Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation
Abstract
:1. Introduction
2. NRF2-Antioxidant Defense System Pathway
3. NRF2 Signaling in Oxidative Stress-Mediated Diseases
3.1. Diabetes Mellitus
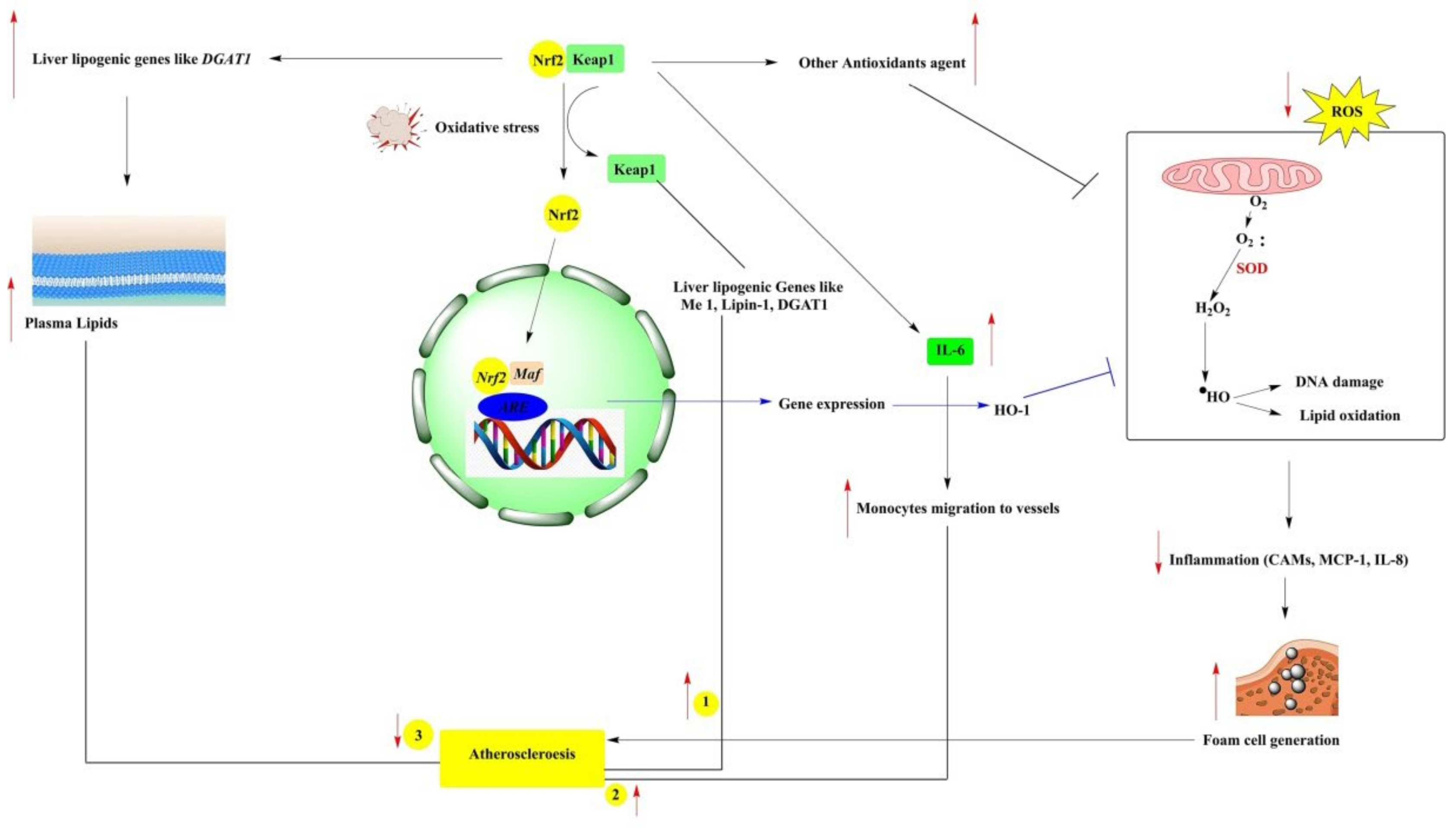
3.2. Atherosclerosis
3.3. Alzheimer’s and Parkinson’s Disease
3.4. Cancer
3.5. Acute and Chronic Kidney Disease
4. Phytochemicals and Regulation of NRF2 Signaling in Various Diseases
4.1. Curcumin
4.2. Quercetin
4.3. Epigallocatechin-3-Gallate (EGCG)
4.4. Resveratrol
4.5. Sulforaphane
4.6. Apigenin
4.7. Ursolic Acid
4.8. Naringenin
4.9. Pterostilbene
4.10. Rutin
4.11. Cinnamaldehyde
4.12. Xanthohumol
5. Epigenetic Regulation of Phytochemicals on the NRF2-Signaling Pathway
| S. No | Phytochemicals | Epigenetic Modification and Mechanism | Cell/Animal Model | Function | Refs |
|---|---|---|---|---|---|
| 1. | Naringenin | Histone acetylation-dependent inhibition of thioredoxin-interacting protein expression; Regulating AMPK-mediated p300 inactivation | diabetic db/db mouse and INS-1 pancreatic β cell line | Protects pancreatic beta cells and inhibit the progression of type II diabetes | [146] |
| 2. | Pterostilbene | Apoptosis in cancer cells is regulated by theMTA1/HDAC1/NuRD complex | SMMC-7721 | suppressed the growth, and invasion of hepatocellular carcinoma | [147] |
| 3. | 3,4-dihydroxytoluene, a rutin metabolite | Inhibited p300 histone acetyltransferase activity and induced hypoacetylation at H3K9, H3K36, H4K8 and H4K16. Decreased lipogenesis-related genes and attenuated lipid synthesis | HepG2 cells ob/ob mice | Suppressed the progression of nonalcoholic fatty liver disease | [148] |
| 4. | Cinnamaldehyde | Regulates PERK-CHOP signaling, Inhibits G9a histone methyltransferase, Mediates autophagic cell death | Gastric cancer cells | Induced autophagy-mediated cell death through ER stress and enhanced epigenetic modification in gastric cancer cells | [149] |
| 5. | Xanthohumol | Increased the expression Nrf2, HMOX1 and NQO1 | Marc-145 cells. | Reduces PRRSV-induced oxidative stress and inhibits PRRSV growth | [150] |
| 6. | Ganoderic acid | Increased expression of PKR, PERK, PRDX3, NRF2 | Senescent human amniotic mesenchymal stem cell | Acts as an anti-aging agent | [151] |
| 7. | Celastrol | Deregulation of various miRNA | Hepatocellular carcinoma | Inhibits the progression of hepatocellular carcinoma | [152] |
| 8. | Polydatin | Increases miR200a expression and regulates KEAP1/NRF2 signaling pathway | HepG2 and BRL-3A cells | Reduces fructose-induced liver inflammation and lipid accumulation | [127] |
| 9. | Pelargonidin | inhibits DNA recognition and catalytic binding by DNMT1 and DNMT3A | HT29 cells | Regulates cell cycle and inhibits proliferation of colorectal carcinoma cells | [153] |
| 10. | Delphinidin | Modulate protein expression of DNMT1, DNMT3a, and class I/II HDACs activates the NRF2-ARE pathway | Mouse epidermal JB6 P+ cells | Inhibits neoplastic transformation and acts as an effective skin cancer chemo preventive agent | [129] |
| 11. | Luteolin | regulates NRF2/ARE pathway via modulating DNMTs and HDACs | Human colon cancer cells HCT116 | Exerts anti-tumor activity by blocking cell transformation | [154] |
| 12. | Fucoxanthin | Activated NRF2 signaling and reduced DNMT activity | Mouse skin epidermal JB6 P+ cells | Involves in skin cancer prevention and inhibits cell transformation | [155] |
| 13. | Corosolic acid | Modules global CpG methylation at tumor promoter | Mouse epidermal JB6 P+ cells | Acts as an effective agent against skin cancer | [156] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, Q. Transcriptional responses to oxidative stress: Pathological and toxicological implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.; Dash, R.; Sohag, A.A.M.; Haque, M.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkBsignaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Zhou, D.D.; Xie, T.; Malik, T.H.; Lu, C.B.; Li, H.J.; Hao, J.L. Nrf2, a potential therapeutic target against oxidative stress in corneal diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2326178. [Google Scholar] [CrossRef] [Green Version]
- Farooqui, Z.; Mohammad, R.S.; Lokhandwala, M.F.; Banday, A.A. Nrf2 inhibition induces oxidative stress, renal inflammation and hypertension in mice. Clin. Exp. Hypertens. 2021, 43, 175–180. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Pan, H.; Zhou, X.; Ruan, Q.; Kong, D.; Yao, P. Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front. Pharmacol. 2019, 10, 1099. [Google Scholar] [CrossRef]
