Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Animal Treatment and Energy Balance Assessment
2.3. Evaluation of Inflammatory Markers
2.4. Heart Lipid Content Measurement
2.5. Mitochondrial Isolation Procedure
2.6. Measurement of Mitochondrial Oxygen Consumption
2.7. Evaluation of Redox Status
2.8. Real-Time PCR
2.9. Light Microscopy
2.10. Statistical Analyses
3. Results
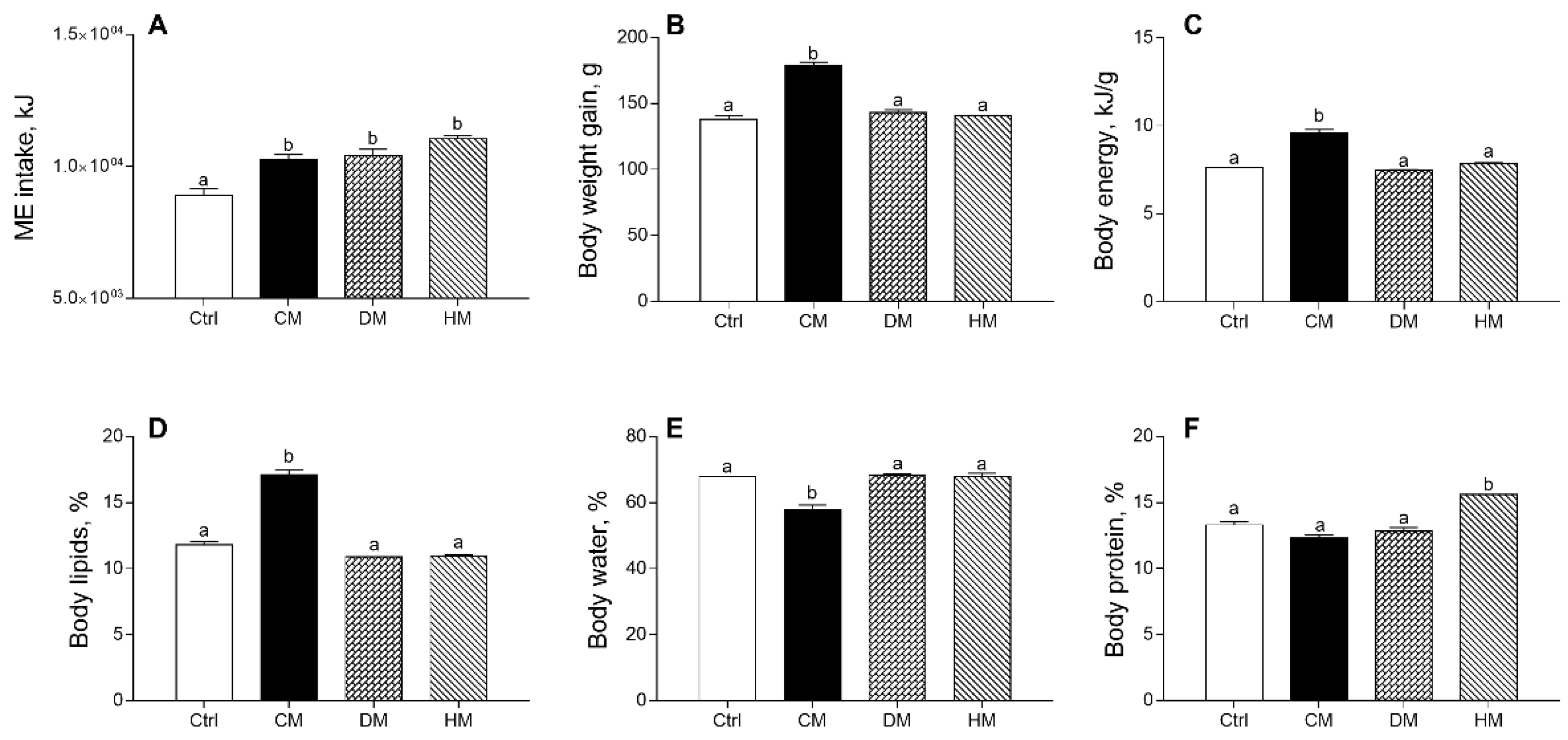
3.1. Distinct Milk Administration Differently Modulates Body Weight Gain, Composition and Efficiency
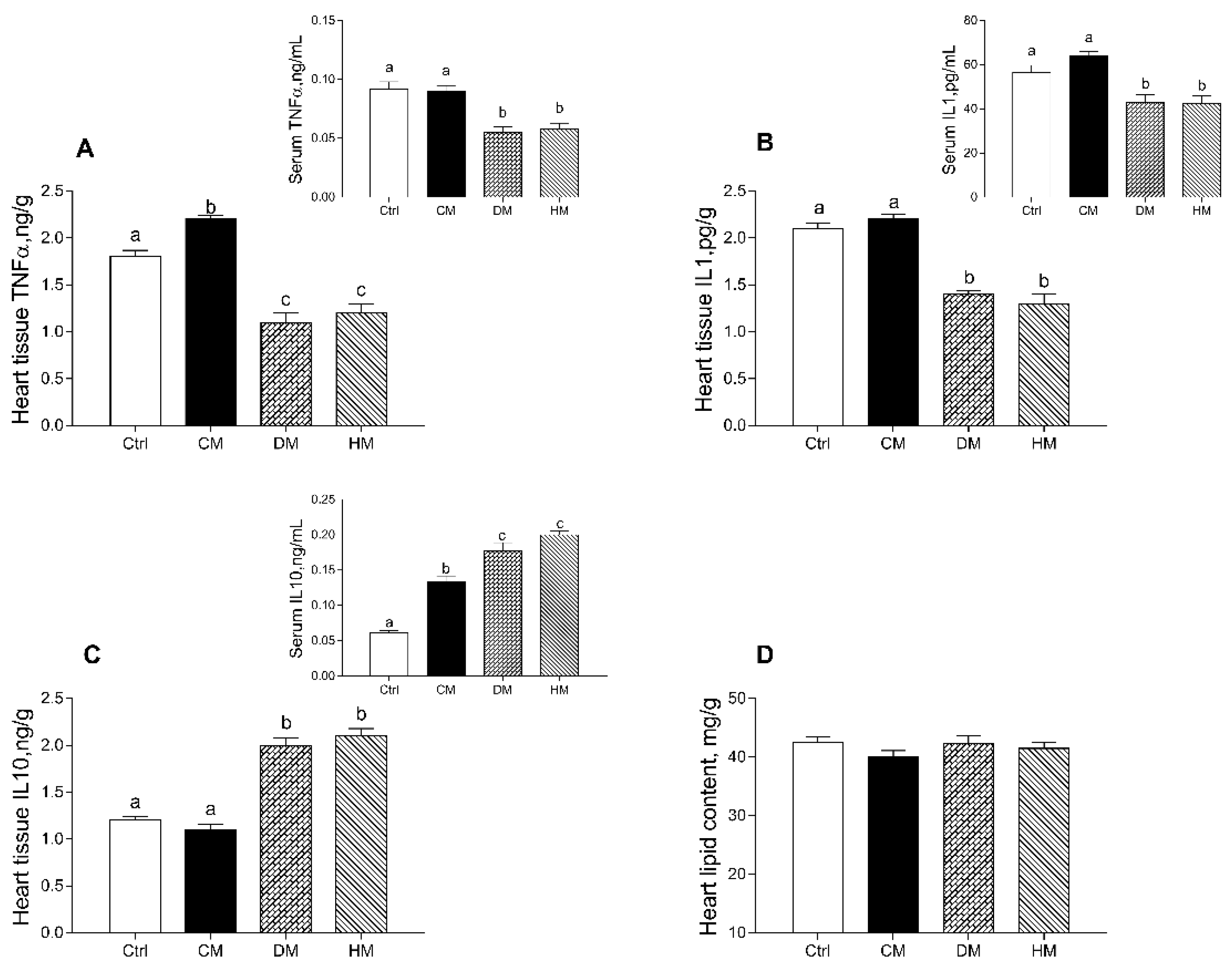
3.2. Modulation of Serum and Tissue Inflammatory Profile in Rats Fed with Different Milk
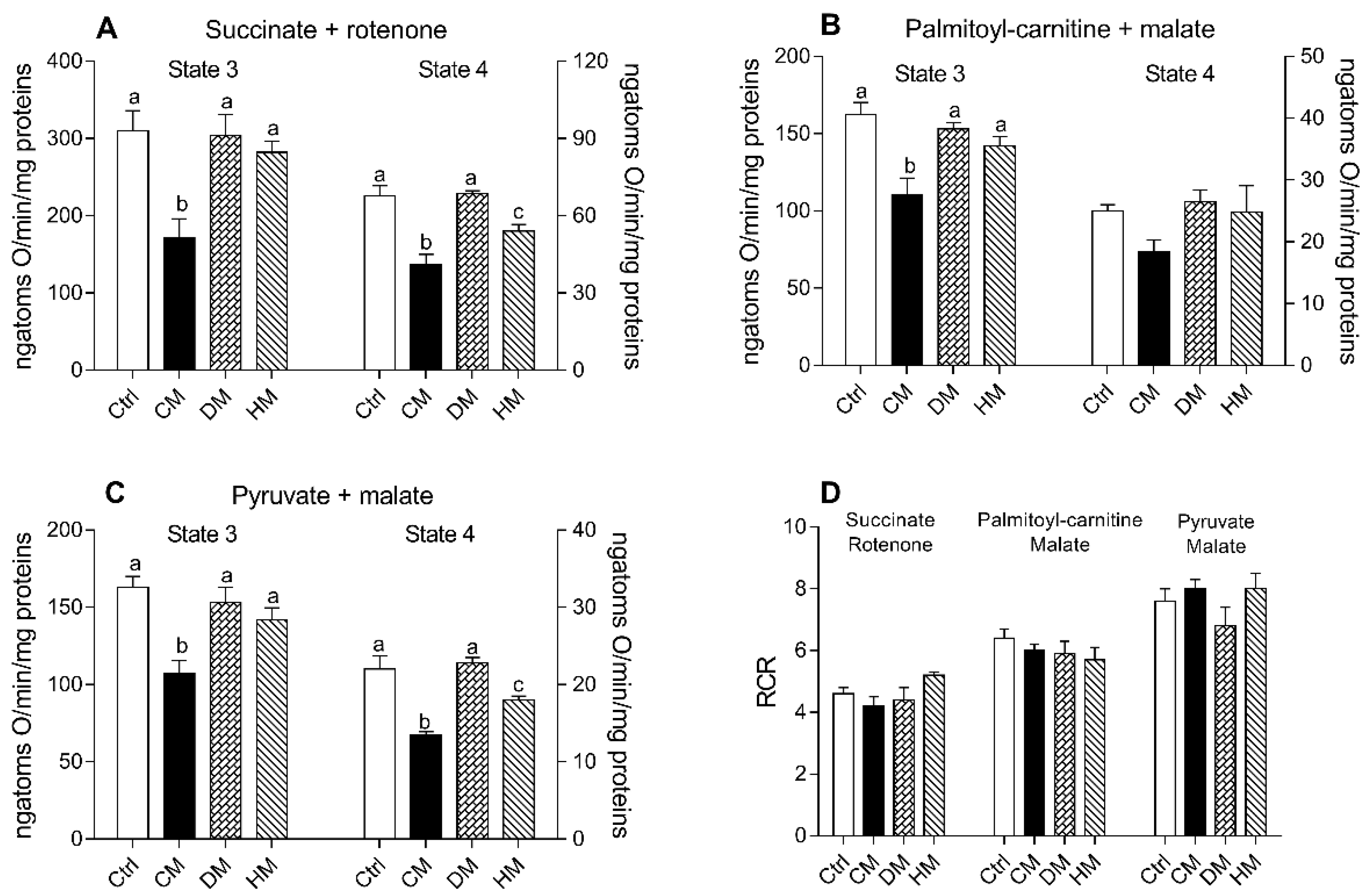
3.3. Heart Mitochondrial Oxidative Capacity Is Modulated by the Administration of Milk from Different Animal Species
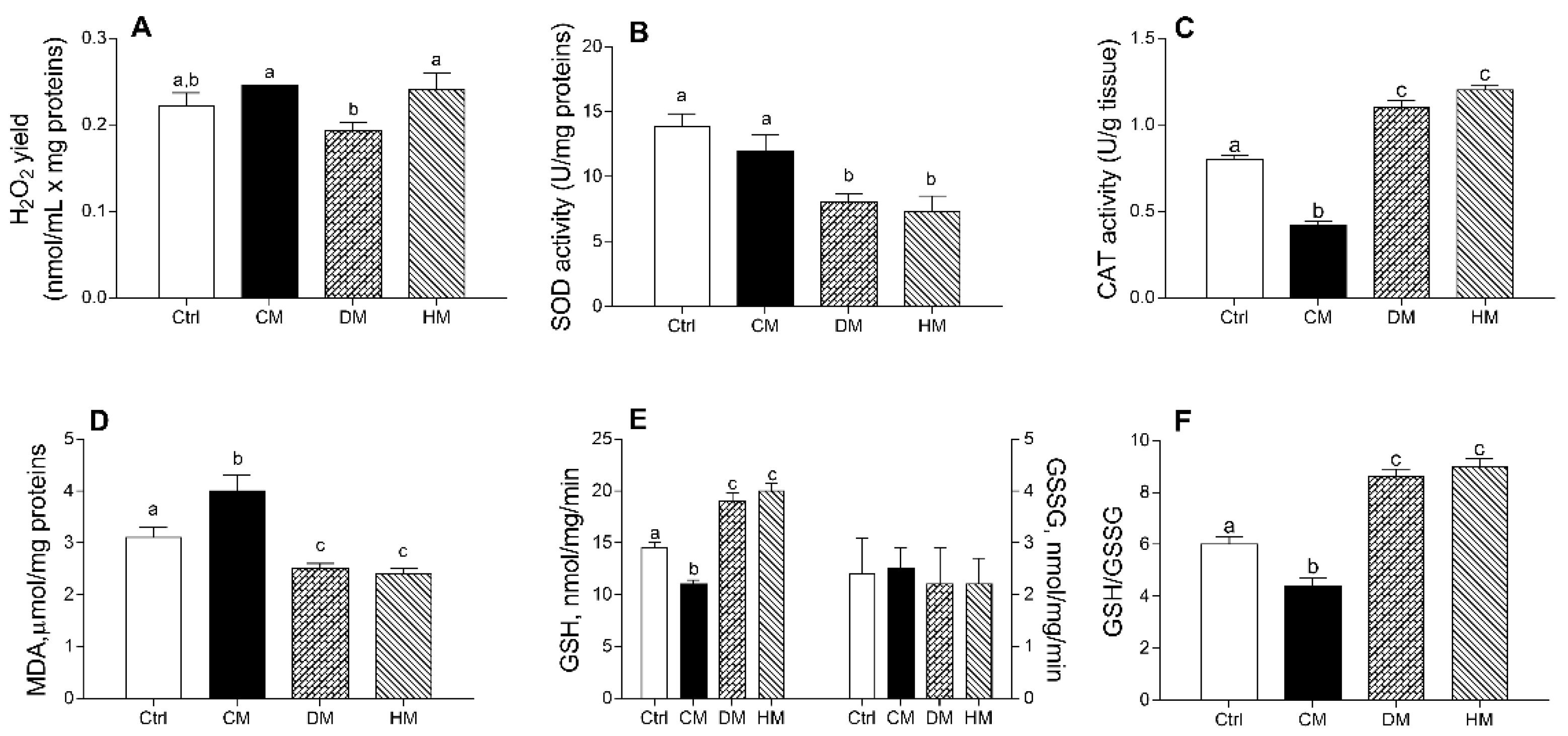
3.4. Cardiac Redox Status Is Modulated by Different Milk Administration in Rats
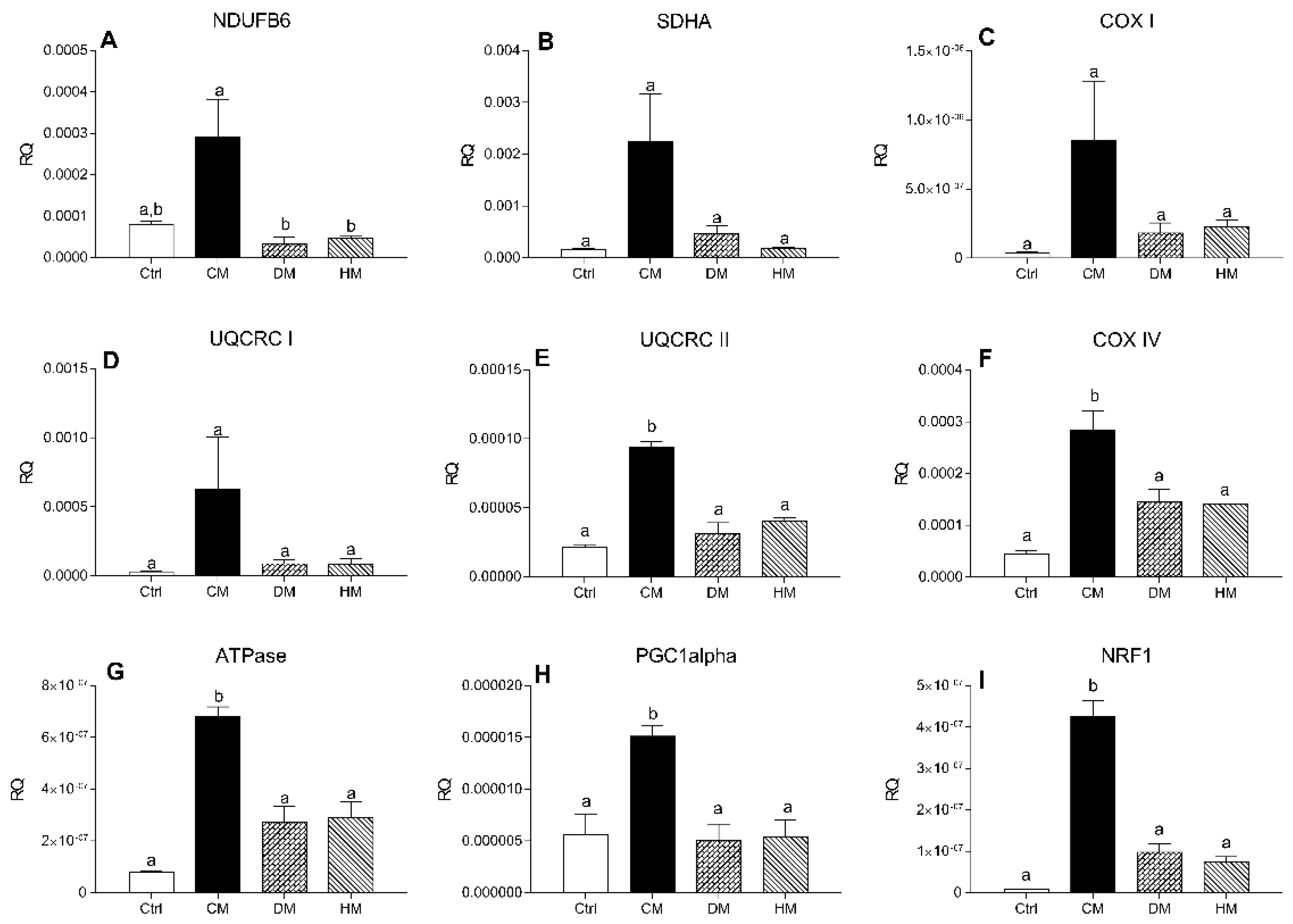
3.5. Distinct Milk Administration Differently Modulates Cardiac Expression of the Mitochondrial Respiratory Chain Complexes Genes
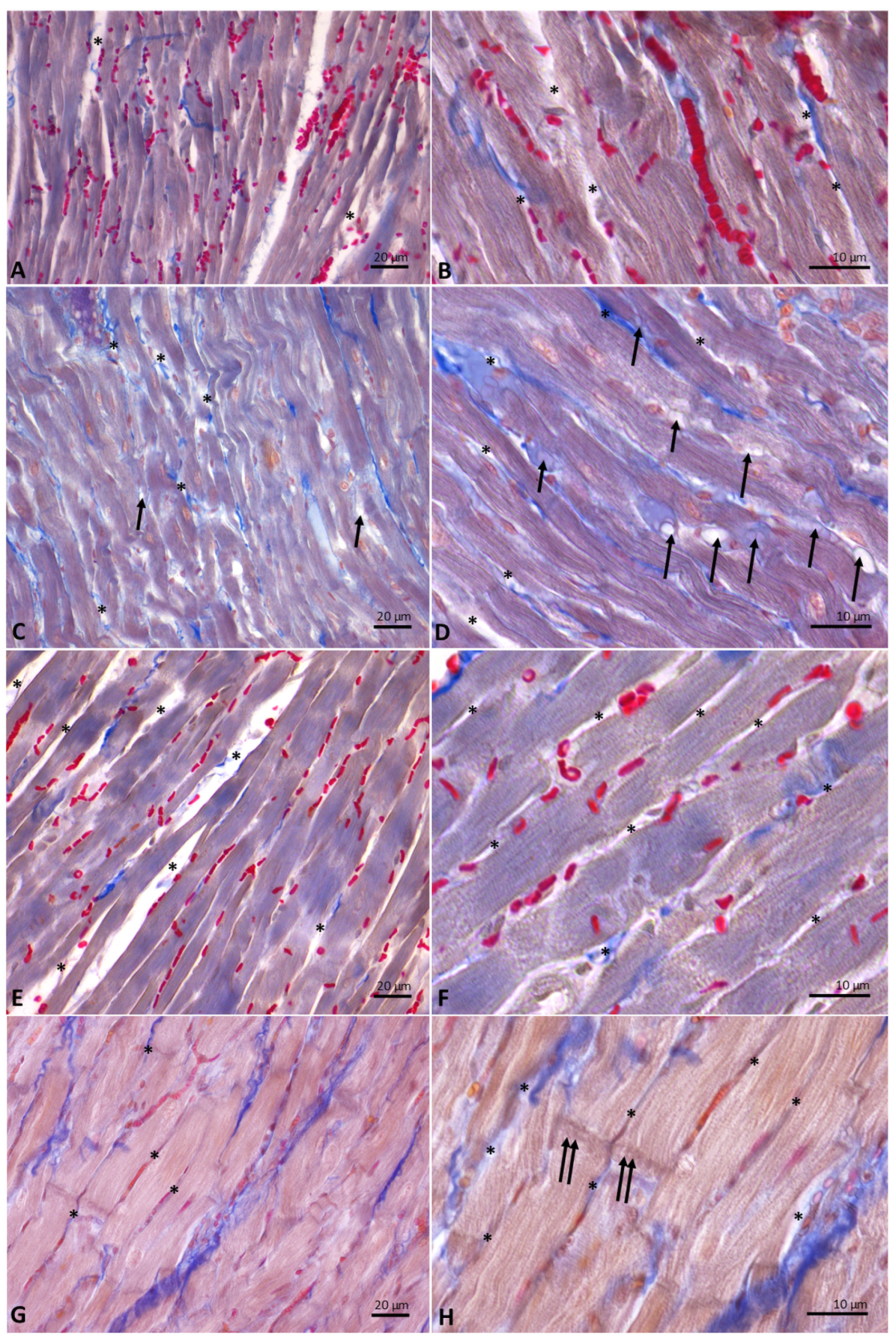
3.6. Distinct Milk Administration Affects the Histological Features of the Heart Muscle Fibers
3.7. Distinct Milk Administration Modifies Lipid Profile and Endocannabinoidome in the Cardiac Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Gao, A.W.; Cantó, C.; Houtkooper, R.H. Mitochondrial response to nutrient availability and its role in metabolic disease. EMBO Mol. Med. 2014, 6, 580–589. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Ingwall, J.S.; DeLuca, M.; Sybers, H.D.; Wildenthal, K. Fetal mouse hearts: A model for studying ischemia. Proc. Natl. Acad. Sci. USA 1975, 72, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Hearse, D.J.; Stewart, D.A.; Braimbridge, M.V. Hypothermic arrest and potassium arrest: Metabolic and myocardial protection during elective cardiac arrest. Circ. Res. 1975, 36, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Hearse, D.J. Oxygen deprivation and early myocardial contractile failure: A reassessment of the possible role of adenosine triphosphate. Am. J. Cardiol. 1979, 44, 1115–1121. [Google Scholar] [CrossRef]
- Gresser, M.; Cardon, J.; Rosen, G.; Boyer, P.D. Demonstration and quantitation of catalytic and noncatalytic bound ATP in submitochondrial particles during oxidative phosphorylation. J. Biol. Chem. 1979, 254, 10649–10653. [Google Scholar] [CrossRef]
- Kadenbach, B.; Barth, J.; Akgün, R.; Freund, R.; Linder, D.; Possekel, S. Regulation of mitochondrial energy generation in health and disease. Biochim. Biophys. Acta 1995, 1271, 103–109. [Google Scholar] [CrossRef]
