Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke
Abstract
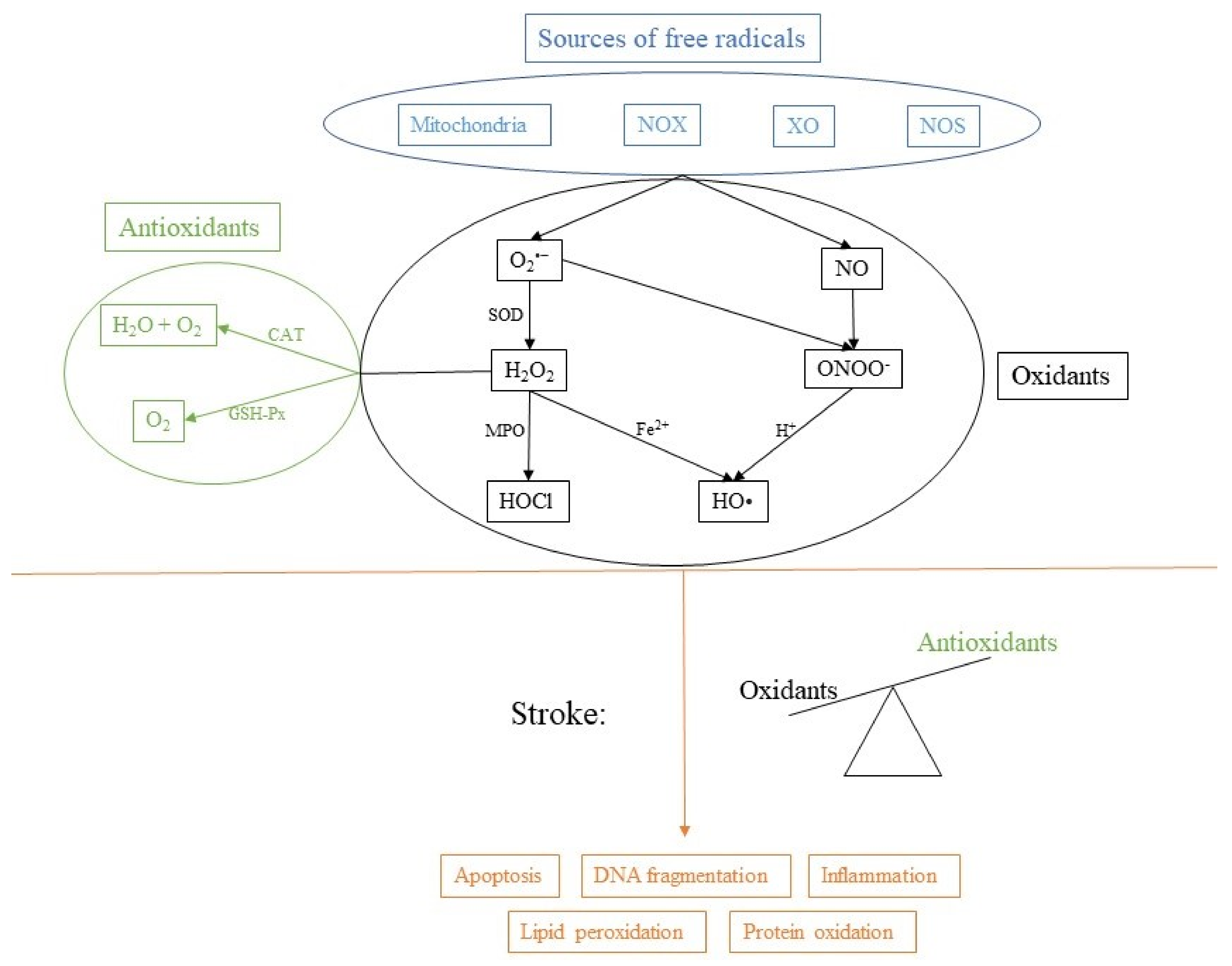
:1. Introduction to Physiology of Free Radicals
2. Oxidative Stress
3. Oxidative Stress in the Brain
4. Antioxidant Treatment Strategies of Oxidative Stress in Stroke
4.1. Inhibition of ROS-Producing Enzymes
4.2. Free Radical Scavengers
4.3. Free Radical Degradation
4.4. Mitochondrial Targeted Antioxidants
4.5. Antioxidant Supplementation to Scavenge ROS
4.6. Antioxidant Treatment of Oxidative Stress in Hemorrhagic Stroke
| Compound | Origin | Stroke Type | Target | Outcome | References |
|---|---|---|---|---|---|
| allopurinol + ** ± | synthetic | ischemic stroke | XO | stroke volume and cerebral edema reduction | [44] |
| ascorbate (vitamin C) ++ * ± | natural | ischemic stroke, hemorrhagic stroke | NOS, NOX, vitamin E, free radical scavenger | reduced risk of stroke; lower levels of peroxidation markers; reduced infarct size | [63] |
| CAPE + ** ± | synthetic | ischemic stroke | XO | XO inhibition | [45] |
| curcumin ++ * ± | natural | ischemic stroke | MPO, cytokines | inhibition of NF-κB; reduced inflammation and brain damage | [50] |
| dauricine + ** ± | natural | ischemic stroke | MPO, cytokines | reduced activity of MPO; reduced inflammation | [51] |
| Edaravone ++ ** ± | synthetic | ischemic stroke | free radical scavenger | improvement of functional outcome | [54] |
| GKT136901 + ** ± | synthetic | ischemic stroke | NOX1, NOX2, NOX4, NOX5 | NOX inhibition | [37] |
| lubeluzole ++ * ±± | synthetic | ischemic stroke | NOS | reduced infarct volume | [58,59] |
| M13 + ** ± | synthetic | ischemic stroke | NOX1, NOX4 | NOX inhibition | [39] |
| melatonin + ** ± | natural | ischemic stroke, hemorrhagic stroke | HO-1 | increased HO-1 expression; amelioration of brain edema, BBB impairment, apoptosis and neurological deficits | [78] |
| mitoquinone + ** ± | synthetic | ischemic stroke | mitochondria | recovery of O2consumption and complex I activity | [62] |
| ML090 + ** ± | synthetic | ischemic stroke | NOX1, NOX4, NOX5 | NOX inhibition | [38] |
| ML171 + ** ± | synthetic | ischemic stroke | NOX1, NOX4, NOX5 | NOX inhibition | [38] |
| N-Acetylcysteine ++ ** ± | natural | ischemic stroke | free radical scavenger, GSH | reduction of infarct volume; reduction of expression of pro-inflammatory cytokines; reduced cell death | [71] |
| neuroglobin + ** ± | natural | ischemic stroke | mitochondria | improved neurological outcome; reduced hypoxia-induced oxidative stress | [82] |
| pyrroloquinoline quinone + ** ± | natural | hemorrhagic stroke | NOS, mitochondria | alleviation of hematoma volumes; reduced expansion of brain edema and production of ROS | [77] |
| quercetin + ** ± | natural | ischemic stroke | MPO, SOD, CAT | reduction of infarct size and MPO levels; increase in SOD and CAT levels | [47] |
| resveratrol ++ ** ± | natural | ischemic stroke, hemorrhagic stroke | MPO, MMP-9, cytokines | reduction of infarct size, neuronal injury, MPO activity, MMPs; reduction of inflammation | [48,52,67] |