- Onodera, Y.; Motohashi, H.; Takagi, K.; Miki, Y.; Shibahara, Y.; Watanabe, M.; Suzuki, T. NRF2 immunolocalization in human breast cancer patients as a prognostic factor. Endocr.-Relat. Cancer 2014, 21, 241–252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Review Antioxidant phytochemical for the prevention and treatment of chronic disease. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Järvinen, R.; Reunanen, A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Park, J.H.; Jung, J.W.; Ahn, Y.J.; Kwon, H.W. Neuroprotective properties of phytochemicals against paraquat-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Pestic. Biochem. Physiol. 2012, 104, 118–125. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Na, H.K.; Kim, S.H.; Surh, Y.J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008, 46, 1279–1287. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Z.; Huang, L.; Meng, B.; Zhou, X.; Wen, X.; Ren, D. Naringenin reduces oxidative stress and improves mitochondrial dysfunction via activation of the Nrf2/ARE signaling pathway in neurons. Int. J. Mol. Med. 2017, 40, 1582–1590. [Google Scholar] [CrossRef] [Green Version]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [Green Version]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Activation of Nrf2 signaling pathway by natural and synthetic chalcones: A therapeutic road map for oxidative stress. Expert Rev. Clin. Pharmacol. 2021, 14, 465–480. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Cui, T. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress–induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Cheng, X.; Siow, R.C.; Mann, G.E. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: A role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid. Redox Signal. 2011, 14, 469–487. [Google Scholar] [CrossRef]
- Uruno, A.; Furusawa, Y.; Yagishita, Y.; Fukutomi, T.; Muramatsu, H.; Negishi, T.; Yamamoto, M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol. Cell. Biol. 2013, 33, 2996–3010. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Whitman, S.A.; Wu, W.; Wondrak, G.T.; Wong, P.K.; Fang, D.; Zhang, D.D. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes 2011, 60, 3055–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, R.; Himori, N.; Taguchi, K.; Ishikawa, Y.; Uesugi, K.; Ito, M.; Nishida, K. The role of the Nrf2-mediated defense system in corneal epithelial wound healing. Free Radic. Biol. Med. 2013, 61, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Mimura, J.; Itoh, K. Role of Nrf2 in the pathogenesis of atherosclerosis. Free Radic. Biol. Med. 2015, 88, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Barajas, B.; Che, N.; Yin, F.; Rowshanrad, A.; Orozco, L.D.; Gong, K.W.; Araujo, J.A. NF-E2–related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Ruotsalainen, A.K.; Inkala, M.; Partanen, M.E.; Lappalainen, J.P.; Kansanen, E.; Mäkinen, P.I.; Levonen, A.L. The absence of macrophage Nrf2 promotes early atherogenesis. Cardiovasc. Res. 2013, 98, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Xu, S.; Duan, H.; Zhu, Z.; Yang, Z.; Cao, J.; Duan, J. A novel, highly-water-soluble apigenin derivative provides neuroprotection following ischemia in male rats by regulating the ERK/Nrf2/HO-1 pathway. Eur. J. Pharmacol. 2019, 855, 208–215. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T. Oxidative stress in Alzheimer’s disease: Molecular hallmarks of underlying vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Springer: Singapore, 2019; pp. 91–115. [Google Scholar]
- Branca, C.; Ferreira, E.; Nguyen, T.V.; Doyle, K.; Caccamo, A.; Oddo, S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 4823–4835. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [Green Version]
- Skibinski, G.; Hwang, V.; Ando, D.M.; Daub, A.; Lee, A.K.; Ravisankar, A.; Finkbeiner, S. Nrf2 mitigates LRRK2-and α-synuclein–induced neurodegeneration by modulating proteostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Wang, F.S.L.; Hu, X.; Chen, F.; Chan, H.M. Acrylamide-induced neurotoxicity in primary astrocytes and microglia: Roles of the Nrf2-ARE and NF-κB pathways. Food Chem. Toxicol. 2017, 106, 25–35. [Google Scholar] [CrossRef]
- Ali, T.; Rehman, S.U.; Shah, F.A.; Kim, M.O. Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain. J. Neuroinflamm. 2018, 15, 1–19. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhang, T.; Du, J. Ursolic acid sensitizes cisplatin-resistant HepG2/DDP cells to cisplatin via inhibiting Nrf2/ARE pathway. Drug Des. Develop. Ther. 2016, 10, 3471. [Google Scholar] [CrossRef] [Green Version]
- De la Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Syu, J.P.; Chi, J.T.; Kung, H.N. Nrf2 is the key to chemotherapy resistance in MCF7 breast cancer cells under hypoxia. Oncotarget 2016, 7, 14659. [Google Scholar] [CrossRef] [Green Version]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Sun, Y.; Liu, Z.; Lu, Y.; Zhu, X.; Lan, B.; Chen, Y. Activation of NRF2 ameliorates oxidative stress and cystogenesis in autosomal dominant polycystic kidney disease. Sci. Transl. Med. 2020, 12, eaba3613. [Google Scholar] [CrossRef]
- Rush, B.M.; Bondi, C.D.; Stocker, S.D.; Barry, K.M.; Small, S.A.; Ong, J.; Tan, R.J. Genetic or pharmacologic Nrf2 activation increases proteinuria in chronic kidney disease in mice. Kidney Int. 2021, 99, 102–116. [Google Scholar] [CrossRef]
- Fujiki, T.; Ando, F.; Murakami, K.; Isobe, K.; Mori, T.; Susa, K.; Uchida, S. Tolvaptan activates the Nrf2/HO-1 antioxidant pathway through PERK phosphorylation. Sci. Rep. 2019, 9, 9245. [Google Scholar] [CrossRef] [Green Version]