- Valkovič, L.; Clarke, W.T.; Schmid, A.I.; Raman, B.; Ellis, J.; Watkins, H.; Robson, M.D.; Neubauer, S.; Rodgers, C.T. Measuring inorganic phosphate and intracellular pH in the healthy and hypertrophic cardiomyopathy hearts by in vivo 7T. J. Cardiovasc. Magn. Reson. 2019, 21, 19. [Google Scholar] [CrossRef]
- Nijtmans, L.G.; Henderson, N.S.; Attardi, G.; Holt, I.J. Impaired ATP synthase assembly associated with a mutation in the human ATP synthase subunit 6 gene. J. Biol. Chem. 2001, 276, 6755–6762. [Google Scholar] [CrossRef] [PubMed]
- Szendrei, L.; Turoczi, T.; Kovacs, P.; Vecsernyes, M.; Das, D.K.; Tosaki, A. Mitochondrial gene expression and ventricular fibrillation in ischemic/reperfused nondiabetic and diabetic myocardium. Biochem. Pharmacol. 2002, 63, 543–552. [Google Scholar] [CrossRef]
- Carbajo, R.J.; Silvester, J.A.; Runswick, M.J.; Walker, J.E.; Neuhaus, D. Solution structure of subunit F(6) from the peripheral stalk region of ATP synthase from bovine heart mitochondria. J. Mol. Biol. 2004, 342, 593–603. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Zhang, L.; Zhu, M.; Gao, L. A high-fat diet impairs mitochondrial biogenesis, mitochondrial dynamics, and the respiratory chain complex in rat myocardial tissues. J. Cell Biochem. 2018, 119, 9602. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Sorriento, D.; Ciccarelli, M.; Del Giudice, C.; Fiordelisi, A.; Napolitano, L.; Trimarco, B.; Iaccarino, G.; Santulli, G. Functional Role of Mitochondria in Arrhythmogenesis. Adv. Exp. Med. Biol. 2017, 982, 191–202. [Google Scholar] [CrossRef]
- Sorriento, D.; Gambardella, J.; Fiordelisi, A.; Trimarco, B.; Ciccarelli, M.; Iaccarino, G.; Santulli, G. Mechanistic Role of Kinases in the Regulation of Mitochondrial Fitness. Adv. Exp. Med. Biol. 2017, 982, 521–528. [Google Scholar] [CrossRef]
- Sorriento, D.; Gambardella, J.; Fiordelisi, A.; Iaccarino, G.; Illario, M. GRKs and β-Arrestins: “Gatekeepers” of Mitochondrial Function in the Failing Heart. Front. Pharmacol. 2019, 10, 64. [Google Scholar] [CrossRef]
- Franco, A.; Sorriento, D.; Gambardella, J.; Pacelli, R.; Prevete, N.; Procaccini, C.; Matarese, G.; Trimarco, B.; Iaccarino, G.; Ciccarelli, M. GRK2 moderates the acute mitochondrial damage to ionizing radiation exposure by promoting mitochondrial fission/fusion. Cell Death Discov. 2018, 4, 25. [Google Scholar] [CrossRef]
- Sorriento, D.; Ciccarelli, M.; Santulli, G.; Illario, M.; Trimarco, B.; Iaccarino, G. Trafficking GRK2: Cellular and Metabolic consequences of GRK2 subcellular localization. Transl. Med. UniSa 2014, 10, 3–7. [Google Scholar]
- Nauta, A.J.; Ben Amor, K.; Knol, J.; Garssen, J.; van der Beek, E.M. Relevance of pre- and postnatal nutrition to development and interplay between the microbiota and metabolic and immune systems. Am. J. Clin. Nutr. 2013, 98, 586S–593S. [Google Scholar] [CrossRef] [PubMed]