| sulforaphane + ** ± | natural | ischemic stroke, hemorrhagic stroke | HO-1, NOX, GSH | increase in HO-1; inhibition of NOX | [76] |
| tirilazad ++ * ±± | synthetic | ischemic stroke | lipid peroxidation | reduced infarct volume; increase death rate | [53] |
| Trigonelline + ** ± | natural | ischemic stroke | MPO, GSH | reduction of infarction; inhibition of MPO and GSH | [49] |
| ursolic acid + ** ± | natural | ischemic stroke, hemorrhagic stroke | free radical scavenger | attenuation of cerebral edema, BBB disruption, neuronal cell death and neurological deficit | [79] |
| VAS2870 + ** ± | synthetic | ischemic stroke | NOX1, NOX2, NOX4, NOX5 | NOX inhibition | [36] |
| α-tocopherol (vitamin E) ++ * ± | natural | ischemic stroke, hemorrhagic stroke | prevent the propagation of ROS chain reaction | reduced risk of ischemic stroke | [65] |
5. Combination Therapy in Stroke Treatment
6. Limitations of Current Antioxidant Therapy in Stroke
7. Conclusions
Funding
Conflicts of Interest
References
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozkul, A.; Akyol, A.; Yenisey, C.; Arpaci, E.; Kiylioglu, N.; Tataroglu, C. Oxidative Stress in Acute Ischemic Stroke. J. Clin. Neurosci. 2007, 14, 1062–1066. [Google Scholar] [CrossRef]
- Sanderson, T.H.; Reynolds, C.A.; Kumar, R.; Przyklenk, K.; Hüttemann, M. Molecular Mechanisms of Ischemia–Reperfusion Injury in Brain: Pivotal Role of the Mitochondrial Membrane Potential in Reactive Oxygen Species Generation. Mol. Neurobiol. 2013, 47, 9–23. [Google Scholar] [CrossRef] [Green Version]
- Kahles, T.; Brandes, R.P. NADPH Oxidases as Therapeutic Targets in Ischemic Stroke. Cell. Mol. Life Sci. 2012, 69, 2345–2363. [Google Scholar] [CrossRef] [PubMed]
- Vergeade, A.; Mulder, P.; Vendeville, C.; Ventura-Clapier, R.; Thuillez, C.; Monteil, C. Xanthine Oxidase Contributes to Mitochondrial ROS Generation in an Experimental Model of Cocaine-Induced Diastolic Dysfunction. J. Cardiovasc. Pharmacol. 2012, 60, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Nitric Oxide and the Immune Response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Montfort, W.R.; Wales, J.A.; Weichsel, A. Structure and Activation of Soluble Guanylyl Cyclase, the Nitric Oxide Sensor. Antioxid. Redox Signal. 2017, 26, 107–121. [Google Scholar] [CrossRef]
- Picón-Pagès, P.; Garcia-Buendia, J.; Muñoz, F.J. Functions and Dysfunctions of Nitric Oxide in Brain. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1949–1967. [Google Scholar] [CrossRef] [PubMed]
- Hishiki, T.; Yamamoto, T.; Morikawa, T.; Kubo, A.; Kajimura, M.; Suematsu, M. Carbon Monoxide: Impact on Remethylation/Transsulfuration Metabolism and Its Pathophysiologic Implications. J. Mol. Med. 2012, 90, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.L.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial Dysfunction and Oxidative Stress in Aging and Cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- Kagan, V.E.; Tyurina, Y.Y. Recycling and Redox Cycling of Phenolic Antioxidants. Annals N. Y. Acad. Sci. 1998, 854, 425–434. [Google Scholar] [CrossRef]
