Dysregulated Autophagy Leads to Oxidative Stress and Aberrant Expression of ABC Transporters in Women with Early Miscarriage
Abstract
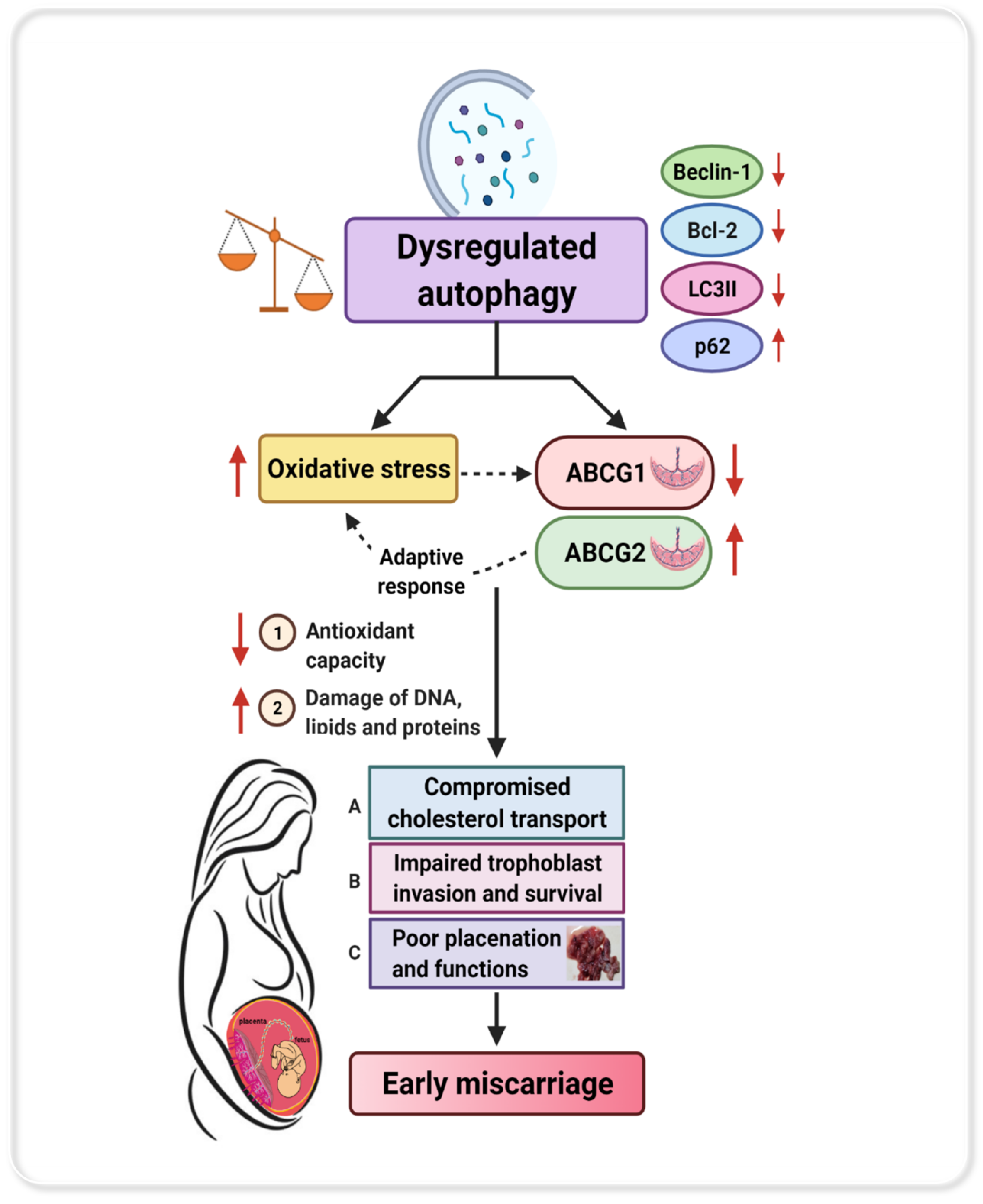
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Groups