- Guardamagna, O.; Abello, F.; Cagliero, P.; Lughetti, L. Impact of nutrition since early life on cardiovascular prevention. Ital. J. Pediatr. 2012, 38, 73. [Google Scholar] [CrossRef]
- Parikh, N.I.; Hwang, S.J.; Ingelsson, E.; Benjamin, E.J.; Fox, C.S.; Vasan, R.S.; Murabito, J.M. Breastfeeding in infancy and adult cardiovascular disease risk factors. Am. J. Med. 2009, 122, 656–663.e1. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Owen, C.G.; Strachan, D.P. The effect of breastfeeding on cardiorespiratory risk factors in adult life. Pediatrics 2007, 119, e1107–e1115. [Google Scholar] [CrossRef]
- Martin, R.M.; Ness, A.R.; Gunnell, D.; Emmett, P.; Davey Smith, G.; Team, A.S. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation 2004, 109, 1259–1266. [Google Scholar] [CrossRef]
- Martin, R.M.; Ben-Shlomo, Y.; Gunnell, D.; Elwood, P.; Yarnell, J.W.; Davey Smith, G. Breast feeding and cardiovascular disease risk factors, incidence, and mortality: The Caerphilly study. J. Epidemiol. Community Health 2005, 59, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Gilg, J.A.; Cook, D.G. Effect of breast feeding in infancy on blood pressure in later life: Systematic review and meta-analysis. BMJ 2003, 327, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Whincup, P.H.; Odoki, K.; Gilg, J.A.; Cook, D.G. Infant feeding and blood cholesterol: A study in adolescents and a systematic review. Pediatrics 2002, 110, 597–608. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Dis. Child. 2000, 82, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.G.; Martin, R.M.; Whincup, P.H.; Davey-Smith, G.; Gillman, M.W.; Cook, D.G. The effect of breastfeeding on mean body mass index throughout life: A quantitative review of published and unpublished observational evidence. Am. J. Clin. Nutr. 2005, 82, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, L.; Cavaliere, G.; Bergamo, P.; Trinchese, G.; De Filippo, C.; Gifuni, G.; Gaita, M.; Pignalosa, A.; Donizzetti, I.; Putti, R.; et al. Diet supplementation with donkey milk upregulates liver mitochondrial uncoupling, reduces energy efficiency and improves antioxidant and antiinflammatory defences in rats. Mol. Nutr. Food Res. 2012, 56, 1596–1600. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, E.; Mollica, M.P.; Lionetti, L.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Chieffi, S.; Gaita, M.; Barletta, A.; De Luca, B.; et al. Effects of an High-Fat Diet Enriched in Lard or in Fish Oil on the Hypothalamic Amp-Activated Protein Kinase and Inflammatory Mediators. Front. Cell Neurosci. 2016, 10, 150. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef]