- Lauridsen, C. From Oxidative Stress to Inflammation: Redox Balance and Immune System. Poult. Sci. 2019, 98, 4240–4246. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermöhlen, O.; Krut, O.; Krönke, M.; Schramm, M. Mitochondrial Reactive Oxygen Species Enable Proinflammatory Signaling through Disulfide Linkage of NEMO. Sci. Signal. 2019, 12, eaar5926. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Barry, R.E. Free Radical Generation by Neutrophils: A Potential Mechanism of Cellular Injury in Acute Alcoholic Hepatitis. Gut 1987, 28, 1157–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørklund, G.; Chirumbolo, S. Role of Oxidative Stress and Antioxidants in Daily Nutrition and Human Health. Nutrition 2017, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Antioxidants in Personalized Nutrition and Exercise. Adv. Nutr. 2018, 9, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Orellana-Urzúa, S.; Claps, G.; Rodrigo, R. Improvement of a Novel Proposal for Antioxidant Treatment Against Brain Damage Occurring in Ischemic Stroke Patients. CNS Neurol. Disord. Drug Targets 2021, 20, 3–21. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The Free Radical Theory of Aging Matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirley, R.; Ord, E.; Work, L. Oxidative Stress and the Use of Antioxidants in Stroke. Antioxidants 2014, 3, 472–501. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, R.A.; Windelborn, J.A.; Kasprzak, J.M.; Franklin, J.L. A Bax-Induced Pro-Oxidant State Is Critical for Cytochrome c Release during Programmed Neuronal Death. J. Neurosci. 2002, 22, 6480–6490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E. Oxidative Stress and Cancer. Curr. Pharm. Des. 2019, 24, 4771–4778. [Google Scholar] [CrossRef]
- Saeed, S.A.; Shad, K.F.; Saleem, T.; Javed, F.; Khan, M.U. Some New Prospects in the Understanding of the Molecular Basis of the Pathogenesis of Stroke. Exp. Brain Res. 2007, 182, 1–10. [Google Scholar] [CrossRef]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative Stress and Pathophysiology of Ischemic Stroke: Novel Therapeutic Opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Margaill, I.; Plotkine, M.; Lerouet, D. Antioxidant Strategies in the Treatment of Stroke. Free. Radic. Biol. Med. 2005, 39, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, Q.; Hu, Z.; Tang, X. Potential Neuroprotective Treatment of Stroke: Targeting Excitotoxicity, Oxidative Stress, and Inflammation. Front. Neurosci. 2019, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Genovese, T.; Mazzon, E.; Paterniti, I.; Esposito, E.; Bramanti, P.; Cuzzocrea, S. Modulation of NADPH Oxidase Activation in Cerebral Ischemia/Reperfusion Injury in Rats. Brain Res. 2011, 1372, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ye, K.; Yang, X.; Zheng, J. Apocynin Attenuates Cerebral Infarction after Transient Focal Ischaemia in Rats. J. Int. Med. Res. 2007, 35, 517–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.H.W.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin Is Not an Inhibitor of Vascular NADPH Oxidases but an Antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef]