- Rubio-Navarro, A.; Vázquez-Carballo, C.; Guerrero-Hue, M.; García-Caballero, C.; Herencia, C.; Gutiérrez, E.; Moreno, J.A. Nrf2 plays a protective role against intravascular hemolysis-mediated acute kidney injury. Front. Pharmacol. 2019, 10, 740. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y.; Chun, Y.S.; Kim, J.K.; Lee, J.O.; Lee, Y.J.; Ku, S.K.; Shim, S.M. Curcumin ameliorated oxidative stress and inflammation-related muscle disorders in C2C12 myoblast cells. Antioxidants 2021, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in RAW264. 7 cells by increasing the activity of antioxidant enzymes and activating the Nrf2-Keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J. Diabetes 2012, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Zhang, Q.; Chi, C.; Wu, G.; Lin, Z.; Li, J.; Chen, G. Curcumin analog A13 alleviates oxidative stress by activating Nrf2/ARE pathway and ameliorates fibrosis in the myocardium of high-fat-diet and streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Sandhir, R.; Yadav, A.; Mehrotra, A.; Sunkaria, A.; Singh, A.; Sharma, S. Curcumin nanoparticles attenuate neurochemical and neurobehavioral deficits in experimental model of Huntington’s disease. Neuromol. Med. 2014, 16, 106–118. [Google Scholar] [CrossRef]
- Li, C.W.; Chang, P.Y.; Chen, B.S. Investigating the mechanism of hepatocellular carcinoma progression by constructing genetic and epigenetic networks using NGS data identification and big database mining method. Oncotarget 2016, 7, 79453. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Pan, X.; Fu, H.; Zheng, Y.; Dai, Y.; Yin, Y.; Hou, D. Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci. Rep. 2017, 7, 10114. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Pung, D.; Su, Z.Y.; Guo, Y.; Zhang, C.; Yang, A.Y.; Kong, A.N. Epigenetics reactivation of Nrf2 in prostate TRAMP C1 cells by curcumin analogue FN1. Chem. Res. Toxicol. 2016, 29, 694–703. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Ko, H.M.; Kim, I.S.; Choi, D.K. Recent trends in the development of nanophytobioactive compounds and delivery systems for their possible role in reducing oxidative stress in Parkinson’s disease models. Int. J. Nanomed. 2015, 10, 6757. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Parikh, M.; Shah, H.; Gandhi, T. Modulation of Nrf2 by quercetin in doxorubicin-treated rats. Heliyon 2020, 6, e03803. [Google Scholar] [CrossRef]
- Miyamoto, N.; Izumi, H.; Miyamoto, R.; Kondo, H.; Tawara, A.; Sasaguri, Y.; Kohno, K. Quercetin induces the expression of peroxiredoxins 3 and 5 via the Nrf2/NRF1 transcription pathway. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1055–1063. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Q.; Li, Z.; Dang, M.; Lin, Y.; Hou, X. Activation of Nrf2 signaling by sitagliptin and quercetin combination against β-amyloid induced Alzheimer’s disease in rats. Drug Dev. Res. 2019, 80, 837–845. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Shahidi, S.B.; Abbasi, M.; Tavakoli, Z.; Esmaeili, A. Quercetin-conjugated superparamagnetic iron oxide nanoparticles (QCSPIONs) increases Nrf2 expression via miR-27a mediation to prevent memory dysfunction in diabetic rats. Sci. Rep. 2020, 10, 15957. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, H.; Yang, Y.; Li, M.; Hang, L.; Xu, X. A solid dispersion of quercetin shows enhanced Nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxid. Med. Cell. Longev. 2019, 2019, 1479571. [Google Scholar] [CrossRef]
- Mostafavi Pour, Z.; Ramezani, F.; Keshavarzi, F.; Samadi, N. The role of quercetin and vitamin C in Nrf2 dependent oxidative stress production in breast cancer cells. Oncol. Lett. 2017, 13, 1965–1973. [Google Scholar] [CrossRef] [Green Version]
- Tsai, P.Y.; Ka, S.M.; Chang, J.M.; Chen, H.C.; Shui, H.A.; Li, C.Y.; Chen, A. Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic. Biol. Med. 2011, 51, 744–754. [Google Scholar] [CrossRef]
- Mohan, T.; Narasimhan, K.K.S.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Periandavan, K. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Radic. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef]
- Han, J.; Wang, M.; Jing, X.; Shi, H.; Ren, M.; Lou, H. (−)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014, 39, 1292–1299. [Google Scholar] [CrossRef]
- Kanlaya, R.; Khamchun, S.; Kapincharanon, C.; Thongboonkerd, V. Protective effect of epigallocatechin-3-gallate (EGCG) via Nrf2 pathway against oxalate-induced epithelial mesenchymal transition (EMT) of renal tubular cells. Sci. Rep. 2016, 6, 30233. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Song, X.; Zhao, L.; Li, Z.; Liu, B. Resveratrol prevents diabetic cardiomyopathy by increasing Nrf2 expression and transcriptional activity. BioMed Res. Int. 2018, 2018, 2150218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol attenuates the cytotoxicity induced by amyloid-β 1–42 in PC12 cells by upregulating heme oxygenase-1 via the PI3K/Akt/Nrf2 pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem.-Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef]