- Qi, Z.; Ding, S. Targeting mitochondrial phenotypes for non-communicable diseases. J. Sport Health Sci. 2016, 5, 155–158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mollica, M.P.; Trinchese, G.; Cimmino, F.; Penna, E.; Cavaliere, G.; Tudisco, R.; Musco, N.; Manca, C.; Catapano, A.; Monda, M.; et al. Milk Fatty Acid Profiles in Different Animal Species: Focus on the Potential Effect of Selected PUFAs on Metabolism and Brain Functions. Nutrients 2021, 13, 1111. [Google Scholar] [CrossRef]
- Fontecha, J.; Calvo, M.V.; Juarez, M.; Gil, A.; Martínez-Vizcaino, V. Milk and Dairy Product Consumption and Cardiovascular Diseases: An Overview of Systematic Reviews and Meta-Analyses. Adv. Nutr. 2019, 10, S164–S189. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspal, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; Canani, R.B.; Matamoros, S.; Bergamo, P.; De Filippo, C.; Aceto, S.; Gaita, M.; Cerino, P.; Negri, R.; et al. Human, donkey and cow milk differently affects energy efficiency and inflammatory state by modulating mitochondrial function and gut microbiota. J. Nutr. Biochem. 2015, 26, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Trinchese, G.; Cavaliere, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Chun, J.T.; Penna, E.; Negri, R.; Muredda, L.; Demurtas, A.; et al. Human Milk and Donkey Milk, Compared to Cow Milk, Reduce Inflammatory Mediators and Modulate Glucose and Lipid Metabolism, Acting on Mitochondrial Function and Oleylethanolamide Levels in Rat Skeletal Muscle. Front. Physiol. 2018, 9, 32. [Google Scholar] [CrossRef]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate Regulates Liver Mitochondrial Function, Efficiency, and Dynamics in Insulin-Resistant Obese Mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Lionetti, L.; Mollica, M.P.; Sica, R.; Donizzetti, I.; Gifuni, G.; Pignalosa, A.; Cavaliere, G.; Putti, R. Differential effects of high-fish oil and high-lard diets on cells and cytokines involved in the inflammatory process in rat insulin-sensitive tissues. Int. J. Mol. Sci. 2014, 15, 3040–3063. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Chiang, S.; Gessert, C.; Lowry, O. Colorimetric determination of extracted lipids. An adaptation for microgram amounts of lipids obtained from cerumen. Curr. List Med. Lit. Res. Rep. 1957, 33, 56–113. [Google Scholar]
- Banni, S.; Carta, G.; Contini, M.S.; Angioni, E.; Deiana, M.; Dessì, M.A.; Melis, M.P.; Corongiu, F.P. Characterization of conjugated diene fatty acids in milk, dairy products, and lamb tissues. J. Nutr. Biochem. 1996, 7, 150–155. [Google Scholar] [CrossRef]
- Melis, M.P.; Angioni, E.; Carta, G.; Murru, E.; Scanu, P.; Spada, S.; Banni, S. Characterization of conjugated linoleic acid and its metabolites by RP-HPLC with diode array detector. Eur. J. Lipid Sci. Technol. 2001, 103, 617–621. [Google Scholar] [CrossRef]