- Schluter, T.; Steinbach, A.C.; Steffen, A.; Rettig, R.; Grisk, O. Apocynin-Induced Vasodilation Involves Rho Kinase Inhibition but Not NADPH Oxidase Inhibition. Cardiovasc. Res. 2008, 80, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M. Apocynin, NADPH Oxidase, and Vascular Cells: A Complex Matter. Hypertension 2008, 51, 172–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinschnitz, C.; Grund, H.; Wingler, K.; Armitage, M.E.; Jones, E.; Mittal, M.; Barit, D.; Schwarz, T.; Geis, C.; Kraft, P.; et al. Post-Stroke Inhibition of Induced NADPH Oxidase Type 4 Prevents Oxidative Stress and Neurodegeneration. PLoS Biol. 2010, 8, e1000479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, A.I.; Geuss, E.; Kleikers, P.W.M.; Mencl, S.; Herrmann, A.M.; Buendia, I.; Egea, J.; Meuth, S.G.; Lopez, M.G.; Kleinschnitz, C.; et al. NOX4-Dependent Neuronal Autotoxicity and BBB Breakdown Explain the Superior Sensitivity of the Brain to Ischemic Damage. Proc. Natl. Acad. Sci. USA 2017, 114, 12315–12320. [Google Scholar] [CrossRef] [Green Version]
- Casas, A.I.; Kleikers, P.W.M.; Geuss, E.; Langhauser, F.; Adler, T.; Busch, D.H.; Gailus-Durner, V.; de Angelis, M.H.; Egea, J.; Lopez, M.G.; et al. Calcium-Dependent Blood-Brain Barrier Breakdown by NOX5 Limits Postreperfusion Benefit in Stroke. J. Clin. Investig. 2019, 129, 1772–1778. [Google Scholar] [CrossRef]
- Dao, V.T.-V.; Elbatreek, M.H.; Altenhöfer, S.; Casas, A.I.; Pachado, M.P.; Neullens, C.T.; Knaus, U.G.; Schmidt, H.H.H.W. Isoform-Selective NADPH Oxidase Inhibitor Panel for Pharmacological Target Validation. Free. Radic. Biol. Med. 2020, 148, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Gao, S.; Tu, S.; Lenahan, C.; Shao, A.; Sheng, J. Pathophysiology and Therapeutic Potential of NADPH Oxidases in Ischemic Stroke-Induced Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Laurie, C.; Lee Mosley, R.; Gendelman, H.E. Oxidative Stress and the Pathogenesis of Neurodegenerative Disorders. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 82, pp. 297–325. ISBN 978-0-12-373989-6. [Google Scholar]
- Tsutsumi, Z.; Moriwaki, Y.; Takahashi, S.; Ka, T.; Yamamoto, T. Oxidized Low-Density Lipoprotein Autoantibodies in Patients with Primary Gout: Effect of Urate-Lowering Therapy. Clin. Chim. Acta 2004, 339, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yan, D.; Li, S.; Liu, S.; Zeng, F.; Cheung, C.W.; Liu, H.; Irwin, M.G.; Huang, H.; Xia, Z. Allopurinol Reduces Oxidative Stress and Activates Nrf2/P62 to Attenuate Diabetic Cardiomyopathy in Rats. J. Cell. Mol. Med. 2020, 24, 1760–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martz, D.; Rayos, G.; Schielke, G.P.; Betz, A.L. Allopurinol and Dimethylthiourea Reduce Brain Infarction Following Middle Cerebral Artery Occlusion in Rats. Stroke 1989, 20, 488–494. