Influence of N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Lines
2.3. iPSC Culturing and Obtaining Differentiated Neuronal Cell Cultures Enriched by DA Neurons
2.3.1. Immunofluorescence Staining
2.3.2. Estimation of the Neuroprotective Properties of N-ADA and N-DDA in Differentiated Neuronal Cell Cultures Enriched by DA Neurons under Conditions of OS
2.3.3. MTT Assay
2.4. RNA Isolation and qRT-PCR
2.5. Measurement of BDNF, proBDNF and GDNF Proteins with ELISA
3. Results
3.1. Analysis of Transcription Levels of Neurotrophic Factors and Their Receptors in Differentiated Neuronal Cultures
3.2. Analysis of BDNF, proBDNF and GDNF Protein Levels in Differentiated Neuronal Cultures by ELISA
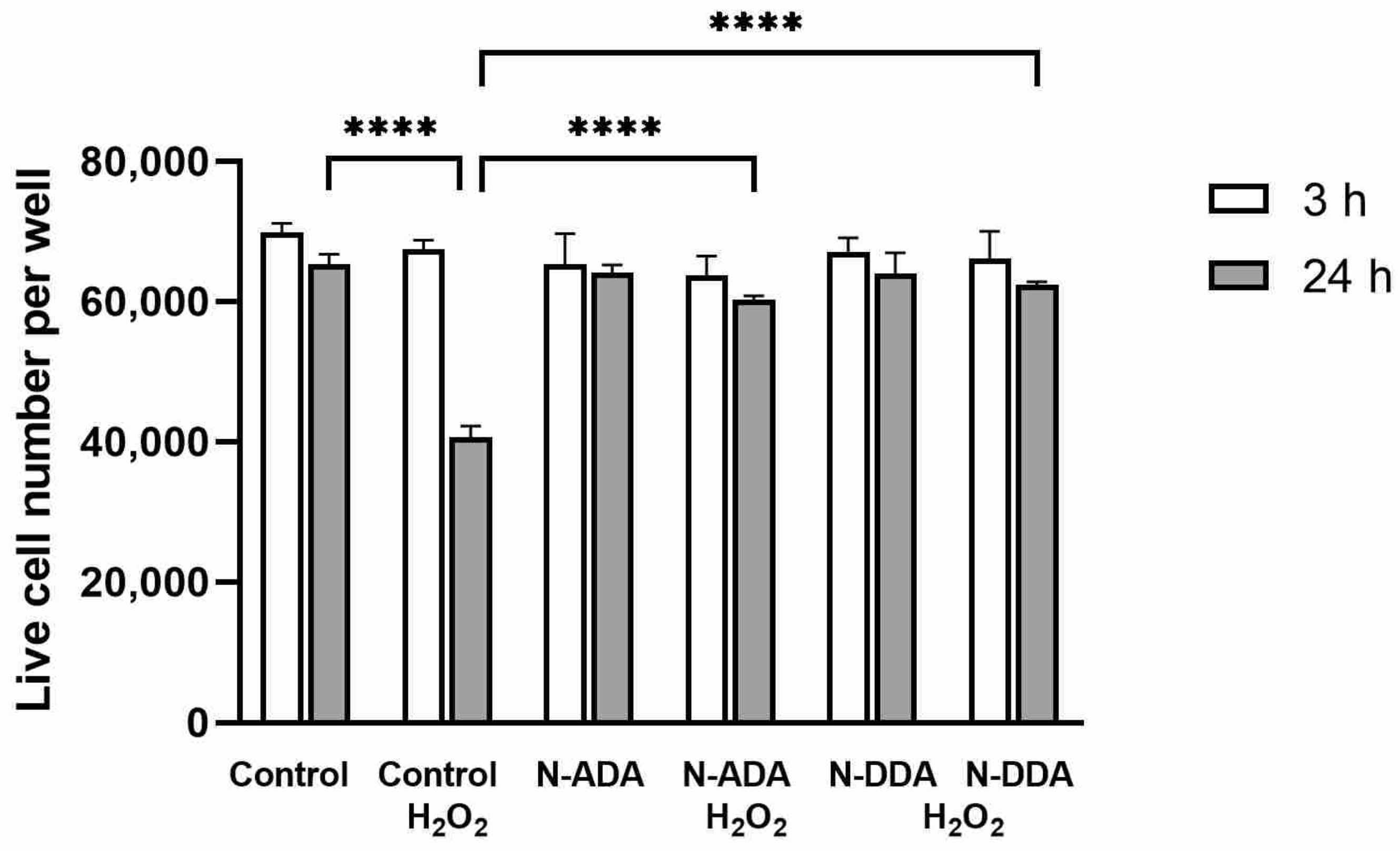
3.3. Assessment of the Effect of N-acyl Dopamines on the Survival of Differentiated Neurons under OS Conditions
3.4. Analysis of BAX and BCL2 Expression Changes in Differentiated Neuronal Cultures under OS Conditions and Application of N-acyl Dopamines
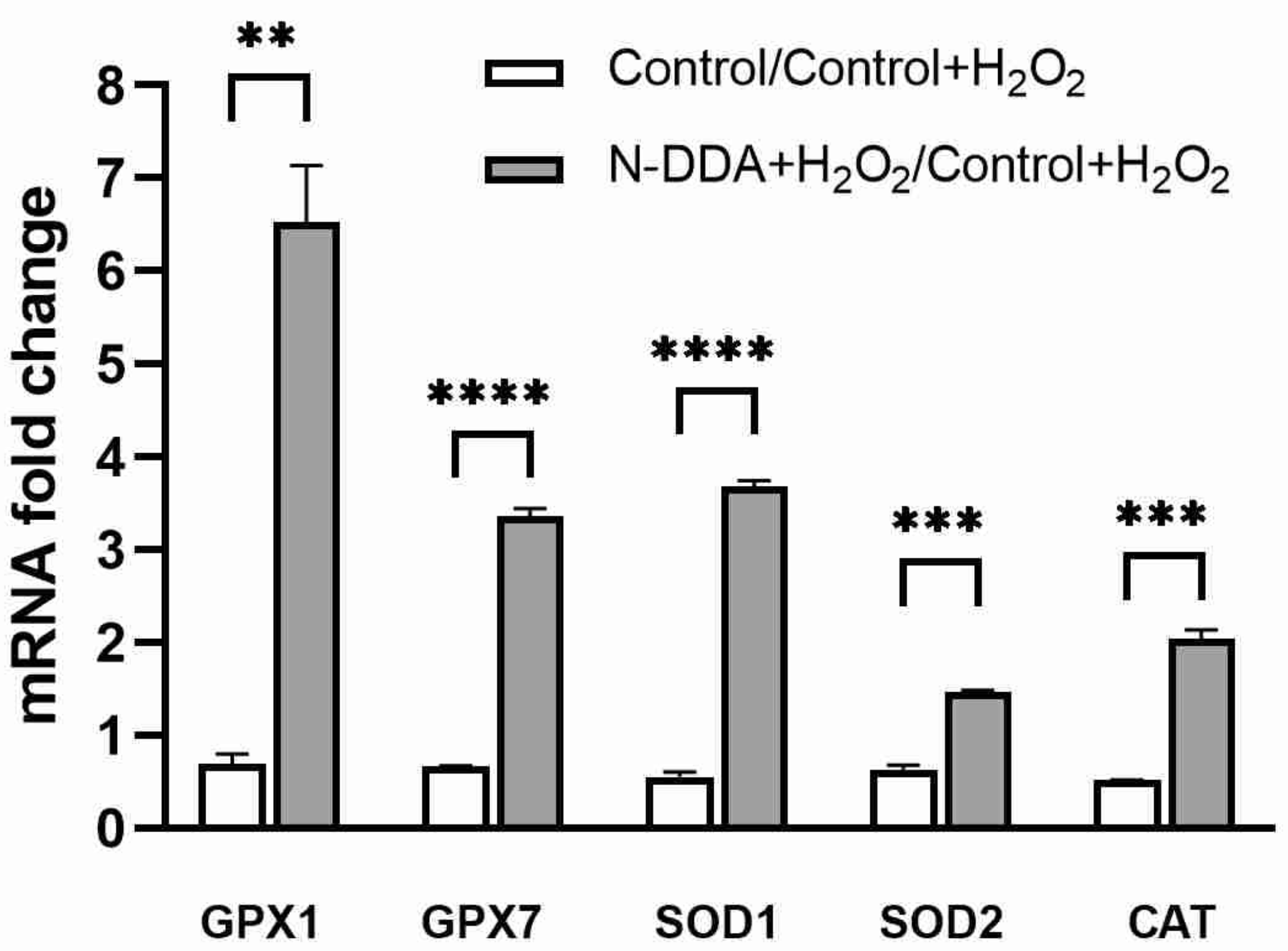
3.5. Analysis of Changes in the Expression of Several Antioxidant Cell Defense Genes in Differentiated Neuronal Cultures upon N-DDA Application under OS Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grabiec, U.; Dehghani, F. N-Arachidonoyl Dopamine: A Novel Endocannabinoid and Endovanilloid with Widespread Physiological and Pharmacological Activities. Cannabis Cannabinoid Res. 2017, 2, 183–196. [Google Scholar] [CrossRef]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef]
- Akimov, M.; Bezuglov, V. N-Acylated dopamine. A new life for the old dopamine. In Dopamine: Functions, Regulation and Health Effects; Nova Science Publishers: New York, NY, USA, 2012; pp. 49–80. [Google Scholar]