- Murru, E.; Lopes, P.A.; Carta, G.; Manca, C.; Abolghasemi, A.; Guil-Guerrero, J.L.; Prates, J.A.M.; Banni, S. Different Dietary N-3 Polyunsaturated Fatty Acid Formulations Distinctively Modify Tissue Fatty Acid and N-Acylethanolamine Profiles. Nutrients 2021, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Barja, G. Mitochondrial free radical production and aging in mammals and birds. Ann. N. Y. Acad Sci. 1998, 854, 224–238. [Google Scholar] [CrossRef]
- Flohé, L.; Otting, F. Superoxide dismutase assays. Methods Enzymol. 1984, 105, 93–104. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, D.M.; Chen, H.L.; Lin, Y.X.; Hang, C.H.; Yin, H.X.; Shi, J.X. N-acetylcysteine suppresses oxidative stress in experimental rats with subarachnoid hemorrhage. J. Clin. Neurosci. 2009, 16, 684–688. [Google Scholar] [CrossRef]
- Bergamo, P.; Maurano, F.; Rossi, M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Radic. Biol. Med. 2007, 43, 71–79. [Google Scholar] [CrossRef]
- Rosati, L.; Agnese, M.; Abagnale, L.; Aniello, F.; Andreuccetti, P.; Prisco, M. The Mussel Mytilus galloprovincialis in the Bay of Naples: New Insights on Oogenic Cycle and Its Hormonal Control. Anat. Rec. 2019, 302, 1039–1049. [Google Scholar] [CrossRef]
- Leverve, X.M.; Fontaine, E. Role of substrates in the regulation of mitochondrial function in situ. IUBMB Life 2001, 52, 221–229. [Google Scholar] [CrossRef]
- Dai, D.F.; Rabinovitch, P.S. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 2009, 19, 213–220. [Google Scholar] [CrossRef]
- Missiroli, S.; Genovese, I.; Perrone, M.; Vezzani, B.; Vitto, V.A.M.; Giorgi, C. The Role of Mitochondria in Inflammation: From Cancer to Neurodegenerative Disorders. J. Clin. Med. 2020, 9, 740. [Google Scholar] [CrossRef]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases-from pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Crispino, M.; Trinchese, G.; Penna, E.; Cimmino, F.; Catapano, A.; Villano, I.; Perrone-Capano, C.; Mollica, M.P. Interplay between Peripheral and Central Inflammation in Obesity-Promoted Disorders: The Impact on Synaptic Mitochondrial Functions. Int. J. Mol. Sci. 2020, 21, 5964. [Google Scholar] [CrossRef]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Caramés, B.; Martín, M.A.; Cillero-Pastor, B.; Lires-Dean, M.; Fuentes-Boquete, I.; Arenas, J.; Blanco, F.J. Mitochondrial activity is modulated by TNFalpha and IL-1beta in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, E.; López-Armada, M.J.; de Andres, M.C.; Caramés, B.; Martín, M.A.; Bonilla, A.; Del Hoyo, P.; Galdo, F.; Arenas, J.; Blanco, F.J. Effect of nitric oxide on mitochondrial respiratory activity of human articular chondrocytes. Ann. Rheum. Dis. 2005, 64, 388–395. [Google Scholar] [CrossRef]