- Cheng, L.; Yan, B.; Chen, K.; Jiang, Z.; Zhou, C.; Cao, J.; Sha, H. Resveratrol-induced downregulation of NAF-1 enhances the sensitivity of pancreatic cancer cells to gemcitabine via the ROS/Nrf2 signaling pathways. Oxid. Med. Cell. Longev. 2018, 2018, 9482018. [Google Scholar] [CrossRef]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, H.; Teimouri, M.; Shabani, M.; Koushki, M.; Khorzoughi, R.B.; Namvarjah, F.; Meshkani, R. Resveratrol alleviates non-alcoholic fatty liver disease through epigenetic modification of the Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2020, 119, 105667. [Google Scholar] [CrossRef]
- Hwang, J.H.; Lim, S.B. Antioxidant and anti-inflammatory activities of broccoli florets in LPS-stimulated RAW 264.7 cells. Prev. Nutr. Food Sci. 2014, 19, 89. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yang, H.; Chen, X. Protective effects of sulforaphane on diabetic retinopathy: Activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liao, G.; Zhou, Q.; Lv, D.; Holthfer, H.; Zou, H. Sulforaphane attenuates contrast-induced nephropathy in rats via Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2016, 2016, 9825623. [Google Scholar] [CrossRef]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Jo, D.G. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Chen, B.; Wang, X.; Wu, L.; Yang, Y.; Cheng, X.; Cao, P. Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2 and autophagy pathways. Sci. Rep. 2016, 6, 32206. [Google Scholar] [CrossRef] [Green Version]
- Xin, Y.; Bai, Y.; Jiang, X.; Zhou, S.; Wang, Y.; Wintergerst, K.A.; Cai, L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ss/Fyn pathway. Redox Biol. 2018, 15, 405–417. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Su, Z.Y.; Kong, A.N.T. Apigenin reactivates Nrf2 anti-oxidative stress signaling in mouse skin epidermal JB6 P+ cells through epigenetics modifications. AAPS J. 2014, 16, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.Y.; Wu, R.; Guo, Y.; Kong, A.N. Fucoxanthin elicits epigenetic modifications, Nrf2 activation and blocking transformation in mouse skin JB6 P+ cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef]
- Li, P.; Bukhari, S.N.A.; Khan, T.; Chitti, R.; Bevoor, D.B.; Hiremath, A.R.; Gubbiyappa, K.S. Apigenin-loaded solid lipid nanoparticle attenuates diabetic nephropathy induced by streptozotocin nicotinamide through Nrf2/HO-1/NF-kB signalling pathway. Int. J. Nanomed. 2020, 15, 9115. [Google Scholar] [CrossRef]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in Lps-stimulated Bv2 microglia through activating Gsk3β/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef]
- Gao, A.M.; Zhang, X.Y.; Ke, Z.P. Apigenin sensitizes BEL-7402/ADM cells to doxorubicin through inhibiting miR-101/Nrf2 pathway. Oncotarget 2017, 8, 82085. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhao, X.; Zhu, H.; Wang, J.; Ma, J.; Gu, M. Apigenin protects against renal tubular epithelial cell injury and oxidative stress by high glucose via regulation of NF-E2-related factor 2 (Nrf2) pathway. Med. Sci. Monit. 2019, 25, 5280. [Google Scholar] [CrossRef]
- Paredes-Gonzalez, X.; Fuentes, F.; Jeffery, S.; Saw, C.L.L.; Shu, L.; Su, Z.Y.; Kong, A.N.T. Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm. Drug Dispos. 2015, 36, 440–451. [Google Scholar] [CrossRef]
- Habtemariam, S. Antioxidant and anti-inflammatory mechanisms of neuroprotection by ursolic acid: Addressing brain injury, cerebral ischemia, cognition deficit, anxiety, and depression. Oxid. Med. Cell. Longev. 2019, 2019, 8512048. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Wang, H.; Zhu, L.; Wei, W. Ursolic acid ameliorates early brain injury after experimental traumatic brain injury in mice by activating the Nrf2 pathway. Neurochem. Res. 2017, 42, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I. Protective effects of ursolic acid on osteoblastic differentiation via activation of IER3/Nrf2. J. Dent. Hyg. Sci. 2019, 19, 198–204. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Protective effects of ursolic acid in an experimental model of liver fibrosis through Nrf2/ARE pathway. Clin. Res. Hepatol. Gastroenterol. 2015, 39, 188–197. [Google Scholar] [CrossRef]
- Kim, H.; Ramirez, C.N.; Su, Z.Y.; Kong, A.N.T. Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells. J. Nutr. Biochem. 2016, 33, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Rajappa, R.; Sireesh, D.; Salai, M.B.; Ramkumar, K.M.; Sarvajayakesavulu, S.; Madhunapantula, S.V. Treatment with naringenin elevates the activity of transcription factor Nrf2 to protect pancreatic β-cells from streptozotocin-induced diabetes In Vitro and In Vivo. Front. Pharmacol. 2019, 9, 1562. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Wu, W.; Ge, S.; Jia, R.; Lin, T.; Yuan, Y.; Zhang, D. Naringin protects against perfluorooctane sulfonate-induced liver injury by modulating NRF2 and NF-κB in mice. Int. Immunopharmacol. 2018, 65, 140–147. [Google Scholar] [CrossRef]
