Plant Extract of Limonium gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion
Abstract
:1. Introduction
2. Materials and Methods
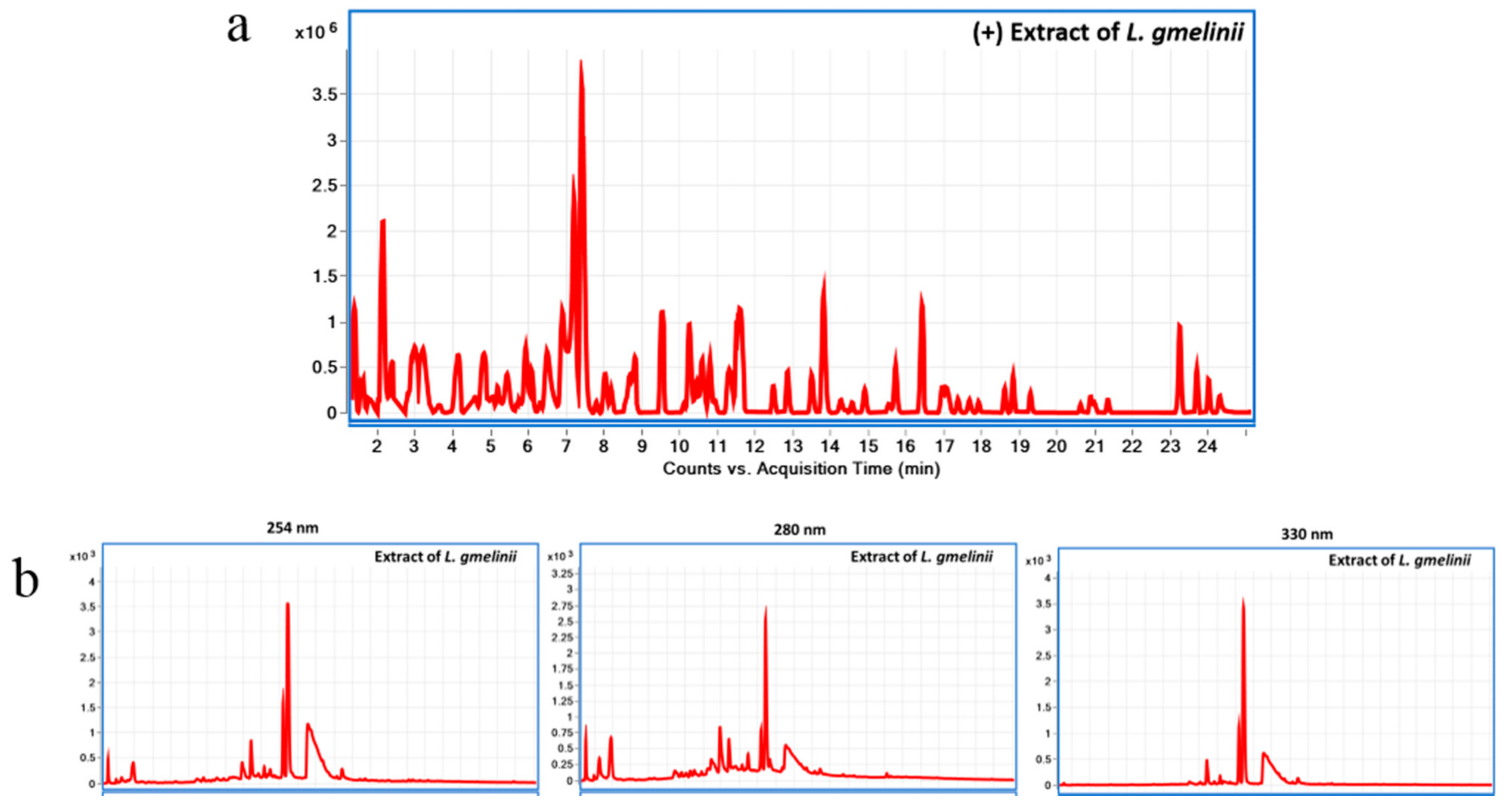
2.1. Preparation and Chemical Characterization of L. gmelinii Roots Extract Using Liquid Chromatography Diode Array Detector-Quadrupole Time-of-Flight Mass Spectrometry (LC-DAD-QToF)
2.2. Cell Culture
2.3. Fluorescent Intracellular ROS Assay
2.4. Activation of NADP Oxidase Assay
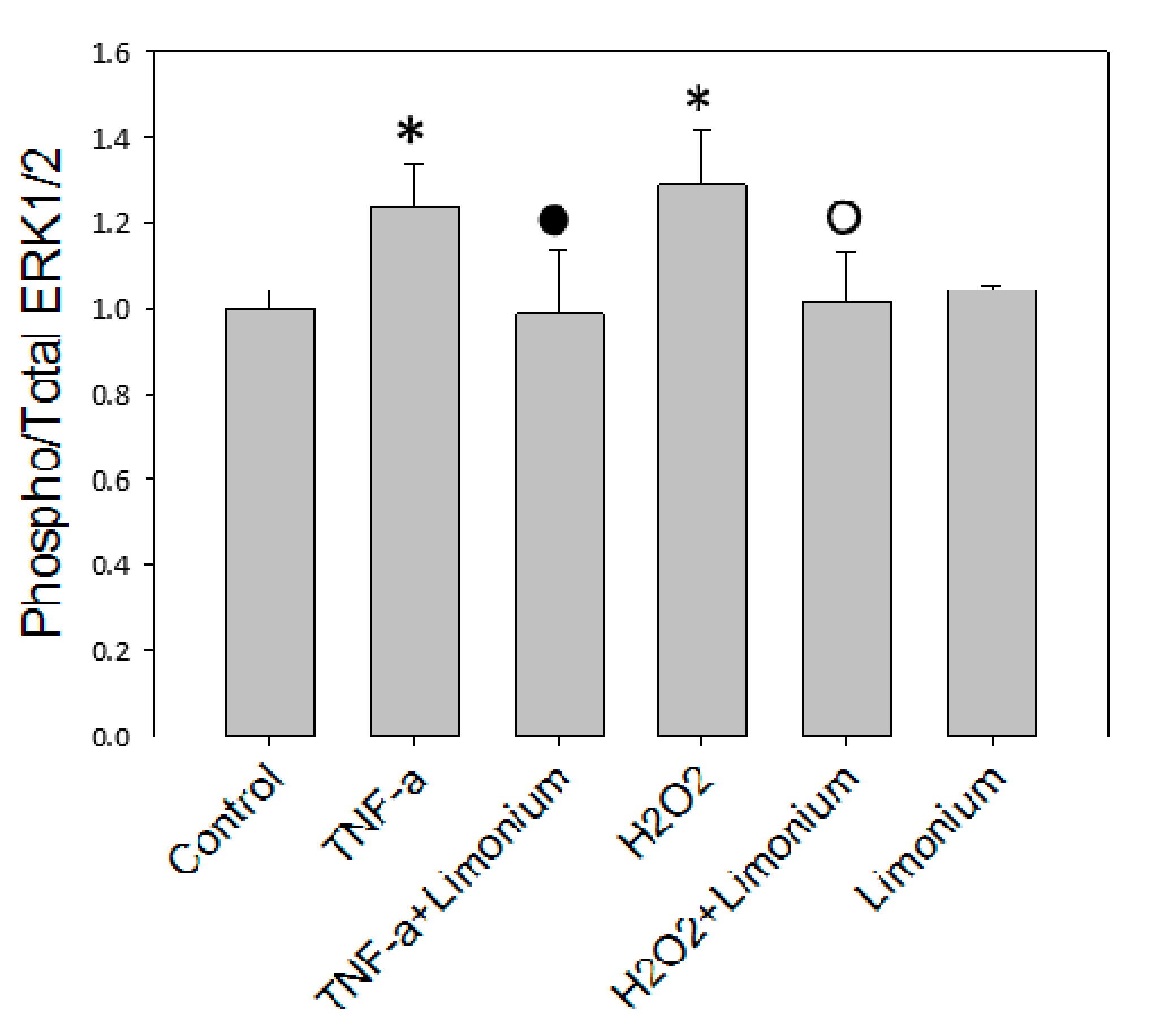
2.5. ERK 1/2 Kinases Assay
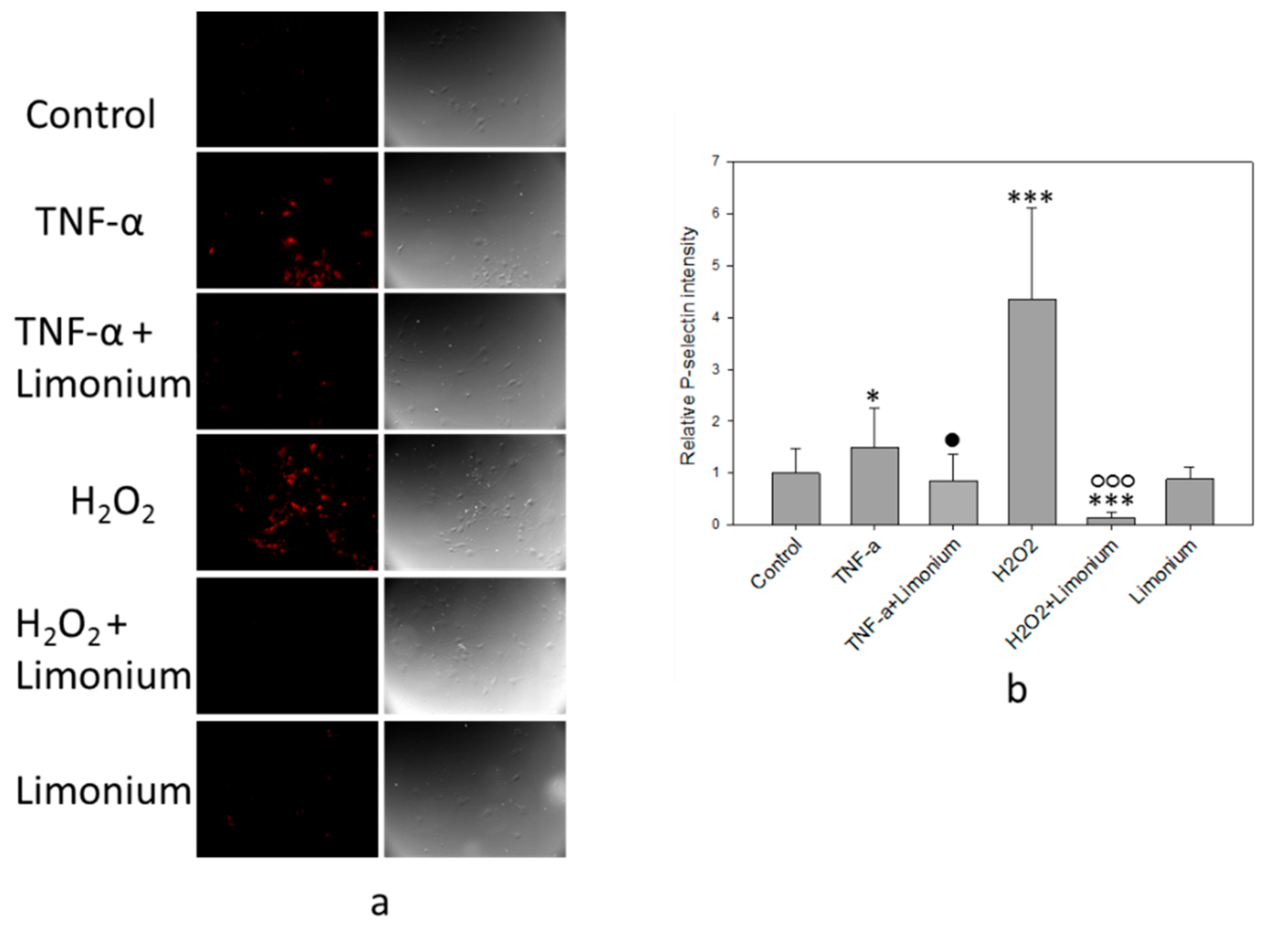
2.6. Immunofluorescent Assay of P-Selectin Expression on the bEnd3 Cells Surface
2.7. In Vivo Study
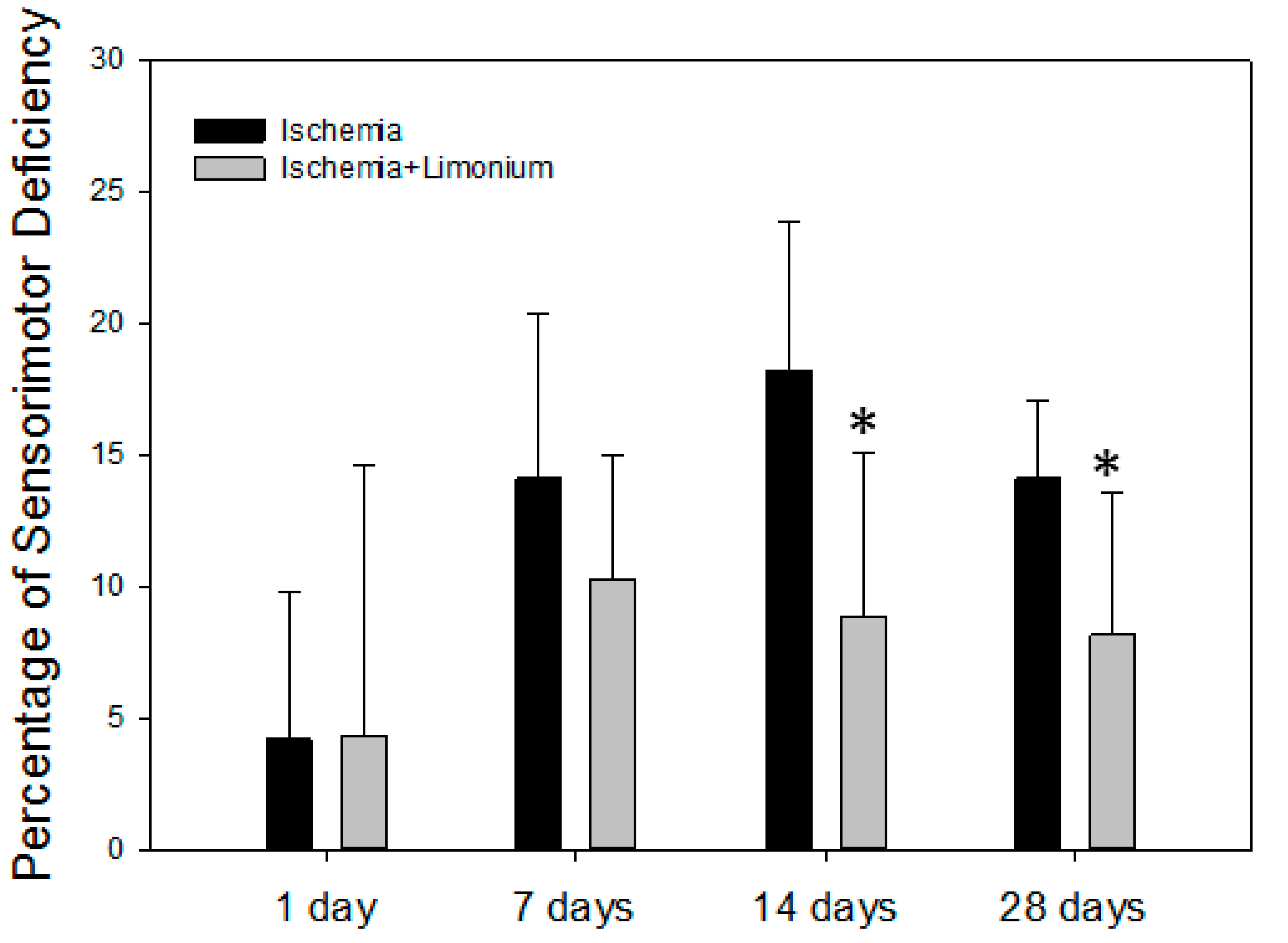
