatic nerve tissue | ↓MDA, ↑GSH, SOD; ↑NGF in sciatic nerve tissue. | -Reduces oxidative stress, risk of nephro-pathy and the level of triglyceride and cholesterol; -Protects from hyperglycemia induced neuronal damage. | [225,226] | |
| 25–50 mg/kg | Diabetes mice model | ↑SIRT1 in kidney tissues | ↑Nrf2, ↓Fibronectin, ICAM 1 | -Reduces renal fibrosis, improves renal function. | [207,224] | |
| S-equol | In vitro 2 µM | MCF-7, MDA-MB-231 | ↓CpGmethylation at BRCA1, BRCA2 promoters | ↑BRCA1, BRCA2 | -Inhibits breast cancer cells’ proliferation. | [192] |
| 12.8 μM | MCF-7, MDA-MB-231 | ↓H3K9, H3K27, H3K4(me3) ↑H3K4ac, H4K8ac at promoter of EZH2,BRCA1, ERα, ERβ, SRC3, p300 genes | ↓EZH2, ↑p300, ↑SRC3 | - | [195] | |
| 50–150 µg/mL | MCF-7 | ↑miR-10a-5p | ↓PI3K p110α, ↓ p-Akt | -Anti-proliferative and pro-apoptotic effect. | [209] | |
| In vivo 10–100 mg/day | Neonatal rats | ↑DNA methylation of H-ras in pancreatic cells | - | - | [227] | |
| II. Prenylflavonoids | ||||||
| 6-PN 8-PN | 1 μM 50–100 μM | MCF-7 SK-MEL-28 | ↓DNMT1 HDAC2,4,7,8 inhibition ↑H3 acetylation | ↓ERα ↑P450 1A1 ↓pS6P, ↓pERK/pP90 | -Activates AhR to attenuate inhibition of CYP1A1 and degradation of ERα; -Antiproliferative effects on melanoma cells. | [228,229] |
| Glabridin | In vitro 10 μM | MDA-MB-231 Hs-578T | ↑miR-148a, ↓DNMT1 ↓DNMT3A, | ↓TGFβ/SMAD2 | -Inhibits the CSCs-like properties of breast cancer cells. | [167] |
| 10–20 μM | MDA-MB-231, Hs-578T; HepG2, Huh-7 | ↑miR-148a | ↓Wnt/β-catenin ↓VEGF ↓SMAD2 | -Attenuates angiogenes in breast cancer cells; -Inhibits the CSCs-like properties of HCC cells. | [166,230] | |
| In vivo 20 mg/kg/d | mouse xenograft | ↑miR-148a, ↓DNMT1 ↓DNMT3A | ↓TGFβ/SMAD2 | -Attenuated the tumor growth, CSCs-like properties in vivo. | [167] | |
| Icariin | 1 nM | hMSCs | ↓DNA methylation at ABCB1 promoter | ↑ABCB1, MMP ↑P-gp protein | -Improves cellular viability, decreases oxidative stress and promotes osteogenesis of MSCs; | [231] |
| 10 nM | Mouse aortic ECs | ↑SIRT6, ↓H3K9ac | ↓NF-κB, TNF-α, ICAM-1, IL-2, IL-6 | -Reduces inflammation in vitro and in vivo; | [232] | |
| 5–10 μM | MDA-MB-231, 4T1 | ↑SIRT6 ↓H3K9ac | ↓NF-κB p65, ↓MMP2, ↓N-cadherin ↓TNFα, ↑E-cadherin | -Suppresses migration, invasion, decreases ROS level in breast cancer cells; | [233] | |
| 2–16 μM | IMR-90 | ↑SIRT6, SIRT1 | ↓NF-κB, ↓p-p53, p-p21, ↓Cav1 | -Prevents D-gal-induced aging and cell-cycle arrest in lung fibroblast cells; | [234] | |
| 25–50 μM | A2780 | ↓miR21 | ↑PTEN, RECK, ↓Bcl-2 | -Regulates proliferation and apoptosis of ovarian cancer cells; | [235] | |
| In vivo 100 mg/kg | Rats | ↑SIRT1, H4AcK16 | ↓TNF-α, IL-1β, and IL-6 ↓NF-κB(p65) phosphorylation | -Suppresses paclitaxel-induced neuroinflammation and mechanical allodynia; | [236] | |
| 10−2 μM 100 mg/kg/day | FA HSPCs isolated from mice | ↑SIRT6, ↓H3K9ac | ↓NF-κB | -In vitro progenitor capacity; -In vivo repopulating ability of FA HSCs. | [237] | |