- Kapoor, R.; Sirohi, V.K.; Gupta, K.; Dwivedi, A. Naringenin ameliorates progression of endometriosis by modulating Nrf2/Keap1/HO1 axis and inducing apoptosis in rats. J. Nutr. Biochem. 2019, 70, 215–226. [Google Scholar] [CrossRef]
- Adil, M.; Kandhare, A.D.; Ghosh, P.; Bodhankar, S.L. Sodium arsenite-induced myocardial bruise in rats: Ameliorative effect of naringin via TGF-β/Smad and Nrf/HO pathways. Chem.-Biol. Interact. 2016, 253, 66–77. [Google Scholar] [CrossRef]
- Chen, R.J.; Kuo, H.C.; Cheng, L.H.; Lee, Y.H.; Chang, W.T.; Wang, B., Jr.; Cheng, H.C. Apoptotic and nonapoptotic activities of pterostilbene against cancer. Int. J. Mol. Sci. 2018, 19, 287. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene activates the Nrf2-dependent antioxidant response to ameliorate arsenic-induced intracellular damage and apoptosis in human keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef]
- Xue, E.X.; Lin, J.P.; Zhang, Y.; Sheng, S.R.; Liu, H.X.; Zhou, Y.L.; Xu, H. Pterostilbene inhibits inflammation and ROS production in chondrocytes by activating Nrf2 pathway. Oncotarget 2017, 8, 41988. [Google Scholar] [CrossRef] [Green Version]
- Elango, B.; Dornadula, S.; Paulmurugan, R.; Ramkumar, K.M. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing antioxidant signaling pathways mediated by Nrf2. Chem. Res. Toxicol. 2016, 29, 47–57. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.Q.; Chen, H.Y.; Liu, B.H. Involvement of the Nrf2 pathway in the regulation of pterostilbene-induced apoptosis in HeLa cells via ER stress. J. Pharmacol. Sci. 2014, 126, 216–229. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, K.; AlSharif, D.; Mazza, C.; Syar, P.; Al Sharif, M.; Fata, J.E. Resveratrol and pterostilbene exhibit anticancer properties involving the downregulation of HPV oncoprotein E6 in cervical cancer cells. Nutrients 2018, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Cytoprotective effect of ascorbic acid and rutin against oxidative changes in the proteome of skin fibroblasts cultured in a three-dimensional system. Nutrients 2020, 12, 1074. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects t butyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef]
- Mittal, R.; Kumar, A.; Singh, D.P.; Bishnoi, M.; Nag, T.C. Ameliorative potential of rutin in combination with nimesulide in STZ model of diabetic neuropathy: Targeting Nrf2/HO-1/NF-kB and COX signalling pathway. Inflammopharmacology 2018, 26, 755–768. [Google Scholar] [CrossRef]
- Oluranti, O.I.; Alabi, B.A.; Michael, O.S.; Ojo, A.O.; Fatokun, B.P. Rutin prevents cardiac oxidative stress and inflammation induced by bisphenol A and dibutyl phthalate exposure via NRF-2/NF-κB pathway. Life Sci. 2021, 284, 119878. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Wang, K.; Zhu, X.; Ali, Y.; Shu, W.; Zhou, F. Procyanidin B2 and rutin in Ginkgo biloba extracts protect human retinal pigment epithelial (RPE) cells from oxidative stress by modulating Nrf2 and Erk1/2 signalling. Exp. Eye Res. 2021, 207, 108586. [Google Scholar] [CrossRef] [PubMed]
- Thabet, N.M.; Moustafa, E.M. Protective effect of rutin against brain injury induced by acrylamide or gamma radiation: Role of PI3K/AKT/GSK-3β/NRF-2 signalling pathway. Arch. Physiol. Biochem. 2018, 124, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Abou El-ezz, D.; Maher, A.; Sallam, N.; El-Brairy, A.; Kenawy, S. Trans-cinnamaldehyde modulates hippocampal Nrf2 factor and inhibits amyloid beta aggregation in LPS-induced neuroinflammation mouse model. Neurochem. Res. 2018, 43, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, Y.; Wang, D.; Yang, Q.; Wan, J.; Liu, S.; Yang, Y. Cinnamaldehyde ameliorates vascular dysfunction in diabetic mice by activating Nrf2. Am. J. Hypertens. 2020, 33, 610–619. [Google Scholar] [CrossRef]
- Ryu, J.S.; Kang, H.Y.; Lee, J.K. Effect of treadmill exercise and trans-cinnamaldehyde against d-galactose-and aluminum chloride-induced cognitive dysfunction in mice. Brain Sci. 2020, 10, 793. [Google Scholar] [CrossRef]
- Uchi, H.; Yasumatsu, M.; Morino-Koga, S.; Mitoma, C.; Furue, M. Inhibition of aryl hydrocarbon receptor signaling and induction of NRF2-mediated antioxidant activity by cinnamaldehyde in human keratinocytes. J. Dermatol. Sci. 2017, 85, 36–43. [Google Scholar] [CrossRef]
- Huang, T.C.; Chung, Y.L.; Wu, M.L.; Chuang, S.M. Cinnamaldehyde enhances Nrf2 nuclear translocation to upregulate phase II detoxifying enzyme expression in HepG2 cells. J. Agric. Food Chem. 2011, 59, 5164–5171. [Google Scholar] [CrossRef]
- Wang, D.; Hou, J.; Yang, Y.; Zhou, P.; Liu, S.; Wan, J.; Wang, P. Cinnamaldehyde ameliorates high-glucose–induced oxidative stress and cardiomyocyte injury through transient receptor potential ankyrin 1. J. Cardiovasc. Pharmacol. 2019, 74, 30–37. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, X.C.; Zhang, H.; Sun, Q.; Wu, Q.L.; Qu, L. Combination of quercetin, cinnamaldehyde and hirudin protects rat dorsal root ganglion neurons against high glucose-induced injury through Nrf-2/HO-1 activation and NF-κB inhibition. Chin. J. Integr. Med. 2017, 23, 663–671. [Google Scholar] [CrossRef]