2.8. Evaluation of Sensorimotor Activity in Laboratory Animals
2.9. Statistical Analysis
3. Results
3.1. Chemical Characterization of the Plant Substance
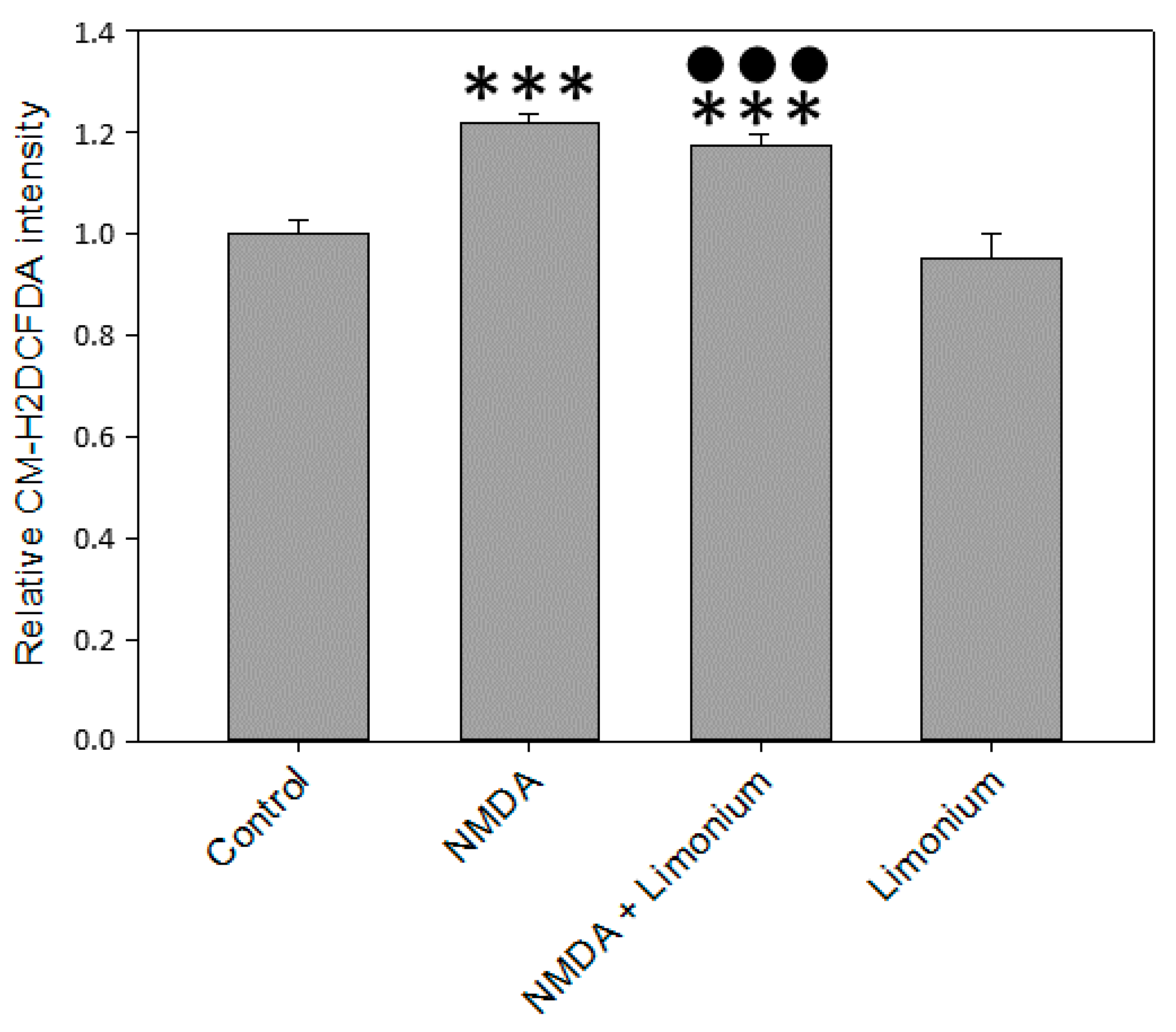
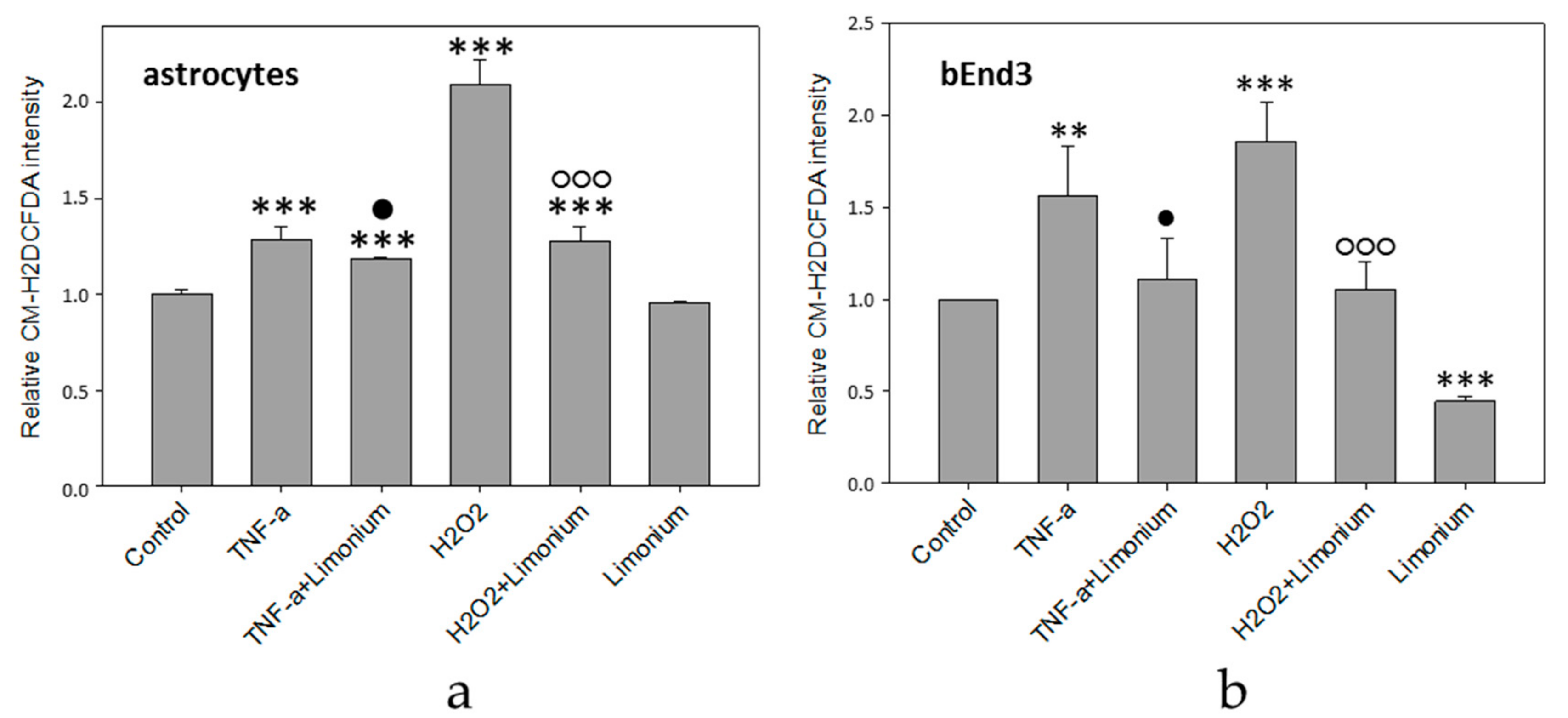
3.2. L. gmelinii Extract Attenuated NMDA-, H2O2, and TNF-α Induced Oxidative Response in Neurons, Astrocytes, and CECs
3.3. Effect of L. gmelinii Extract on the Activity of NADPH Oxidase in Human Primary Astrocytes
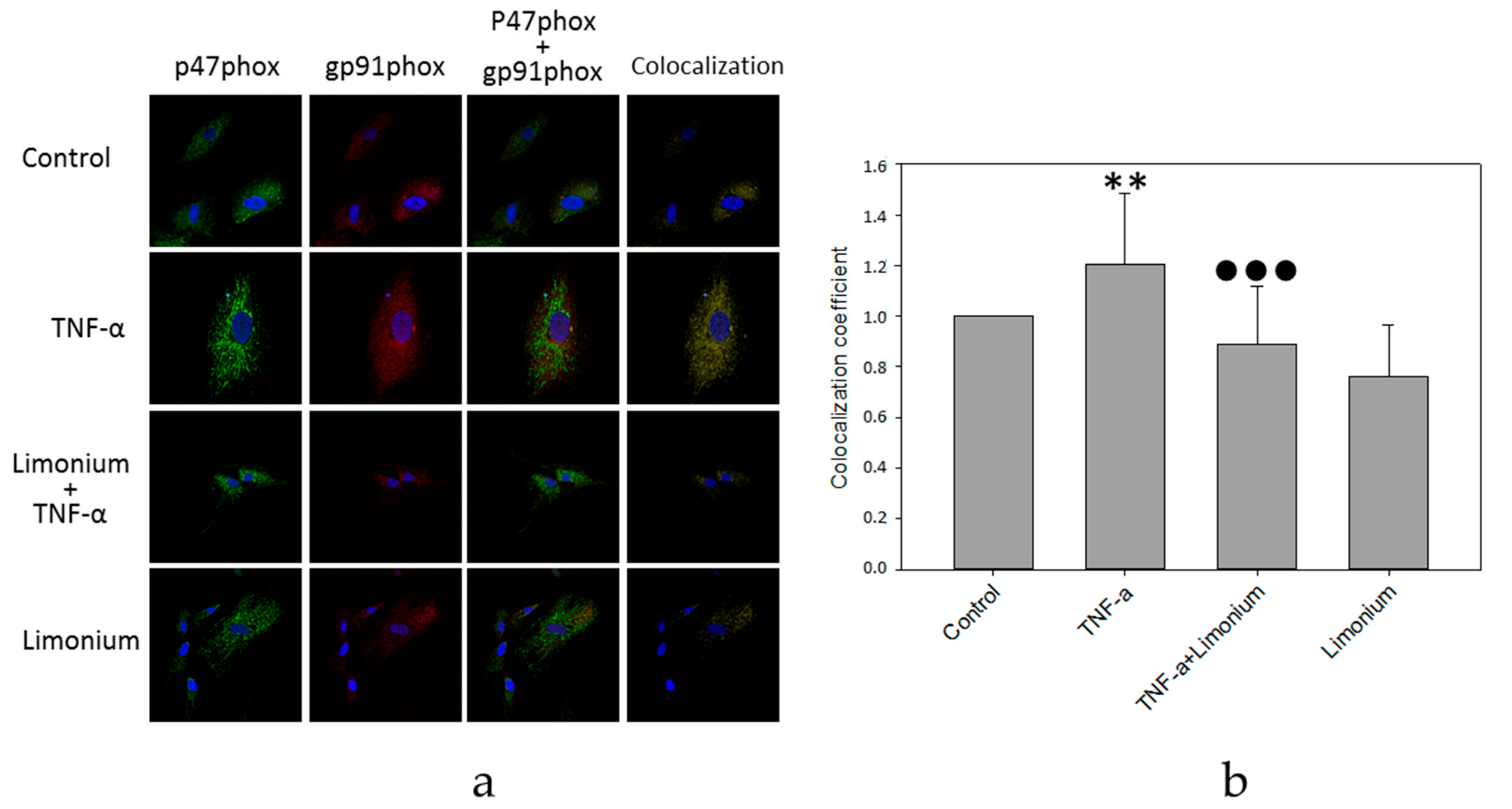
3.4. Pretreatment with L. gmelinii Extract Suppressed Induced by TNF-α Pro-Inflammatory Responses in Astrocytes and CECs
3.5. L. gmelinii Extract Improved Motor Activity in Rats with MCAO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.; Moreira, P.I. Oxidative