- Guidarelli, A.; Cerioni, L.; Cantoni, O. Inhibition of complex III promotes loss of Ca2+ dependence for mitochondrial superoxide formation and permeability transition evoked by peroxynitrite. J. Cell Sci. 2007, 120, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Xu, M.; Xo, R.; Mates, A.; Wilson, G.L.; Pearsall, A.W.; Grishko, V. Mitochondrial DNA damage is involved in apoptosis caused by pro-inflammatory cytokines in human OA chondrocytes. Osteoarthr. Cartil. 2010, 18, 424–432. [Google Scholar] [CrossRef] [PubMed]
- López-Armada, M.J.; Riveiro-Naveira, R.R.; Vaamonde-García, C.; Valcárcel-Ares, M.N. Mitochondrial dysfunction and the inflammatory response. Mitochondrion 2013, 13, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.; Pérez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 2000, 106, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.O.; Wende, A.R.; Crum, A.; Hunter, D.; Olsen, C.D.; Rawlings, T.; Riehle, C.; Ward, W.F.; Abel, E.D. Maintaining PGC-1α expression following pressure overload-induced cardiac hypertrophy preserves angiogenesis but not contractile or mitochondrial function. FASEB J. 2014, 28, 3691–3702. [Google Scholar] [CrossRef]
- Sack, M.N.; Kelly, D.P. The energy substrate switch during development of heart failure: Gene regulatory mechanisms (Review). Int. J. Mol. Med. 1998, 1, 17–24. [Google Scholar] [CrossRef]
- Tong, M.; Sadoshima, J. Mitochondrial autophagy in cardiomyopathy. Curr. Opin. Genet. Dev. 2016, 38, 8–15. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef]
- Garlick, P.B.; Davies, M.J.; Hearse, D.J.; Slater, T.F. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ. Res. 1987, 61, 757–760. [Google Scholar] [CrossRef]
- Tosaki, A.; Braquet, P. DMPO and reperfusion injury: Arrhythmia, heart function, electron spin resonance, and nuclear magnetic resonance studies in isolated working guinea pig hearts. Am. Heart J. 1990, 120, 819–830. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Lipid peroxidation, antioxidants and cardiovascular disease: How should we move forward? Cardiovasc. Res. 2000, 47, 410–418. [Google Scholar] [CrossRef]
- Schrauwen, P.; Schrauwen-Hinderling, V.; Hoeks, J.; Hesselink, M.K. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta 2010, 1801, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Merkel, M.; Eckel, R.H.; Goldberg, I.J. Lipoprotein lipase: Genetics, lipid uptake, and regulation. J. Lipid Res. 2002, 43, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, P. Myocardial, perivascular, and epicardial fat. Diabetes Care 2011, 34 (Suppl. S2), S371–S379. [Google Scholar] [CrossRef] [PubMed]
- Marchington, J.M.; Mattacks, C.A.; Pond, C.M. Adipose tissue in the mammalian heart and pericardium: Structure, foetal development and biochemical properties. Comp. Biochem. Physiol. B 1989, 94, 225–232. [Google Scholar] [CrossRef]