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Villegas, V.; Istre, H.; Heppler, B.; Gonzalez, N.; Brusman, N.; Snider, L.; Hogle, E.; Tucker, J.; Oñate, A.; et al. Synthesis and Characterization of CAPE Derivatives as Xanthine Oxidase Inhibitors with Radical Scavenging Properties. Bioorganic Chem. 2019, 86, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, H.; Du, Q.; Shen, J. Targeting Myeloperoxidase (MPO) Mediated Oxidative Stress and Inflammation for Reducing Brain Ischemia Injury: Potential Application of Natural Compounds. Front. Physiol. 2020, 11, 433. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, A.; Ansari, M.A.; Manjunath, P.M. Partial Role of Multiple Pathways in Infarct Size Limiting Effect of Quercetin and Rutin against Cerebral Ischemia-Reperfusion Injury in Rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 491–500. [Google Scholar] [PubMed]
- Lei, J.; Tu, X.; Wang, Y.; Tu, D.; Shi, S. Resveratrol Downregulates the TLR4 Signaling Pathway to Reduce Brain Damage in a Rat Model of Focal Cerebral Ischemia. Exp. Ther. Med. 2019, 17, 3215–3221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pravalika, K.; Sarmah, D.; Kaur, H.; Vats, K.; Saraf, J.; Wanve, M.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; et al. Trigonelline Therapy Confers Neuroprotection by Reduced Glutathione Mediated Myeloperoxidase Expression in Animal Model of Ischemic Stroke. Life Sci. 2019, 216, 49–58. [Google Scholar] [CrossRef]
- Tu, X.; Yang, W.; Chen, J.; Chen, Y.; Ouyang, L.; Xu, Y.; Shi, S. Curcumin Inhibits TLR2/4-NF-ΚB Signaling Pathway and Attenuates Brain Damage in Permanent Focal Cerebral Ischemia in Rats. Inflammation 2014, 37, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Jiang, S.-Q.; Zhang, L.; Liu, Q.-N.; Gong, P.-L. Inhibitory Effect of Dauricine on Inflammatory Process Following Focal Cerebral Ischemia/Reperfusion in Rats. Am. J. Chin. Med. 2007, 35, 477–486. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol Attenuates Endothelial Oxidative Injury by Inducing Autophagy via the Activation of Transcription Factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef]
- Sena, E.; Wheble, P.; Sandercock, P.; Macleod, M. Systematic Review and Meta-Analysis of the Efficacy of Tirilazad in Experimental Stroke. Stroke 2007, 38, 388–394. [Google Scholar] [CrossRef] [Green Version]
- Otomo, E.; Tohgi, H.; Takakura, K.; Hirai, S. Gotoh Effect of a Novel Free Radical Scavenger, Edaravone (MCI-186), on Acute Brain Infarction. Cerebrovasc. Dis. 2003, 15, 222–229. [Google Scholar] [CrossRef]
- Fujimura, M.; Morita-Fujimura, Y.; Noshita, N.; Sugawara, T.; Kawase, M.; Chan, P.H. The Cytosolic Antioxidant Copper/Zinc-Superoxide Dismutase Prevents the Early Release of Mitochondrial Cytochrome c in Ischemic Brain after Transient Focal Cerebral Ischemia in Mice. J. Neurosci. 2000, 20, 2817–2824. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, T.; Lewén, A.; Gasche, Y.; Yu, F.; Chan, P.H. Overexpression of SOD1 Protects Vulnerable Motor Neurons after Spinal Cord Injury by Attenuating Mitochondrial Cytochrome c Release. FASEB J. 2002, 16, 1997–1999. [Google Scholar] [CrossRef]