Stress: A Major Player in Cerebrovascular Alterations Associated to Neurodegenerative Events. Front. Physiol. 2018, 9, 806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yoshioka, H.; Kim, G.S.; Jung, J.E.; Okami, N.; Sakata, H.; Maier, C.M.; Narasimhan, P.; Goeders, C.E.; Chan, P.H. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox. Signal 2011, 14, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Simonyi, A.; Wang, Q.; Miller, R.L.; Yusof, M.; Shelat, P.B.; Sun, A.Y.; Sun, G.Y. Polyphenols in cerebral ischemia: Novel targets for neuroprotection. Mol. Neurobiol. 2005, 31, 135–147. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Chan, M.H.; Zong, Y.; Sheng, W.; He, Y.; Jiang, J.H.; Simonyi, A.; Gu, Z.; Fritsche, K.L.; Cui, J.; et al. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J. Neuroinflammation 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Z.; Wang, P.; Yu, B.; Liu, Y.; Xue, Y. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement. Altern. Med. 2013, 13, 187. [Google Scholar] [CrossRef] [Green Version]
- Panickar, K.S.; Jang, S. Dietary and plant polyphenols exert neuroprotective effects and improve cognitive function in cerebral ischemia. Recent Pat. Food Nutr. Agric. 2013, 5, 128–143. [Google Scholar] [CrossRef]
- Abib, R.T.; Quincozes-Santos, A.; Nardin, P.; Wofchuk, S.T.; Perry, M.L.; Goncalves, C.A.; Gottfried, C. Epicatechin gallate increases glutamate uptake and S100B secretion in C6 cell lineage. Mol. Cell Biochem. 