- Wang, F.; Pu, C.; Zhou, P.; Wang, P.; Liang, D.; Wang, Q.; Hu, Y.; Li, B.; Hao, X. Cinnamaldehyde prevents endothelial dysfunction induced by high glucose by activating Nrf2. Cell. Physiol. Biochem. 2015, 36, 315–324. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Cykowiak, M.; Szaefer, H.; Kleszcz, R.; Baer-Dubowska, W. Combination of xanthohumol and phenethyl isothiocyanate inhibits NF-κB and activates Nrf2 in pancreatic cancer cells. Toxicol. In Vitro 2020, 65, 104799. [Google Scholar] [CrossRef]
- Li, F.; Yao, Y.; Huang, H.; Hao, H.; Ying, M. Xanthohumol attenuates cisplatin-induced nephrotoxicity through inhibiting NF-κB and activating Nrf2 signaling pathways. Int. Immunopharmacol. 2018, 61, 277–282. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. Xanthohumol induces phase II enzymes via Nrf2 in human hepatocytes in vitro. Toxicol. In Vitro 2013, 27, 149–156. [Google Scholar] [CrossRef]
- Lee, I.S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a polyphenol chalcone present in hops, activating Nrf2 enzymes to confer protection against oxidative damage in PC12 cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; Rada, P.; Cuadrado, A.; López, M.G.; García, A.G.; León, R. Melatonin-sulforaphane hybrid ITH12674 induces neuroprotection in oxidative stress conditions by a ‘drug-prodrug’ mechanism of action. Br. J. Pharmacol. 2015, 172, 1807–1821. [Google Scholar] [CrossRef] [Green Version]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current landscape of NRF2 biomarkers in clinical trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Tan, X.; Yang, Y.; Xu, J.; Zhang, P.; Deng, R.; Mao, Y.; He, J.; Chen, Y.; Zhang, Y.; Ding, J.; et al. Luteolin exerts neuroprotection via modulation of the p62/Keap1/Nrf2 pathway in intracerebral hemorrhage. Front. Pharmacol. 2020, 10, 1551. [Google Scholar] [CrossRef]
- Tidke, P.S.; Patil, C.R. Nrf2 activator corosolic acid meliorates alloxan induced diabetic nephropathy in mice. Asian Pac. J. Trop. Biomed. 2017, 7, 797. [Google Scholar] [CrossRef]
- Hong, B.; Su, Z.; Zhang, C.; Yang, Y.; Guo, Y.; Li, W.; Kong, A.N. Reserpine inhibit the JB6 P+ cell transformation through epigenetic reactivation of Nrf2-mediated anti-oxidative stress pathway. AAPS J. 2016, 18, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, J.; Shree, A.; Vafa, A.; Afzal, S.M.; Sultana, S. Taxifolin ameliorates Benzo[a]pyrene-induced lung injury possibly via stimulating the Nrf2 signalling pathway. Int. Immunopharmacol. 2021, 96, 107566. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Kumar, S.; Navgeet. Ganoderic acid targeting nuclear factor erythroid 2-related factor 2 in lung cancer. Tumor Biol. 2017, 39, 1010428317695530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divya, T.; Dineshbabu, V.; Soumyakrishnan, S.; Sureshkumar, A.; Sudhandiran, G. Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem.-Biol. Int. 2016, 246, 52–62. [Google Scholar] [CrossRef]
- Zhao, X.J.; Yu, H.W.; Yang, Y.Z.; Wu, W.Y.; Chen, T.Y.; Jia, K.K.; Kang, L.L.; Jiao, R.Q.; Kong, L.D. Polydatin prevents fructose-induced liver inflammation and lipid deposition through increasing miR-200a to regulate Keap1/Nrf2 pathway. Redox Biol. 2018, 18, 124–137. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wang, C.; Wu, R.; Yin, R.; Kuo, H.C.; Wang, L.; Kong, A.N. Pelargonidin reduces the TPA induced transformation of mouse epidermal cells-potential involvement of Nrf2 promoter demethylation. Chem.-Biol. Interact. 2019, 309, 108701. [Google Scholar] [CrossRef]
- Kuo, H.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.N. Anthocyanin delphinidin prevents neoplastic transformation of mouse skin JB6 P+ cells: Epigenetic reactivation of Nrf2-ARE pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef]
- Okada, K.; Shoda, J.; Taguchi, K.; Maher, J.M.; Ishizaki, K.; Inoue, Y.; Yamamoto, M. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G735–G747. [Google Scholar] [CrossRef]
- Lee, B.H.; Hsu, W.H.; Hsu, Y.W.; Pan, T.M. Dimerumic acid attenuates receptor for advanced glycation end products signal to inhibit inflammation and diabetes mediated by Nrf2 activation and promotes methylglyoxal metabolism into d-lactic acid. Free Radic. Biol. Med. 2013, 60, 7–16. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.F.; Liu, X.T.; Li, Y.C.; Zhu, H.M.; Sun, M.R.; Yang, H. Songorine promotes cardiac mitochondrial biogenesis via Nrf2 induction during sepsis. Redox Biol. 2021, 38, 101771. [Google Scholar] [CrossRef]
- Ohnuma, T.; Sakamoto, K.; Shinoda, A.; Takagi, C.; Ohno, S.; Nishiyama, T.; Hiratsuka, A. Procyanidins from Cinnamomi cortex promote proteasome-independent degradation of nuclear Nrf2 through phosphorylation of insulin-like growth factor-1 receptor in A549 cells. Arch. Biochem. Biophys. 2017, 635, 66–73. [Google Scholar] [CrossRef]