- Atochin, D.N.; Wang, A.; Liu, V.W.T.; Critchlow, J.D.; Dantas, A.P.V.; Looft-Wilson, R.; Murata, T.; Salomone, S.; Shin, H.K.; Ayata, C.; et al. The Phosphorylation State of ENOS Modulates Vascular Reactivity and Outcome of Cerebral Ischemia in Vivo. J. Clin. Invest. 2007, 117, 1961–1967. [Google Scholar] [CrossRef] [Green Version]
- Aronowski, J.; Strong, R.; Grotta, J.C. Treatment of Experimental Focal Ischemia in Rats with Lubeluzole. Neuropharmacology 1996, 35, 689–693. [Google Scholar] [CrossRef]
- Diener, H.C. Multinational Randomised Controlled Trial of Lubeluzole in Acute Ischaemic Stroke. Cerebrovasc. Dis. 1998, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Niatsetskaya, Z.V.; Sosunov, S.A.; Matsiukevich, D.; Utkina-Sosunova, I.V.; Ratner, V.I.; Starkov, A.A.; Ten, V.S. The Oxygen Free Radicals Originating from Mitochondrial Complex I Contribute to Oxidative Brain Injury Following Hypoxia-Ischemia in Neonatal Mice. J. Neurosci. 2012, 32, 3235–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.P. Antioxidants as Therapies: Can We Improve on Nature? Free. Radic. Biol. Med. 2014, 66, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.M.; Parnova, R.G. Protective Effect of Mitochondria-Targeted Antioxidants against Inflammatory Response to Lipopolysaccharide Challenge: A Review. Pharmaceutics 2021, 13, 144. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; Tanaka, T.; Pu, F.; Egashira, N.; Iwasaki, K.; Hidaka, R.; Matsunaga, K.; Takata, J.; Karube, Y.; Fujiwara, M. Vitamin E Isoforms A-Tocotrienol and g-Tocopherol Prevent Cerebral Infarction in Mice. Neurosci. Lett. 2003, 5, 56–60. [Google Scholar] [CrossRef]
- Loh, H.C.; Lim, R.; Lee, K.W.; Ooi, C.Y.; Chuan, D.R.; Looi, I.; Kah Hay, Y.; Abdul Karim Khan, N. Effects of Vitamin E on Stroke: A Systematic Review with Meta-Analysis and Trial Sequential Analysis. Stroke Vasc. Neurol. 2021, 6, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, L.; Ning, S.; Liu, Z.; Lin, H.; Chen, S.; Zhu, J. Vitamin E Intake and Risk of Stroke: A Meta-Analysis. Br. J. Nutr. 2018, 120, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wang, K.; Wan, W.; Cheng, Y.; Pu, X.; Ye, X. Resveratrol Provides Neuroprotection by Regulating the JAK2/STAT3/PI3K/AKT/MTOR Pathway after Stroke in Rats. Genes Dis. 2018, 5, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H. Brain Protection by Resveratrol and Fenofibrate against Stroke Requires Peroxisome Proliferator-Activated Receptor α in Mice. Neurosci. Lett. 2003, 352, 203–206. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Dringen, R. Glutathione Metabolism and Oxidative Stress in Neurodegeneration. Eur. J. Biochem. 2000, 267, 4903. [Google Scholar] [CrossRef]