2008, 310, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Mahler, A.; Mandel, S.; Lorenz, M.; Ruegg, U.; Wanker, E.E.; Boschmann, M.; Paul, F. Epigallocatechin-3-gallate: A useful, effective and safe clinical approach for targeted prevention and individualised treatment of neurological diseases? EPMA J. 2013, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Shalakhmetova, T.; Zhusupova, G.; Askarova, S. Antioxidative and hepatoprotective properties of phyto medicine extracted from Limonium gmelinii. Int. J. Biol. Chem. 2010, 1, 61–66. [Google Scholar]
- Maryam, M.; Lack, H.W.; Maria, L.; Hossein, A. The discovery, naming and typification of Limonium gmelini (Plumbaginaceae). Willdenowia 2017, 47, 99–106. [Google Scholar]
- Askarova, S.; Sun, Z.; Sun, G.Y.; Meininger, G.A.; Lee, J.C. Amyloid-β peptide on sialyl-Lewis(X)-selectin-mediated membrane tether mechanics at the cerebral endothelial cell surface. PLoS ONE 2013, 8, e60972. [Google Scholar] [CrossRef] [Green Version]
- Abduakhitovich, A.Z.; Dyusembaevich, R.K.; Eventaevna, Z.G. Method for obtaining a total polyphenolic complex from the roots of Limonium gmelinii. RK Patent No. 14418 from 15.10.2007. bul. No. 10 (rus.). Available online: https://kzpatents.com/5-14418-sposob-polucheniya-summarnogo-polifenolnogo-kompleksa-iz-kornejj-kermeka-gmelina.html (accessed on 6 October 2021).