- McKinney, M.K.; Cravatt, B.F. Structure and function of fatty acid amide hydrolase. Annu. Rev. Biochem. 2005, 74, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Horváth, B.; Rajesh, M.; Matsumoto, S.; Saito, K.; Bátkai, S.; Patel, V.; Tanchian, G.; Gao, R.Y.; Cravatt, B.F.; et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic. Biol. Med. 2011, 50, 179–195. [Google Scholar] [CrossRef]
- Hiley, C.R. Endocannabinoids and the heart. J. Cardiovasc. Pharmacol. 2009, 53, 267–276. [Google Scholar] [CrossRef]
| Gene | Accession n° (NCBI Database) | Sense Primer Sequence | Anti-Sense Primer Sequence |
|---|---|---|---|
| 18 S | NR_003278 | 5’-GTAACCCGTTGAACCCCATT-3’ | 5’CCATCCAATCGGTAGTAGCG-3’ |
| NDUFB 6 | NM_001033305.3 | 5’-ATAACTTTTTGCGGGACGGG-3’ | 5’-CAGGAAAATCTCTCATTGGTG-3’ |
| SDHA | NM_023281.1 | 5’-CATACTGTTGCAGCAGCACAGG-3’ | 5’-CCACCAAATGCACGCTGATA-3’ |
| ATPASE | NM_001302213.1 | 5’-TGTGGAAGGAAGTGGGCAA-3’ | 5’-CCACTATGAGCTGGAGCCGT-3’ |
| COX 1 | NP_904330.1 | 5’-GAAGAGACAGTGTTTCATGTGGTGT-3’ | 5’-TCCTGGGCCTTTCAGGAATA-3’ |
| COX 4 | NM_001293559.1 | 5’-GAGCACATGGGAGTGTTGTG-3’ | 5’-CTGTCTTCCATTCATTGGTGCC-3’ |
| UQCRC I | NM_025407.2 | 5’-CCTACGCACTCGAGAGCAC-3’ | 5’-AGGTGTGCCCTGGAATGCTG-3’ |
| UQCRC II | NM_025899.2 | 5’-TCCCTCAAAGTTGCCCC-3’ | 5’-GCAAGACGTAGTAAATGTGAG-3’ |
| PGC1 ALPHA | NM_008904.2 | 5’-AAACTTGCTAGCGGTCCTCA-3’ | 5’-TGGCTGGTGCCAGTAAGAG-3’ |
| NRF1 | NM_001164226.1 | 5’-GCACCTTTGGAGAATGTGGT-3’ | 5’-GGGTCATTTTGTCCACAGAGA-3’ |
| nmol/g Tissue | Control | CM-Treated | DM-Treated | HM-Treated |
|---|---|---|---|---|
| ALA,18:3n3 | 258.57 ± 21.35 | 235.50 ± 38.53 | 262.00 ± 26.20 | 238.72 ± 23.99 |
| EPA,20:5n3 | 180.42 ± 12.61 a,b | 149.34 ± 12.61 a | 187.97 ± 24.62 b | 183.21 ± 6.77 a,b |
| DPA,22:5n3 | 902.76 ± 62.55 a,b | 831.42 ± 90.42 a | 974.87 ± 86.88 a,b | 906.75 ± 42.24 b |
| DHA,22:6n3 | 8938.36 ± 436.47 a | 9616.58 ± 158.38 a | 11,132.58 ± 336.50 b | 10,290.31 ± 295.78 a,b |
| LA,18:2n6 | 21,911.79 ± 372.92 a,b,c | 18,689.10 ± 902.29 b,c | 23,642.50 ± 847.93 a | 19,785.46 ± 379.40 b |
| ETA,20:3n6 | 632.89 ± 60.42 | 674.66 ± 32.68 | 653.45 ± 60.58 | 747.09 ± 41.46 |
| AA,20:4n6 | 13,445.11 ± 404.61 | 13,201.11 ± 651.81 | 13,963.23 ± 1144.02 | 14,381.91 ± 573.23 |
| DPA,22:5n6 | 604.56 ± 31.19 a | 693.92 ± 7.68 b | 655.69 ± 11.55 a,b | 676.23 ± 25.97 a,b |
| DTA,22:4n6 | 756.65 ± 59.39 | 777.80 ± 58.09 | 761.79 ± 60.48 | 730.88 ± 35.32 |
| 14:1 n7 | 156.33 ± 10.34 a | 157.32 ± 3.01 a | 101.23 ± 1.50 b | 115.61 ± 8.00 a |
| POA,16:1n7 | 647.98 ± 71.88 | 678.39 ± 189.96 | 546.32 ± 90.22 | 461.51 ± 70.58 |
| OA,18:1n9 | 7238.21 ± 296.97 | 6939.20 ± 820.32 | 7438.93 ± 771.68 | 7596.36 ± 375.55 |
| 18:1n7 | 5054.95 ± 167.26 | 4401.26 ± 324.03 | 4313.26 ± 200.96 | 4433.04 ± 123.26 |
| 8:0 | 106.20 ± 17.43 | 136.47 ± 17.36 | 99.76 ± 17.58 | 141.32 ± 31.38 |
| 10:0 | 42.73 ± 9.50 | 68.62 ± 27.13 | 44.59 ± 11.27 | 45.39 ± 8.22 |