| Icaritin | 40 μM | CD4+T cells from SLE patients | ↑H3K4me3 at Foxp3 gene ↑H3K9me3 at IL17a gene | ↑Foxp3 ↓IL17a | -Reduced autoreactivity of CD4+Tcells. | [238] |
| III. Coumestans | ||||||
| Coumestrol | In vitro 1–10 μM | Muscle cells | ↑SIRT1 | ↑NDUFA9, SDHA, UQCRC2, COX1, PGC1, Nrf1 | -Increases mitochondria number, respiratory chain proteins and mitochondrial function; | [239] |
| 10–50 μM | ES2 | ↓DNMT3A phospho | ↓CK2, PCNA, ERBB2, p-AKT, p70S6K, ERK1/2, JNK1/2, p90RSK | -Preventive effects on epithelial ovarian cancer cells; | [161] | |
| 20–40 μM | HCT116 | ↓H3Tr3phos | ↓Haspin kinase | -Suppresses colon cancer cells’ proliferation; | [162] | |
| In vivo 10–100 mg/day | Neonatal rats | ↑DNA methylation of H-ras | - | - | [227] | |
| Wedelolactone | 0.1–10 μM | Mino | ↓EZH2, PRC2, HTM ↓H3K27me3 | ↓PRC2, EZH2 | -Inhibition of B cell non-Hodgkin’s lymphoma cells’ proliferation; | [240] |
| 50 μM | HepG2, THP1, K562 | ↓EZH2, PRC2 | ↑DAB2IP, ADRB2, CDKN2A, GADD45A | -Inhibits proliferation and migration, and induces apoptosis and cell-cycle arrest of PRC2-dependent cancers. | [241] | |
| IV. Lignans | ||||||
| Arctigenin | In vitro 0.268 μM 0.5–1 μM 5 μM 20–100 μM | Rats’ neurons; SH-SY5Y; MDA-MB-231 H9C2, Rats cardiomyocyte | ↑SIRT1 ↑miR-16 ↑miR-199a ↑H3K9 me3 at AP-1, Bcl-2 promoters ↑SIRT1 | ↓NLRP3 ↓IL-1β, ↓IL-18, ↓ASC ↓caspases-1 p20; ↓IKKα ↓IKKβ, ↓NF-κB ↓TNF-α ↓IL-6, ↑IL-10; ↓Bcl-2, ↑ phos ATF-2 ↑AMPK, ↑I-κB, ↓NFkB | -Protection against ischemic stroke, neuroprotection; -Induces anti-inflammatory, anti-apoptotic mechanisms to prevent secondary damage; -Supressed cardiomyocytes apoptosis, inflammation and oxidative stress; | [169,242,243,244] |
| In vivo 100 µM/kg; 4 mg/kg/day; 20 mg/kg; 50–100 mg/kg | Rats—myocardial ischemia; xenograft mice; Rats—cerebral ischemia | ↑SIRT1; ↑pho-p38; ↑SIRT1, ↑miR-96-5p ↓miR-126-5p, miR-21-5p, ↑miR-135a-5p, miR-205-5p, miR-22-3p, miR-455-5p | ↑AMPK, ↑I-κB, ↓NFkB ↓Bcl-2; ↓NLRP3 IL-1β, IL-18; ↓ASC, caspases-1 p20 TIMP3, ZNF185 ↓VEGF, EGF, FGF-β, ↑Bax/Bcl-2 ratio | -Inhibition of oxidative Stress and inflammation after acute myocardial ischemia; -Protects against ischemic stroke; -Inhibited prostate tumor cell growth both in vitro and in vivo. | [169,242,244,245] | |
| Arctiin | 5 μM 10 μM | HaCaT NHDF | ↑miR-125a-5p, -205-3p, -21-3p, -29b-1-5p ↓miR-3652, -494, -1246; ↑miR-602, -762, -150-3p, -4327, -584-5p, -874, -3665 ↓miR-3679-5p, -1290, -575 | -Possible regulation of members of MAPK pathways and cell growth signaling pathways. | -Enhances wound healing, DNA repair in UVB-exposed keratinocytes; -Inhibits the UVB-mediated cell growth defect, apoptosis, DNA damage. | [246,247] |
| SGD | In vitro 50–100 µM | 3T3L1 | ↓DNMTs, HDACs, MBD2 | - | -Antioxidant effect, epigenetic modification in murine adipocytes. | [248] |
| In vivo Flaxseed diet | Female mice Mice pneumonopathy | ↑miR-30b, -324-5p ↓miR-382, -423; ↓miR-142-3p, -150 ↑miR-34a | Changes in mammary gland miRNome; ↓Bcl2, FGFR1 | -Prevents breast cancer development during adulthood. -Antioxidant and anti-inflammatory effects. | [249,250] | |