- Samuelson, D.J.; Powell, M.B.; Lluria-Prevatt, M.; Romagnolo, D.F. Transcriptional activation of the gp91phox NADPH oxidase subunit by TPA in HL-60 cells. J. Leukoc. Biol. 2001, 69, 161–168. [Google Scholar]
- DeLeo, F.R.; Allen, L.A.H.; Apicella, M.; Nauseef, W.M. NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 1999, 163, 6732–6740. [Google Scholar]
- Yang, X.G.; Askarova, S.; Sheng, W.W.; Chen, J.K.; Sun, A.Y.; Sun, G.Y.; Yao, G.; Lee, J.C.M. Low Energy Laser Light (632.8 nm) Suppresses Amyloid-Beta Peptide-Induced Oxidative and Inflammatory Responses in Astrocytes. Biophys. J. 2011, 100, 624. [Google Scholar] [CrossRef] [Green Version]
- Uluç, K.; Miranpuri, A.; Kujoth, G.C.; Aktüre, E.; Başkaya, M.K. Focal cerebral ischemia model by endovascular suture occlusion of the middle cerebral artery in the rat. J. Vis. Exp. 2011, e1978. [Google Scholar] [CrossRef] [Green Version]
- Zhusupova, G.E.; Abil’kaeva, S.A. Dimeric prodelphinidins from Limonium gmelinii roots. III. Chem. Nat. Compd. 2006, 42, 164–168. [Google Scholar] [CrossRef]
- Shelat, P.; Chalimoniuk, M.; Wang, J.-H.; Strosznajder, J.; Lee, J.; Simonyi, A.; Sun, G. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Askarova, S.; Yang, X.; Sheng, W.; Sun, G.Y.; Lee, J.C.M. Role of Aβ-receptor for advanced glycation endproducts interaction in oxidative stress and cytosolic phospholipase A₂ activation in astrocytes and cerebral endothelial cells. Neuroscience 2011, 199, 375–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoy, A.; Saliev, T.; Abzhanova, E.; Turgambayeva, A.; Kaiyrlykyzy, A.; Akishev, M.; Saparbayev, S.; Umbayev, B.; Askarova, S. The Effects of Mobile Phone Radiofrequency Electromagnetic Fields on β-Amyloid-Induced Oxidative Stress in Human and Rat Primary Astrocytes. Neuroscience 2019, 408, 46–57. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH Oxidase: An Update. Blood 1999, 93, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Jiu-Hong, K.; Rong-Liang, Z. NADPH oxidase produces reactive oxygen species and maintains survival of rat astrocytes. Cell Biochem. Funct. 2005, 23, 93–100. [Google Scholar]
- Zhu, D.; Hu, C.; Sheng, W.; Tan, K.S.; Haidekker, M.A.; Sun, A.Y.; Sun, G.Y.; Lee, J.C.-M. NAD(P)H oxidase-mediated reactive oxygen species production alters astrocyte membrane molecular order via phospholipase A2. Biochem J. 2009, 421, 201–210. [Google Scholar] [CrossRef]
- Sheng, W.S.; Hu, S.; Feng, A.; Rock, R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013, 38, 2148–2159. [Google Scholar] [CrossRef] [Green Version]
- Tsoy, A.; Shalakhmetova, T.; Umbayev, B.; Askarova, S. Role of ros in aβ42 mediated cell surface p-selectin expression and actin polymerization. Neurol. Asia 2014, 19, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complementary Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A 2016, 33, 1761–1774. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Mansuri, M.L.; Parihar, P.; Solanki, I.; Parihar, M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014, 9, 400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lee, H.-G.; Raina, A.K.; Perry, G.; Smith, M.A. The Role of Mitogen-Activated Protein Kinase Pathways in Alzheimer’s Disease. Neurosignals 2002, 11, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Kim, S.; Chung, H.T.; Pae, H.O. Reactive oxygen species in the activation of MAP kinases. Methods Enzym. 2013, 528, 27–48. [Google Scholar]
- Salman, M.M.; Kitchen, P.; Woodroofe, M.N.; Bill, R.M.; Conner, A.C.; Heath, P.R.; Conner, M.T. Transcriptome Analysis of Gene Expression Provides New Insights into the Effect of Mild Therapeutic Hypothermia on Primary Human Cortical Astrocytes Cultured under Hypoxia. Front. Cell Neurosci. 2017, 11, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salman, M.M.; Sheilabi, M.A.; Bhattacharyya, D.; Kitchen, P.; Conner, A.C.; Bill, R.M.; Woodroofe, M.N.; Conner, M.T.; Princivalle, A.P. Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur. J. Neurosci. 2017, 46, 2121–2132. [Google Scholar] [CrossRef]