- Khan, M.; Sekhon, B.; Giri, S.; Jatana, M.; Gilg, A.G.; Ayasolla, K.; Elango, C.; Singh, A.K.; Singh, I. S -Nitrosoglutathione Reduces Inflammation and Protects Brain against Focal Cerebral Ischemia in a Rat Model of Experimental Stroke. J. Cereb. Blood Flow Metab. 2005, 25, 177–192. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, M.M.; Javed, H.; Raza, S.S.; Ishrat, T.; Badruzzaman Khan, M.; Safhi, M.M.; Islam, F. Edaravone Ameliorates Oxidative Stress Associated Cholinergic Dysfunction and Limits Apoptotic Response Following Focal Cerebral Ischemia in Rat. Mol. Cell Biochem. 2012, 367, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Ritz, M.-F.; Curin, Y.; Mendelowitsch, A.; Andriantsitohaina, R. Acute Treatment with Red Wine Polyphenols Protects from Ischemia-Induced Excitotoxicity, Energy Failure and Oxidative Stress in Rats. Brain Res. 2008, 1239, 226–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Peng, D.; Zhu, H.; Wang, X. Experimental Evidence of Ginkgo Biloba Extract EGB as a Neuroprotective Agent in Ischemia Stroke Rats. Brain Res. Bull. 2012, 87, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wen, Z.; Shen, H.; Shen, M.; Chen, G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxidative Med. Cell. Longev. 2016, 2016, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective Effect of Sulforaphane against Oxidative Stress: Recent Advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef]
- Lu, H.; Shen, J.; Song, X.; Ge, J.; Cai, R.; Dai, A.; Jiang, Z. Protective Effect of Pyrroloquinoline Quinone (PQQ) in Rat Model of Intracerebral Hemorrhage. Cell. Mol. Neurobiol. 2015, 35, 921–930. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, C.; Meng, C.-J.; Zhu, G.-Q.; Sun, X.-B.; Huo, L.; Zhang, J.; Liu, H.-X.; He, W.-C.; Shen, X.-M.; et al. Melatonin Activates the Nrf2-ARE Pathway When It Protects against Early Brain Injury in a Subarachnoid Hemorrhage Model: Melatonin and Nrf2-ARE Pathway in SAH. J. Pineal Res. 2012, 53, 129–137. [Google Scholar] [CrossRef]
- Zhang, T. Ursolic Acid Reduces Oxidative Stress to Alleviate Early Brain Injury Following Experimental Subarachnoid Hemorrhage. Neurosci. Lett. 2014, 579, 12–17. [Google Scholar] [CrossRef]
- Jelinek, M.; Jurajda, M.; Duris, K. The Role of Oxidative Stress in Early Brain Injury after Subarachnoid Hemorrhage. Oxidative Med. Cell. Longev. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Xie, Y.-K.; Zhou, X.; Yuan, H.-T.; Qiu, J.; Xin, D.-Q.; Chu, X.-L.; Wang, D.-C.; Wang, Z. Resveratrol Reduces Brain Injury after Subarachnoid Hemorrhage by Inhibiting Oxidative Stress and Endoplasmic Reticulum Stress. Neural Regen. Res. 2019, 14, 1734. [Google Scholar] [CrossRef]
- Ord, E.N.; Shirley, R.; McClure, J.D.; McCabe, C.; Kremer, E.J.; Macrae, I.M.; Work, L.M. Combined Antiapoptotic and Antioxidant Approach to Acute Neuroprotection for Stroke in Hypertensive Rats. J. Cereb. Blood Flow Metab. 2013, 33, 1215–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alper, B.S.; Foster, G.; Thabane, L.; Rae, A.; Malone, M.; Manheimer, E. Thrombolysis with Alteplase 3–4.5 Hours after Acute Ischaemic Stroke: Trial Reanalysis Adjusted for Baseline Imbalances. BMJ Evid. Based Med. 2020, 25, 8. [Google Scholar] [CrossRef]