- Bobrov, M.Y.; Lizhin, A.A.; Andrianova, E.L.; Gretskaya, N.M.; Frumkina, L.E.; Khaspekov, L.G.; Bezuglov, V.V. Antioxidant and neuroprotective properties of N-arachidonoyldopamine. Neurosci. Lett. 2008, 431, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, M.Y.; Lyzhin, A.A.; Andrianova, E.L.; Gretskaya, N.M.; Zinchenko, G.N.; Frumkina, L.E.; Khaspekov, L.G.; Bezuglov, V.V. Antioxidant and neuroprotective properties of N-docosahexaenoyl dopamine. Bull. Exp. Biol. Med. 2006, 142, 425–427. [Google Scholar] [CrossRef]
- Grabiec, U.; Koch, M.; Kallendrusch, S.; Kraft, R.; Hill, K.; Merkwitz, C.; Ghadban, C.; Lutz, B.; Straiker, A.; Dehghani, F. The endocannabinoid N-arachidonoyldopamine (NADA) exerts neuroprotective effects after excitotoxic neuronal damage via cannabinoid receptor 1 (CB(1)). Neuropharmacology 2012, 62, 1797–1807. [Google Scholar] [CrossRef]
- Vedunova, M.V.; Mitroshina, E.V.; Sakharnova, T.A.; Bobrov, M.Y.; Bezuglov, V.V.; Khaspekov, L.G.; Mukhina, I.V. Effect of N-arachidonoyl dopamine on activity of neuronal network in primary hippocampus culture upon hypoxia modelling. Bull. Exp. Biol. Med. 2014, 156, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Sukhanova, I.A.; Sebentsova, E.A.; Khukhareva, D.D.; Vysokikh, M.Y.; Bezuglov, V.V.; Bobrov, M.Y.; Levitskaya, N.G. Early-life N-arachidonoyl-dopamine exposure increases antioxidant capacity of the brain tissues and reduces functional deficits after neonatal hypoxia in rats. Int. J. Dev. Neurosci. 2019, 78, 7–18. [Google Scholar] [CrossRef]
- Soler-Torronteras, R.; Lara-Chica, M.; García, V.; Calzado, M.A.; Muñoz, E. Hypoximimetic activity of N-acyl-dopamines. N-arachidonoyl-dopamine stabilizes HIF-1α protein through a SIAH2-dependent pathway. Biochim. Biophys. Acta 2014, 1843, 2730–2743. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, E.V.; Arsenyeva, E.L.; Manuilova, E.S.; Khaspekov, L.G.; Bobrov, M.Y.; Bezuglov, V.V.; Illarioshkin, S.N.; Grivennikov, I.A. Neuroprotective Properties of Endocannabinoids N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine Examined in Neuronal Precursors Derived from Human Pluripotent Stem Cells. Biochemistry 2017, 82, 1367–1372. [Google Scholar] [CrossRef]