- Davies, T.G.; Wixted, W.E.; Coyle, J.E.; Griffiths-Jones, C.; Hearn, K.; McMenamin, R.; Kerns, J.K. Monoacidic inhibitors of the Kelch-like ECH-associated protein 1: Nuclear factor erythroid 2-related factor 2 (KEAP1: NRF2) protein–protein interaction with high cell potency identified by fragment-based discovery. J. Med. Chem. 2016, 59, 3991–4006. [Google Scholar] [CrossRef]
- Lu, M.C.; Jiao, Q.; Liu, T.; Tan, S.J.; Zhou, H.S.; You, Q.D.; Jiang, Z.Y. Discovery of a head-to-tail cyclic peptide as the Keap1-Nrf2 protein-protein interaction inhibitor with high cell potency. Eur. J. Med. Chem. 2018, 143, 1578–1589. [Google Scholar] [CrossRef]
- Hassanein, E.H.; Sayed, A.M.; Hussein, O.E.; Mahmoud, A.M. Coumarins as modulators of the Keap1/Nrf2/ARE signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1675957. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Kim, G.J.; Kim, H.J.; Nam, J.W.; Kim, J.; Chin, J.; Park, K.D. Identification and evaluation of a napyradiomycin as a potent Nrf2 activator: Anti-oxidative and anti-inflammatory activities. Bioorg. Chem. 2020, 105, 104434. [Google Scholar] [CrossRef]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Biswal, S. Small molecule inhibitor of NRF2 selectively intervenes therapeutic resistance in KEAP1-deficient NSCLC tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Kim, E.H.; Lee, J.; Roh, J.L. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic. Biol. Med. 2018, 129, 454–462. [Google Scholar] [CrossRef]
- Zhou, J.W.; Wang, M.; Sun, N.X.; Qing, Y.; Yin, T.F.; Li, C.; Wu, D. Sulforaphane induced epigenetic regulation of Nrf2 expression by DNA methyltransferase in human Caco 2 cells. Oncol. Lett. 2019, 18, 2639–2647. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.Y.; Zhang, C.; Lee, J.H.; Shu, L.; Wu, T.Y.; Khor, T.O.; Kong, A.N.T. Requirement and epigenetics reprogramming of Nrf2 in suppression of tumor promoter TPA-induced mouse skin cell transformation by sulforaphane. Cancer Prev. Res. 2014, 7, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Khor, T.O.; Huang, Y.; Wu, T.Y.; Shu, L.; Lee, J.; Kong, A.N.T. Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem. Pharmacol. 2011, 82, 1073–1078. [Google Scholar] [CrossRef]
- Singh, B.; Shoulson, R.; Chatterjee, A.; Ronghe, A.; Bhat, N.K.; Dim, D.C.; Bhat, H.K. Resveratrol inhibits estrogen-induced breast carcinogenesis through induction of NRF2-mediated protective pathways. Carcinogenesis 2014, 35, 1872–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Khor, T.O.; Shu, L.; Saw, C.L.L.; Wu, T.Y.; Suh, N.; Kong, A.N.T. A γ-tocopherol-rich mixture of tocopherols maintains Nrf2 expression in prostate tumors of TRAMP mice via epigenetic inhibition of CpG methylation. J. Nutr. 2012, 142, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Z.Y.; Khor, T.O.; Shu, L.; Lee, J.H.; Saw, C.L.L.; Wu, T.Y.; Kong, A.N.T. Epigenetic reactivation of Nrf2 in murine prostate cancer TRAMP C1 cells by natural phytochemicals Z-ligustilide and Radix angelica sinensis via promoter CpG demethylation. Chem. Res. Toxicol. 2013, 26, 477–485. [Google Scholar] [CrossRef]
- Wang, S.W.; Sheng, H.; Bai, Y.F.; Weng, Y.Y.; Fan, X.Y.; Zheng, F.; Zhang, F. Inhibition of histone acetyltransferase by naringenin and hesperetin suppresses Txnip expression and protects pancreatic β cells in diabetic mice. Phytomedicine 2021, 88, 153454. [Google Scholar] [CrossRef]
- Qian, Y.Y.; Liu, Z.S.; Yan, H.J.; Yuan, Y.F.; Levenson, A.S.; Li, K. Pterostilbene inhibits MTA1/HDAC1 complex leading to PTEN acetylation in hepatocellular carcinoma. Biomed. Pharmacother. 2018, 101, 852–859. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.H.; Chung, M.Y.; Lee, J.H.; Nam, T.G.; Park, J.H.; Choi, H.K. 3, 4-dihydroxytoluene, a metabolite of rutin, suppresses the progression of nonalcoholic fatty liver disease in mice by inhibiting p300 histone acetyltransferase activity. Acta Pharmacol. Sin. 2020, 42, 1449–1460. [Google Scholar] [CrossRef]
- Kim, T.W. Cinnamaldehyde induces autophagy-mediated cell death through ER stress and epigenetic modification in gastric cancer cells. Acta Pharmacol. Sin. 2021, 1–12. [Google Scholar] [CrossRef]
- Liu, X.; Song, Z.; Bai, J.; Nauwynck, H.; Zhao, Y.; Jiang, P. Xanthohumol inhibits PRRSV proliferation and alleviates oxidative stress induced by PRRSV via the Nrf2–HMOX1 axis. Vet. Res. 2019, 50, 61. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Yuan, H.; Luo, Y.; Zhao, Y.J.; Xiao, J.H. Ganoderic acid D protects human amniotic mesenchymal stem cells against oxidative stress-induced senescence through the PERK/NRF2 signaling pathway. Oxid. Med. Cell. Longev. 2020, 2020, 8291413. [Google Scholar] [CrossRef]
- Li, W.; Suwanwela, N.C.; Patumraj, S. Curcumin by down-regulating NF-kB and elevating Nrf2, reduces brain edema and neurological dysfunction after cerebral I/R. Microvasc. Res. 2016, 106, 117–127. [Google Scholar] [CrossRef]
- Karthi, N.; Karthiga, A.; Kalaiyarasu, T.; Stalin, A.; Manju, V.; Singh, S.K.; Lee, S.M. Exploration of cell cycle regulation and modulation of the DNA methylation mechanism of pelargonidin: Insights from the molecular modeling approach. Comput. Biol. Chem. 2017, 70, 175–185. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, R.; Xiao, X.; Yang, C.; Yang, Y.; Wang, C.; Kong, A.N. The dietary flavone luteolin epigenetically activates the Nrf2 pathway and blocks cell transformation in human colorectal cancer HCT116 cells. J. Cell. Biochem. 2018, 119, 9573–9582. [Google Scholar] [CrossRef]