- Baker, A.H.; Sica, V.; Work, L.M.; Williams-Ignarro, S.; de Nigris, F.; Lerman, L.O.; Casamassimi, A.; Lanza, A.; Schiano, C.; Rienzo, M.; et al. Brain Protection Using Autologous Bone Marrow Cell, Metalloproteinase Inhibitors, and Metabolic Treatment in Cerebral Ischemia. Proc. Natl. Acad. Sci. USA 2007, 104, 3597–3602. [Google Scholar] [CrossRef] [Green Version]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Howells, D.W. Evaluation of Combination Therapy in Animal Models of Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2012, 32, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhao, M.; Chen, H.; Lenahan, C.; Zhou, X.; Ou, Y.; He, Y. The Role of Nanomaterials in Stroke Treatment: Targeting Oxidative Stress. Oxidative Med. Cell. Longev. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the Surface Modification, Size, and Shape on Cellular Uptake of Nanoparticles: Cellular Uptake of Nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Baranoski, J.F.; Ducruet, A.F. Nanoparticle-Facilitated Delivery of Antioxidant Therapy Following Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2019, 85, E174–E175. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.-G.; Cha, B.G.; Kang, D.-W.; Kim, D.Y.; Ki, S.K.; Kim, S.I.; Han, J.h.; Yang, W.; Kim, C.K.; Kim, J.; et al. Ceria Nanoparticles Synthesized With Aminocaproic Acid for the Treatment of Subarachnoid Hemorrhage. Stroke 2018, 49, 3030–3038. [Google Scholar] [CrossRef]
- He, J.; Liu, J.; Huang, Y.; Tang, X.; Xiao, H.; Hu, Z. Oxidative Stress, Inflammation, and Autophagy: Potential Targets of Mesenchymal Stem Cells-Based Therapies in Ischemic Stroke. Front. Neurosci. 2021, 15, 641157. [Google Scholar] [CrossRef] [PubMed]
- Boshuizen, M.C.S.; Steinberg, G.K. Stem Cell–Based Immunomodulation After Stroke: Effects on Brain Repair Processes. Stroke 2018, 49, 1563–1570. [Google Scholar] [CrossRef]
- Tadokoro, K.; Fukui, Y.; Yamashita, T.; Liu, X.; Tsunoda, K.; Shang, J.; Morihara, R.; Nakano, Y.; Tian, F.; Sasaki, R.; et al. Bone Marrow Stromal Cell Transplantation Drives Molecular Switch from Autophagy to the Ubiquitin-Proteasome System in Ischemic Stroke Mice. J. Stroke Cerebrovasc. Dis. 2020, 29, 104743. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R. Why Have Antioxidants Failed in Clinical Trials? Am. J. Cardiol. 2008, 101, S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palacio, C.; Mooradian, A.D. Clinical Trials and Antioxidant Outcomes. In Oxidative Stress and Antioxidant Protection; Armstrong, D., Stratton, R.D., Eds.; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2016; pp. 493–506. ISBN 978-1-118-83243-1. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. https://doi.org/10.3390/antiox10121886
Jelinek M, Jurajda M, Duris K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants. 2021; 10(12):1886. https://doi.org/10.3390/antiox10121886
Chicago/Turabian StyleJelinek, Matyas, Michal Jurajda, and Kamil Duris. 2021. "Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke" Antioxidants 10, no. 12: 1886. https://doi.org/10.3390/antiox10121886
APA StyleJelinek, M., Jurajda, M., & Duris, K. (2021). Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants, 10(12), 1886. https://doi.org/10.3390/antiox10121886