- Novosadova, E.; Antonov, S.; Arsenyeva, E.; Kobylanskiy, A.; Vanyushina, Y.; Malova, T.; Khaspekov, L.; Bobrov, M.; Bezuglov, V.; Tarantul, V.; et al. Neuroprotective and neurotoxic effects of endocannabinoid-like compounds, N-arachidonoyl dopamine and N-docosahexaenoyl dopamine in differentiated cultures of induced pluripotent stem cells derived from patients with Parkinson’s disease. Neurotoxicology 2021, 82, 108–118. [Google Scholar] [CrossRef]
- Popova, N.K.; Ilchibaeva, T.V.; Naumenko, V.S. Neurotrophic Factors (BDNF and GDNF) and the Serotonergic System of the Brain. Biochemistry 2017, 82, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. NGF, BDNF, NT3, and NT4. Handb. Exp. Pharmacol. 2014, 220, 3–15. [Google Scholar] [CrossRef]
- Dechant, G.; Barde, Y.A. The neurotrophin receptor p75(NTR): Novel functions and implications for diseases of the nervous system. Nat. Neurosci. 2002, 5, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.Y.; Lee, F.S.; et al. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Eggert, S.; Kins, S.; Endres, K.; Brigadski, T. Brothers in arms: ProBDNF/BDNF and sAPPα/Aβ-signaling and their common interplay with ADAM10, TrkB, p75NTR, sortilin, and sorLA in the progression of Alzheimer’s disease. Biol. Chem. 2022, 403, 43–71. [Google Scholar] [CrossRef]
- Treanor, J.J.; Goodman, L.; de Sauvage, F.; Stone, D.M.; Poulsen, K.T.; Beck, C.D.; Gray, C.; Armanini, M.P.; Pollock, R.A.; Hefti, F.; et al. Characterization of a multicomponent receptor for GDNF. Nature 1996, 382, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Shishkina, T.V.; Vedunova, M.V.; Mishchenko, T.A.; Mukhina, I.V. The Role of Glial Cell Line-Derived Neurotrophic Factor in the Functioning of the Nervous System (Review). Sovrem. Tehnol. Med. 2015, 7, 211–220. [Google Scholar] [CrossRef][Green Version]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549–557. [Google Scholar] [CrossRef]
- Paloczi, J.; Varga, Z.V.; Hasko, G.; Pacher, P. Neuroprotection in Oxidative Stress-Related Neurodegenerative Diseases: Role of Endocannabinoid System Modulation. Antioxid. Redox Signal. 2018, 29, 75–108. [Google Scholar] [CrossRef]