| Sesamin | 10 µM | THP-1 | ↓H3/H4 acetylation at MDC promoter area, ↓CBP | ↓MAPK-p38, NFkB-p65, MDC, IP-10 | -Supresses allergy and asthma-related chemokines expression; -Anti-inflammatory effect. | [251] |
| ENL | 20 μM | RWPE-1, WPE1-NA22, -NB14, -NB11, -NB26, LNCaP | ↓miR-106b cluster (miR-106b, -93, -25) | ↓GMNN, CDT1, MCM2, MCM 7 ↑PTEN | -Anti-proliferative effect on mid and late prostate cancers; | [252] |
| 50–100 µM | 3T3L1 | ↓DNMTs, HDACs, MBD2; | - | -Antioxidant activities, downregulates epigenetic-modification-associated gene expression in murine adipocytes. | [248] | |
| V. Stibenes | ||||||
| Resveratrol | In vitro 1 μM | HaCaT | ↑ SIRT1, HDAC2, ↓H4K16Ac | ↑eNOS | -Accelerates wound healing repair in vitro and in vivo skin-wound models; | [253] |
| 5 μM | Canine-bone tissue cells MC3T3-E1 | ↑ SIRT1 ↓p300 | ↓NF-κB acetylation, IκBα phosphorylation, IKK activity kinase activity, ↑Cbfa-1 | -Anti-osteoclastogenic, activates the bone-tissue cells to osteoblast and osteogenesis; | [254] | |
| 10 μM | MDA-MB-468 A2058, M223; HCT116, SW480; HUVEC; ARPE-19 | ↓CpG methylation at ERα promoter; ↑SIRT1 ↑DNMT1, ↑LINE-1 methylation | ↓STAT3 acethylation ↑ERα expression; ↓NF-Κb, CXCR4, MMP9; ↑eNOS acetylation | -Anti-proliferative, reduces viability and induces mesenchymal to epithelial transition phenotype in breast cancer and CRC cells; -Regulates endothelial function during oxidative stress; -Ameliorates viability and ROS production in retinal pigment epithelia cells under oxidative and inflammatory conditions; | [255,256,257,258] | |
| 14–15 μM | MCF10A, MCF7, HCC1806, MDA-MB-157 | ↑CpG methylation at KCNJ4, RNF169, BCHE, DAOA ↓CpG methylation of HOXA9, KRTAP2-1, TAGAP, RUNX3; ↓CpG methylation at PTEN promoter, ↓DNMT1; ↓SIRT1, DNMT3B, ↓DNMTs activity; ↑HDACs, HATs, ↑H3Ac, H4Ac, H3K9Ac at ERα promoter | ↓KCNJ4, DAOA ↑BCHE, KRTAP2-1, TAGAP; ↑PTEN, p21; ↓γ-H2AX, ↓hTERT; ↑ERα | -No cytotoxic effect on normal mammary gland cells; -Antiproliferative effect on breast cancer cells; -Induces apoptosis and cell-cycle arrest on TNBC cells. | [259,260,261,262] | |
| 10–20 μM | MCF-7; MDA-MB-231 | ↓DMNT1, MBD2, H3K9me3 at BRCA1 promoter ↑H4Ac, H3K9Ac at BRCA1 promoter ↓PRMT5, EZH2, KDACs, ↑KAT2A/3B ↑global H3K9ac, H3K27ac ↓H4R3me2s, H3K27me3 at BRCA1, p53, p21 promoters | ↑BRCA-1 ↓AhR and ERα at BRCA1 promoters; ↑BRCA1, p53, p21 | -Attenuates dioxin carcinogenic chemicals-dependent repression of BRCA-1 and induction of DNA damage; -Inhibits breast cancer cells’ proliferation. | [263,264] | |
| 25 μM | C2C12; A549 | ↑SIRT1; ↓lncAK001796 | ↑AMPK ↓LKB1ac, ↓PGC-1α ac ↑Nrf-1, Nrf-2, NDUFS8, SDHb, Uqcrc1, COX5b, ATP5a1 ↑BIRC5, TFDP2, CDC6 ↓ATR, CCNB1, CKS2 | -Increase mitochondrial membrane potential, cellular ATP content in mouse mioblasts; -Inhibits lung cancer cells’ proliferation. | [265,266] | |