- Yang, M.; Jiang, Z.H.; Li, C.G.; Zhu, Y.J.; Li, Z.; Tang, Y.Z.; Ni, C.L. Apigenin prevents metabolic syndrome in high-fructose diet-fed mice by Keap1-Nrf2 pathway. Biomed. Pharmacother. 2018, 105, 1283–1290. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Wu, R.; Su, S.; Zheng, M.; Kong, A.N. Triterpenoid corosolic acid modulates global CpG methylation and transcriptome of tumor promotor TPA induced mouse epidermal JB6 P+ cells. Chem.-Biol. Interact. 2020, 321, 109025. [Google Scholar] [CrossRef]




| S.No | Phytochemicals | Molecular Target | Cell/Animal Model | Function | Refs |
|---|---|---|---|---|---|
| 1. | Luteolin | p62/KEAP1/NRF2 | Adult male Sprague−Dawley rats | Neuroprotection | [120] |
| 2. | Fucoxanthin | NRF2 signaling pathway | Skin JB6 P+ cells | Anticancer effect | [76] |
| 3. | Corosolic acid | Nrf2 expression | Swiss albino mice | Antidiabetic | [121] |
| 4. | Reserpine | Epigenetic modulation of Nrf2 expression | Skin epidermal JB6 P+ cells | Anticancer | [122] |
| 5. | Taxifolin | NRF2 signaling pathway | Male Swiss Albino Mice | Antioxidant and anti-inflammatory | [123] |
| 6. | Ganoderic acid | Nrf2 expression | Lung cancer H460 cells | Anticancer | [124] |
| 7. | Celastrol | Nrf2 expression | Male Wistar albino rat | Antifibrotic | [125] |
| 8. | Polydatin | miR-200a to control KEAP1/NRF2 pathway b | BRL-3A cells | anti-inflammatory and antihyperlipidemic | [126] |
| 9. | Pelargonidin | NRF2 promoter demethylation | Skin epidermal JB6 P+ cells. | Anticancer | [127] |
| 10. | Delphinidin | Epigenetic reactivation of NRF2 | Skin epidermal JB6 P+ cells. | Anticancer | [128] |
| S. No | Compounds | Activator/Inhibitor | Cell/Animal Model/ | Mechanism | Refs |
|---|---|---|---|---|---|
| 1. | Ursodiol | FDA approved drug acting as NRF2 activator | KEAP1- knockdown mice, Nrf2 gene-null mice | Activate NRF2; induces of Mrp family members in livers, stimulates detoxification and antioxidative stress systems | [129] |
| 2. | Dimeric acid | NRF2 activator | Balb/C mice | Activates NRF2, increases hepatic glyoxalase and glutathione, reduces serum and hepatic AGE levels and suppresses inflammatory in MG induced diabetic mice | [130] |
| 3. | Songorine | NRF2 activator | C57BL/6 mice | Activates NRF2/ARE signaling cascades to rescue cardiomyocytes from endotoxin insult and prevents septic heart injury | [131] |
| 4. | Procyanidins | NRF2 inhibitor | A549 cells | Promotes proteasome-independent degradation of nuclear NRF2 via phosphorylating IGF-1 receptor and activating cysteine proteases | [132] |
| 5. | Compound KI-696 | KEAP1 Kelch–NRF2 interactions inhibitor | NHBE cells, Bronchial epithelial cells from human COPD patient lung | Nrf2-regulated genes are expressed in COPD patient-generated bronchial epithelial cells | [133] |
| 6. | Cyclic peptide head to tail | KEAP1–NRF2-specific protein inhibitor | Mouse RAW 264.7 cells; HepG2-ARE-C8 cells. | Upregulates NRF2-dependent antioxidant proteins and enzymesenhance the antioxidant capacity and inhibit inflammation factors in LPS-induced macrophage RAW 264.7 cells | [134] |
| 7. | Coumarins | Inhibits KEAP1/NRF2 protein–protein interactions | Molecular docking simulation studies | Binds with Keap and activate NRF2 signaling | [135] |
| 8. | Napyradiomycin (Compound 1) | potent NRF2 activator | BV-2 microglial cells | Exhibits antioxidant and anti-inflammatory effects | [136] |
| 9. | ML385 Small molecule inhibitor | NRF2 inhibitor | Tumor xenograft mice | Improves chemotherapeutic efficacy by interacting with NRF2 and inhibiting its transcriptional activity | [137] |
| 10. | Trigonelline | NRF2 inhibitor | Head and neck cancer cells (HN3R) | Inhibition of the NRF2-ARE mechanism reverses ferroptosis resistance in HNSCC cells | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. https://doi.org/10.3390/antiox10121859
Thiruvengadam M, Venkidasamy B, Subramanian U, Samynathan R, Ali Shariati M, Rebezov M, Girish S, Thangavel S, Dhanapal AR, Fedoseeva N, et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants. 2021; 10(12):1859. https://doi.org/10.3390/antiox10121859
Chicago/Turabian StyleThiruvengadam, Muthu, Baskar Venkidasamy, Umadevi Subramanian, Ramkumar Samynathan, Mohammad Ali Shariati, Maksim Rebezov, Shabari Girish, Sivakumar Thangavel, Anand Raj Dhanapal, Natalya Fedoseeva, and et al. 2021. "Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation" Antioxidants 10, no. 12: 1859. https://doi.org/10.3390/antiox10121859
APA StyleThiruvengadam, M., Venkidasamy, B., Subramanian, U., Samynathan, R., Ali Shariati, M., Rebezov, M., Girish, S., Thangavel, S., Dhanapal, A. R., Fedoseeva, N., Lee, J., & Chung, I.-M. (2021). Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants, 10(12), 1859. https://doi.org/10.3390/antiox10121859