- Numakawa, T.; Matsumoto, T.; Numakawa, Y.; Richards, M.; Yamawaki, S.; Kunugi, H. Protective Action of Neurotrophic Factors and Estrogen against Oxidative Stress-Mediated Neurodegeneration. J. Toxicol. 2011, 2011, 405194. [Google Scholar] [CrossRef]
- Chen, S.D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef]
- Bezuglov, V.; Bobrov, M.; Gretskaya, N.; Gonchar, A.; Zinchenko, G.; Melck, D.; Bisogno, T.; Di Marzo, V.; Kuklev, D.; Rossi, J.C.; et al. Synthesis and biological evaluation of novel amides of polyunsaturated fatty acids with dopamine. Bioorg. Med. Chem. Lett. 2001, 11, 447–449. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Novosadova, E.V.; Nekrasov, E.D.; Chestkov, I.V.; Surdina, A.V.; Vasina, E.M.; Bogomazova, A.N.; Manuilova, E.S.; Arsenyeva, E.L.; Simonova, V.V.; Konovalova, E.V.; et al. A platform for studying molecular and cellular mechanisms of Parkinson s disease based on human induced pluripotent stem cells. Sovrem. Tehnol. Med. 2016, 8, 157–166. [Google Scholar] [CrossRef]
- Pollock, G.S.; Vernon, E.; Forbes, M.E.; Yan, Q.; Ma, Y.T.; Hsieh, T.; Robichon, R.; Frost, D.O.; Johnson, J.E. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J. Neurosci. 2001, 21, 3923–3931. [Google Scholar] [CrossRef]
- Skibinska, M.; Kapelski, P.; Dmitrzak-Weglarz, M.; Lepczynska, N.; Pawlak, J.; Twarowska-Hauser, J.; Szczepankiewicz, A.; Rajewska-Rager, A. Elevated Epidermal Growth Factor (EGF) as Candidate Biomarker of Mood Disorders-Longitudinal Study in Adolescent and Young Adult Patients. J. Clin. Med. 2021, 10, 4064. [Google Scholar] [CrossRef] [PubMed]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem. Toxicol. 1999, 37, 949–962. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Shao, A.; Lin, D.; Wang, L.; Tu, S.; Lenahan, C.; Zhang, J. Oxidative Stress at the Crossroads of Aging, Stroke and Depression. Aging Dis. 2020, 11, 1537–1566. [Google Scholar] [CrossRef]