| 20–30 μM | PANC-1, MIA PaCa-2, AsPC1; THP1 | ↓SIRT1, SIRT2, SIRT3; ↑miR-663, ↓miR-155 | ↑PTEN, p-JNK, FOXO ↓Ras, p-AKT, p-ERK, AKT kinase activity ↑caspase-3; ↓AP-1, ↑cMaf | -Induces cell-cycle arrest and apoptosis in pancreatic cancer cells; -Inhibits pancreatic tumor growth in vivo; | [267,268] | |
| 20–50 μM | A549, BGC-823, SGC-7901 U266, LP1 | ↓CpG methylation at ZFP36 promoter; ↓DNMT1; ↑SIRT1; ↓lnc NEAT1 | ↑ZFP36, ↓CCND1, MYC, VEGFA; ↓cyclin D1, CDK4, CDK6 ↑p21, p16; ↑β-catenin cytoplasm, ↓c-Myc, MMP7, Survivin | -Inhibits migration and cell proliferation in non-small-cell lung cancer cells; -Inhibits gastric cancer cells’ proliferation and induces cell-cycle arrest; -Inhibits the tumor growth of xenografts; -Inhibits the proliferation, migration and invasion of multiple myeloma cells; | [269,270,271] | |
| 50 µM | MCF-10A, MCF-7, MDA-MB-231 LNCaP, DU145, 22Rv1 SW480 | ↓CpG methylation at Nrf2, ↓miR-93; ↓HDAC activity, ↓HDAC2 at ATP2A3 promoter ↑global H3Ac, H3K27ac ↓DNMTs activity, ↓MeCP2, MBD2; ↓miRs-17-92, -106ab clusters ↓miRs-7, -17, -18b ↑miRs-150, -296-5p ↓miRs-17, -20a, -106a, -106b; ↓HDACs, NuRD complex, ↑p300; ↑miR-663, ↓miR-17, -21, -25, -92a-2 | ↑Nrf2; ↑ATP2A3, SERCA3; ↑PTEN; ↑PTENac, p53, ↓MTA1, PI3K-Akt; ↓TGFβ1, ↑PTEN, PDCD4, SMAD7 | -Protective role against E2-induced mammary carcinogenesis; -Pro-apoptosis effect and changes in Ca2+ homeostasis; -Induces apoptosis and inhibits cell growth, angiogenesis and metastasis in prostate cancer cells; -Induces tumor regression in orthotopic prostate cancer xenografts; -Induces apoptosis, inhibits colon cancer cells growth. | [272,273,274,275,276,277] | |
| In vivo 7 ppm mixed AIN-76A diet | Pregnant female Sprague–Dawley rats | ↓CpG methylation at BRCA1 promoter ↓DNMT1 at BRCA-1 promoter | ↑BRCA1, AhR | -Reduces the risk of breast tumorigenesis in the offspring; | [278] | |
| diet with 0.4% resveratrol | Wild-type mouse | ↑SIRT1 | ↑p-AMPK, NAD+, LKB1 acetylation | -Improves mitochondrial function and increases cellular ATP in skeletal muscle; | [265] | |
| 5–25 mg/kg/day | Female rats bearing breast cancer | ↑miRs -21, -129, -204, -489 ↓DNMT3B in tumor tissues; ↓miRs -21, -129, -204, -489 ↑DNMT3B in normal tissues | -Inhibits breast tumor formation in vivo; | [279] | ||
| 25–50 mg/kg | Mice bearing human melanoma | ↓DNMT1 ↓CpG methylation at PTPN6, CDKN2A, SOCS3 promoters | ↓STAT3 acetylation | -Tumor-growth inhibition; | [255] | |
| 50 mg subcutaneous pellet/month | Female ACI rats | ↓CpG methylation at Nrf2 promoter ↓miR-93 | ↑Nrf2, NQO1, SOD3 OGG1, FMO1, AOX1 ↓MTA1, pAkt | -Decreases tumor incidence and chemoprevention; | [272] | |
| extract containing resveratrol/ 1 year | Peripheral blood male with type-2 diabetes | ↑miR-21, -181b, -663, -30c2 ↓miR-155, -34a | ↓IL-6, CCL3, IL-1β, TNF-α ↑LRRFIP-1 | -Beneficial immunomodulatory effect on hypertensive patients with type 2 diabeties. | [280] | |
| Pterostilbene | 2.5–10 μM | MCF-7 MDA-MB-231 in coculture with TAM | ↑miR488 | ↓NF-κB, Twist1, vimentin ↑E-cadherin | -Suppresses breast EMT and/or generation of CSCs; | [173] |