| 12:0 | 58.49 ± 20.13 | 106.85 ± 25.12 | 29.31 ± 3.36 | 107.30 ± 16.65 |
| MA,14:0 | 218.29 ± 21.86 a | 467.59 ± 57.14 b | 228.89 ± 19.40 a,b | 251.69 ± 15.93 a,b |
| PA,16:0 | 13,368.47 ± 549.03 | 12,908.57 ± 612.14 | 12,195.98 ± 505.56 | 12,508.22 ± 357.77 |
| 17:0 | 494.22 ± 49.96 | 418.46 ± 44.82 | 320.53 ± 17.76 | 297.69 ± 33.37 |
| SA,18:0 | 14,044.16 ± 383.02 | 13,532.07 ± 387.28 | 13,013.23 ± 471.47 | 14,4424.90 ± 327.01 |
| pmol/g Tissue | Control | CM-Treated | DM-Treated | HM-Treated |
|---|---|---|---|---|
| POEA | 12.53 ± 2.69 a,b | 7.80 ± 0.52 b | 13.75 ± 1.13 a | 9.34 ± 1.00 a,b |
| AEA | 55.56 ± 3.55 a | 45.74 ± 3.55 a,b | 21.54 ± 1.84 c | 27.34 ± 0.36 b |
| DHEA | 126.03 ± 3.94 a | 132.81 ± 4.73 a | 78.59 ± 6.18 b | 97.92 ± 5.65 a,b |
| LEA | 100.97 ± 7.36 | 105.19 ± 6.25 | 108.78 ± 8.01 | 103.53 ± 10.09 |
| PEA | 140.32 ± 9.19 | 129.41 ± 11.78 | 125.08 ± 12.16 | 144.48 ± 17.80 |
| OEA | 169.75 ± 11.26 | 153.75 ± 7.07 | 169.28 ± 11.06 | 171.49 ± 13.82 |
| DTEA | 9.70 ± 1.20 | 11.44 ± 3.19 | 7.23 ± 0.73 | 9.05 ± 1.28 |
| SEA | 69.19 ± 4.08 | 68.43 ± 3.38 | 71.08 ± 5.83 | 69.19 ± 7.58 |
| 2AG | 4251.89 ± 307.50 | 5076.72 ± 508.05 | 3446.42 ± 217.36 | 4662.21 ± 773.63 |
| 2LG | 15,045.36 ± 1.472.329 | 17,714.40 ± 1513.11 | 8383.01 ± 1269.33 | 14,721.11 ± 3523.24 |
| 2PG | 287,635.54 ± 126360.68 | 251,591.30 ± 64894.94 | 250,790.67 ± 90116.73 | 294,106.10 ± 72879.22 |
| 2OG | 20,974.23 ± 2234.46 | 29,883.32 ± 3591.74 | 9739.69 ± 1504.49 | 25,774.59 ± 4962.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinchese, G.; Cimmino, F.; Cavaliere, G.; Rosati, L.; Catapano, A.; Sorriento, D.; Murru, E.; Bernardo, L.; Pagani, L.; Bergamo, P.; et al. Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake. Antioxidants 2021, 10, 1807. https://doi.org/10.3390/antiox10111807
Trinchese G, Cimmino F, Cavaliere G, Rosati L, Catapano A, Sorriento D, Murru E, Bernardo L, Pagani L, Bergamo P, et al. Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake. Antioxidants. 2021; 10(11):1807. https://doi.org/10.3390/antiox10111807
Chicago/Turabian StyleTrinchese, Giovanna, Fabiano Cimmino, Gina Cavaliere, Luigi Rosati, Angela Catapano, Daniela Sorriento, Elisabetta Murru, Luca Bernardo, Luciana Pagani, Paolo Bergamo, and et al. 2021. "Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake" Antioxidants 10, no. 11: 1807. https://doi.org/10.3390/antiox10111807
APA StyleTrinchese, G., Cimmino, F., Cavaliere, G., Rosati, L., Catapano, A., Sorriento, D., Murru, E., Bernardo, L., Pagani, L., Bergamo, P., Scudiero, R., Iaccarino, G., Greco, L., Banni, S., Crispino, M., & Mollica, M. P. (2021). Heart Mitochondrial Metabolic Flexibility and Redox Status Are Improved by Donkey and Human Milk Intake. Antioxidants, 10(11), 1807. https://doi.org/10.3390/antiox10111807