- Ogura, Y.; Sato, K.; Kawashima, K.-I.; Kobayashi, N.; Imura, S.; Fujino, K.; Kawaguchi, H.; Nedachi, T. Subtoxic levels of hydrogen peroxide induce brain-derived neurotrophic factor expression to protect PC12 cells. BMC Res. Notes 2014, 7, 840. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.P.; Cope, T.C.; Buck, C.R. CNS neurotrophins are biologically active and expressed by multiple cell types. J. Mol. Histol. 2004, 35, 771–783. [Google Scholar] [CrossRef]
- Azevedo, M.D.; Sander, S.; Tenenbaum, L. GDNF, A Neuron-Derived Factor Upregulated in Glial Cells during Disease. J. Clin. Med. 2020, 9, 456. [Google Scholar] [CrossRef]
- Saavedra, A.; Baltazar, G.; Santos, P.; Carvalho, C.M.; Duarte, E.P. Selective injury to dopaminergic neurons up-regulates GDNF in substantia nigra postnatal cell cultures: Role of neuron-glia crosstalk. Neurobiol. Dis. 2006, 23, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Lovell, M.A.; Furukawa, K.; Markesbery, W.R. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995, 65, 1740–1751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fu, Y.S.; Guo, J.W. Ability of GDNF to diminish free radical production leads to protection against kainate-induced excitotoxicity in hippocampus. Hippocampus 2004, 14, 77–86. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef] [PubMed]





| Genes | Time | Control/H2O2 | N-ADA + H2O2/H2O2 | N-DDA + H2O2/H2O2 |
|---|---|---|---|---|
| TRKA | 3 h | 1.24 ± 0.63 | 0.96 ± 0.41 | 1.8 ± 0.80 |
| 24 h | 2.64 ± 0.4 *** | 1.62 ± 0.50 * | 1.69 ± 0.60 * | |
| NGF | 3 h | 1.66 ± 1.20 | 1.23 ± 0.29 | 1.05 ± 0.37 |
| 24 h | 0.12 ± 0.01 * | 0.59 ± 0.07 * | 0.52 ± 0.03 * | |
| TRKB95 | 3 h | 3.38 ± 2.3 | 1.75 ± 0.70 | 4.00 ± 2.2 * |
| 24 h | 1.92 ± 1.30 | 0.28 ± 0.04 ** | 0.85 ± 0.50 | |
| TRKB145 | 3 h | 1.09 ± 0.36 | 1.14 ± 0.46 | 1.62 ± 0.29 * |
| 24 h | 1.90 ± 0.8 | 1.04 ± 0.03 | 1.23 ± 0.03 | |
| BDNF | 3 h | 2.57 ± 1.29 | 1.74 ± 0.36 ** | 3.01 ± 1.2 * |