- Keshari, R.S.; Verma, A.; Barthwal, M.K.; Dikshit, M. Reactive oxygen species-induced activation of ERK and p38 MAPK mediates PMA-induced NETs release from human neutrophils. J. Cell Biochem. 2013, 114, 532–540. [Google Scholar] [CrossRef]
- McEver, R.P.; Zhu, C. Rolling cell adhesion. Annu. Rev. Cell Dev. Biol. 2010, 26, 363–396. [Google Scholar] [CrossRef]
- Liu, Y.J.; Guo, D.W.; Tian, L.; Shang, D.S.; Zhao, W.D.; Li, B.; Fang, W.G.; Zhu, L.; Chen, Y.H. Peripheral T cells derived from Alzheimer’s disease patients overexpress CXCR2 contributing to its transendothelial migration, which is microglial TNF-alpha-dependent. Neurobiol. Aging 2010, 31, 175–188. [Google Scholar] [CrossRef]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Frijns, C.J.M.; Kappelle, L.J. Inflammatory Cell Adhesion Molecules in Ischemic Cerebrovascular Disease. Stroke 2002, 33, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Savman, K.; Heyes, M.P.; Svedin, P.; Karlsson, A. Microglia/macrophage-derived inflammatory mediators galectin-3 and quinolinic acid are elevated in cerebrospinal fluid from newborn infants after birth asphyxia. Transl. Stroke Res. 2013, 4, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799.e719. [Google Scholar] [CrossRef] [PubMed]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Tornroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2021, awab311. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Iliff, J.J.; Bill, R.M. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat. Rev. Neurosci. 2021, 22, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, D.; Zambonin, L.; Vieceli Dalla Sega, F.; Hrelia, S. Polyphenols as Modulators of Aquaporin Family in Health and Disease. Oxidative Med. Cell. Longev. 2015, 2015, 196914. [Google Scholar] [CrossRef] [Green Version]
- Tesse, A.; Grossini, E.; Tamma, G.; Brenner, C.; Portincasa, P.; Marinelli, R.A.; Calamita, G. Aquaporins as Targets of Dietary Bioactive Phytocompounds. Front. Mol. Biosci. 2018, 5, 30. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurkenov, T.; Tsoy, A.; Olzhayev, F.; Abzhanova, E.; Turgambayeva, A.; Zhussupova, A.; Avula, B.; Ross, S.; Aituarova, A.; Kassymova, D.; et al. Plant Extract of Limonium gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion. Antioxidants 2021, 10, 1814. https://doi.org/10.3390/antiox10111814
Nurkenov T, Tsoy A, Olzhayev F, Abzhanova E, Turgambayeva A, Zhussupova A, Avula B, Ross S, Aituarova A, Kassymova D, et al. Plant Extract of Limonium gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion. Antioxidants. 2021; 10(11):1814. https://doi.org/10.3390/antiox10111814
Chicago/Turabian StyleNurkenov, Tulendy, Andrey Tsoy, Farkhad Olzhayev, Elvira Abzhanova, Anel Turgambayeva, Aizhan Zhussupova, Bharathi Avula, Samir Ross, Aigerim Aituarova, Dariya Kassymova, and et al. 2021. "Plant Extract of Limonium gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion" Antioxidants 10, no. 11: 1814. https://doi.org/10.3390/antiox10111814
APA StyleNurkenov, T., Tsoy, A., Olzhayev, F., Abzhanova, E., Turgambayeva, A., Zhussupova, A., Avula, B., Ross, S., Aituarova, A., Kassymova, D., Zhusupova, G., Shalakhmetova, T., Tokay, T., Lee, J. C., & Askarova, S. (2021). Plant Extract of Limonium gmelinii Attenuates Oxidative Responses in Neurons, Astrocytes, and Cerebral Endothelial Cells In Vitro and Improves Motor Functions of Rats after Middle Cerebral Artery Occlusion. Antioxidants, 10(11), 1814. https://doi.org/10.3390/antiox10111814