| 5 μM | HCC1806 MDA-MB-157 | ↓SIRT1, ↓DNMTs activity ↑HDACs, HATs ↑H3Ac, H4Ac, H3K9Ac at ERα promoter | ↓γ-H2AX, hTERT ↑ERα | -Induces apoptosis and cell-cycle arrest in breast cancer cell lines; | [261,262] | |
| 50 μM | DU145, 22Rv1 | ↓miRs-17, -20a, -106a, -106b | ↑PTEN ↓PI3K-Akt | -Promotes apoptosis, inhibits cell proliferation both in vitro and in vivo, and downregulates circulating tumor-derived oncomiRs in vivo. | [275] | |
| Piceatannol | In vitro 1 μM | U937 | ↓miR-183 | ↓ADAM17, Sp1, Foxp3, TNFα/NFkB ↑ β-TrCP | -InhibitsTNF α-mediated signaling pathway in leukemia cell line; | [281] |
| 10 μM | THP-1, Raw264.7 | ↑SIRT1 ↓miR-183 | ↑HO-1 | -Attenuates osteoclastogenesis in bone-marrow-derived macrophages; | [282] | |
| 30 μM | RAW264.7, A2058, WM266-4, HCT116 | ↑miR-200a ↑miR-181a ↑miR-129 | ↑Nrf2 ↓NLRP3, IL-18, IL-1β, caspase1 ↑Bax, caspase 3, ↓Bcl-2 | -Attenuates oxLDL-induced lipid storage by inhibiting pyroptosis in human macrophage cells; -Induces apoptosis of melanoma cells and CRC cells; | [283,284] | |
| In vivo 50 mg/kg/day | Renal fibrosis mice model | ↓HDAC4, HDAC5 | ↓p38-MAPK, ECM | -Ameliorates renal fibrosis. | [285] | |
| Resveratrol + Pterostibene | In vitro 15 μM + 5 μM | HCC1806 MDA-MB-157 | ↓SIRT1, ↓DNMTs, ↓Global DNA methylation ↑HDACs, HATs ↑ H3Ac, H4Ac, H3K9Ac at ERα promoter | ↓γ-H2AX, hTERT, ↑ERα | -Induces apoptosis and cell-cycle arrest; -Retrieves responsiveness to E2 and 4-hydroxytamoxifen treatments in resensitized breast cancer cells. | [261,262] |
| 15 µM + 7 μM | MCF10A MCF10CA1h MCF10CA1a | ↑CpG methylation at MAML2, GLI2 promoters; ↑DNMT3B | ↓MAML2 ↓NOTCH | -Inhibition of growth of cancer cells with low and high invasive properties; | [286] | |
| In vivo 5–25 mg/kg/day | Rats bearing estrogen-dependent breast tumors | ↓DNMT3B, ↑miR10a,−21, −129, −204, −489 | - | -Delay in mammary tumor formation; -Different pattern of epigenetic changes tumor versus normal tissues; | [279] | |
| CSAA diet + REV 1.2 g or with PTS, 1.34 g/kg/day | Rats | DNA methylation ↓RUNX3, ↑ KCNJ4 | ↑RUNX3, ↓KCNJ4 | -Changes theDNA methylation pattern on long-term dietary exposures. | [259] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. https://doi.org/10.3390/antiox10121893
Ionescu VS, Popa A, Alexandru A, Manole E, Neagu M, Pop S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants. 2021; 10(12):1893. https://doi.org/10.3390/antiox10121893
Chicago/Turabian StyleIonescu, Victor Stefan, Alexandra Popa, Andrei Alexandru, Emilia Manole, Mihaela Neagu, and Sevinci Pop. 2021. "Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health" Antioxidants 10, no. 12: 1893. https://doi.org/10.3390/antiox10121893
APA StyleIonescu, V. S., Popa, A., Alexandru, A., Manole, E., Neagu, M., & Pop, S. (2021). Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants, 10(12), 1893. https://doi.org/10.3390/antiox10121893