| 24 h | 1.40 ± 0.7 | 0.64 ± 0.50 | 1.05 ± 0.6 | |
| TRKC | 3 h | 1.22 ± 0.34 | 1.10 ± 0.46 | 1.69 ± 0.49 * |
| 24 h | 2.35 ± 1.0 | 1.49 ± 0.70 | 1.33 ± 0.09 | |
| NT3 | 3 h | 2.17 ± 1.1 * | 1.53 ± 0.80 | 2.32 ± 1.19 * |
| 24 h | 2.23 ± 1.60 | 0.93 ± 0.60 | 1.17 ± 0.20 | |
| P75 | 3 h | 0.85 ± 0.30 | 0.72 ± 0.06 | 0.68 ± 0.05 |
| 24 h | 1.03 ± 0.30 | 0.60 ± 0.10 ** | 0.87 ± 0.40 | |
| SORTILIN | 3 h | 1.40 ± 0.59 | 0.86 ± 0.09 | 1.58 ± 0.50 |
| 24 h | 2.51 ± 1.70 | 0.96 ± 0.40 | 1.55 ± 0.60 | |
| RET | 3 h | 2.44 ± 1.40 * | 1.07 ± 0.44 | 1.56 ± 0.40 * |
| 24 h | 5.89 ± 1.70 *** | 2.68 ± 1.50 * | 3.91 ± 0.50 ** | |
| GFR α | 3 h | 1.84 ± 0.9 | 0.71 ± 0.38 | 1.19 ± 0.35 |
| 24 h | 7.90 ± 4.80 * | 4.09 ± 2.60 * | 3.15 ± 0.51 *** | |
| GDNF | 3 h | 1.57 ± 1.10 | 1.77 ± 0.40 * | 2.32 ± 0.40 * |
| 24 h | 0.85 ± 0.33 | 1.09 ± 0.40 | 1.12 ± 0.12 |
| Gene Name and Accession Number | Direct Primer | Reverse Primer | Annealing T °C |
|---|---|---|---|
| BAX NM_138764.5 | CGAACTGGACAGTAACATGG | CAGTTTGCTGGCAAAGTAGA | 60 |
| BCL2 NM_000633.3 | AGGATTGTGGCCTTCTTTGAGT | CAGAGACAGCCAGGAGAAATCA | 60 |
| GPX7 NM_015696.5 | TTGGTCCCATCATTCTTGTGG | GGCTGGTGATTCACTGGTCAA | 58 |
| GPX1 NM_000581.4 | TATCGAGAATGTGGCGTCCC | TCTTGGCGTTCTCCTGATGC | 62 |
| SOD1 NM_000454.5 | ACTGGTGGTCCATGAAAAAGC | AACGACTTCCAGCGTTTCCT | 60 |
| SOD2 NM_001024465.3 | GACAAACCTCAGCCCTAACG | GAAACCAAGCCAACCCCAAC | 55 |
| CAT NM_001752.4 | TAAGACTGACCAGGGCATC | CAAACCTTGGTGAGATCGAA | 60 |
| GDNF NM_000514.4 | TGGGTCTGGGCTATGAAACC | ATGCCTGCCCTACTTTGTCA | 60 |
| GFRα NM_005264.8 | GCCTGTGTGCTCCTATGAAG | CTGGCTGGCAGTTGGTAAA | 60 |
| RET NM_001355216.1 | AGCGGCTCTTCAACCTTCTG | CTCCTCTTAACCATCATCTTCTCC | 60 |
| SORTILIN NM_002959.7 | CTGGGTTTGGCACAATCTTT | CACCTTCCTCCTTGGTCAAA | 60 |
| P75 NM_002507.4 | GTGGGACAGAGTCTGGGTGT | AAGGAGGGGAGGTGATAGGA | 60 |
| NT3 NM_001102654.2 | TGGTTACTTTTGCCACGATCT | CCTTAACGTCCACCATCTGCT | 60 |
| TRKC NM_002530.4 | AAGCAGCCATGGTTCCAACT | CCTTGATGTTCAACCGCTGC | 60 |
| BDNF NM_170731.5 | TTTGGTTGCATGAAGGCTGC | GCCGAACTTTCTGGTCCTCA | 60 |
| TRKB145 AF508964.1 | GTTTCATAAGATCCCACTGGA | TGCTGCTTAGCTGCCTGAGAG | 60 |
| TRKB95 AF400441.1 | AGGGCAACCCGCCCACGGAA | GGATCGGTCTGGGGAAAAG | 60 |
| NGF NM_002506.3 | CATACAGGCGGAACCACACT | TTAAACAGCCTGGGGTCCAC | 60 |
| TRKA NM_001012331.2 | TCAACAAATGTGGACGGAGA | GTGGTGAACACAGGCATCAC | 60 |
| 18S KY962518.1 | CGGCTACCACATCCAAGGAA | GCTGGAATTACCGCGGCT | 60 |
| Treatment | BAX/BCL2 | |
|---|---|---|
| 3 h | 24 h | |
| control | 0.66 ± 0.23 | 0.46 ± 0.21 |
| H2O2 | 0.43 ± 0.13 | 3.81 ± 0.29 * |
| N-ADA + H2O2 | 0.50 ± 0.09 | 2.26 ± 0.84 * |
| N-DDA + H2O2 | 0.57 ± 0.30 | 1.56 ± 0.01 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novosadova, E.; Dolotov, O.; Inozemtseva, L.; Novosadova, L.; Antonov, S.; Shimchenko, D.; Bezuglov, V.; Vetchinova, A.; Tarantul, V.; Grivennikov, I.; et al. Influence of N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress. Antioxidants 2022, 11, 142. https://doi.org/10.3390/antiox11010142
Novosadova E, Dolotov O, Inozemtseva L, Novosadova L, Antonov S, Shimchenko D, Bezuglov V, Vetchinova A, Tarantul V, Grivennikov I, et al. Influence of N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress. Antioxidants. 2022; 11(1):142. https://doi.org/10.3390/antiox11010142
Chicago/Turabian StyleNovosadova, Ekaterina, Oleg Dolotov, Ludmila Inozemtseva, Ludmila Novosadova, Stanislav Antonov, Darya Shimchenko, Vladimir Bezuglov, Anna Vetchinova, Vyacheslav Tarantul, Igor Grivennikov, and et al. 2022. "Influence of N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress" Antioxidants 11, no. 1: 142. https://doi.org/10.3390/antiox11010142
APA StyleNovosadova, E., Dolotov, O., Inozemtseva, L., Novosadova, L., Antonov, S., Shimchenko, D., Bezuglov, V., Vetchinova, A., Tarantul, V., Grivennikov, I., & Illarioshkin, S. (2022). Influence of N-Arachidonoyl Dopamine and N-Docosahexaenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress. Antioxidants, 11(1), 142. https://doi.org/10.3390/antiox11010142