and Sample Collection
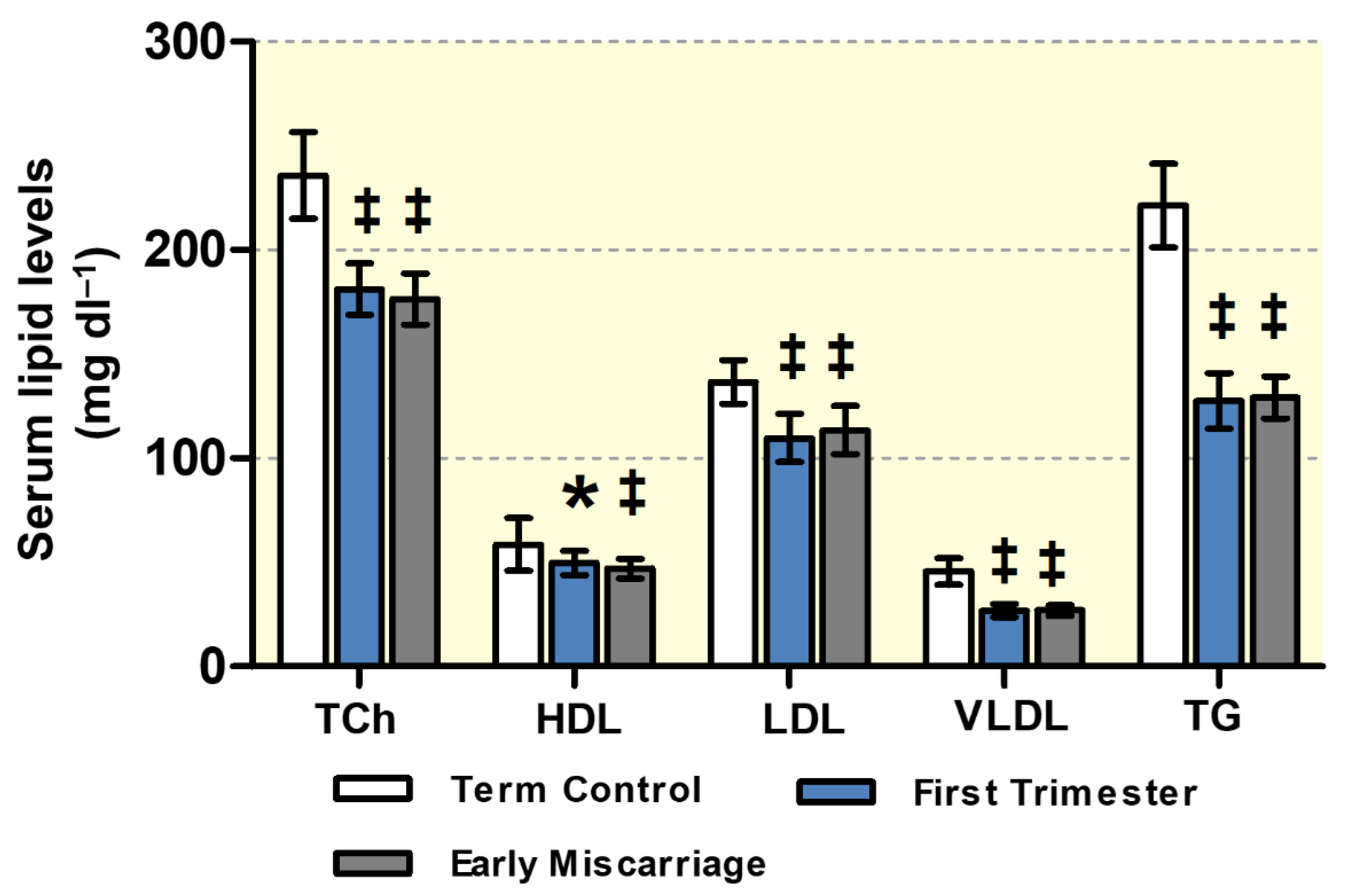
2.3. Determination of Maternal Lipid Levels
2.4. RNA Isolation, Reverse Transcription, and Quantitative RT-PCR Analysis
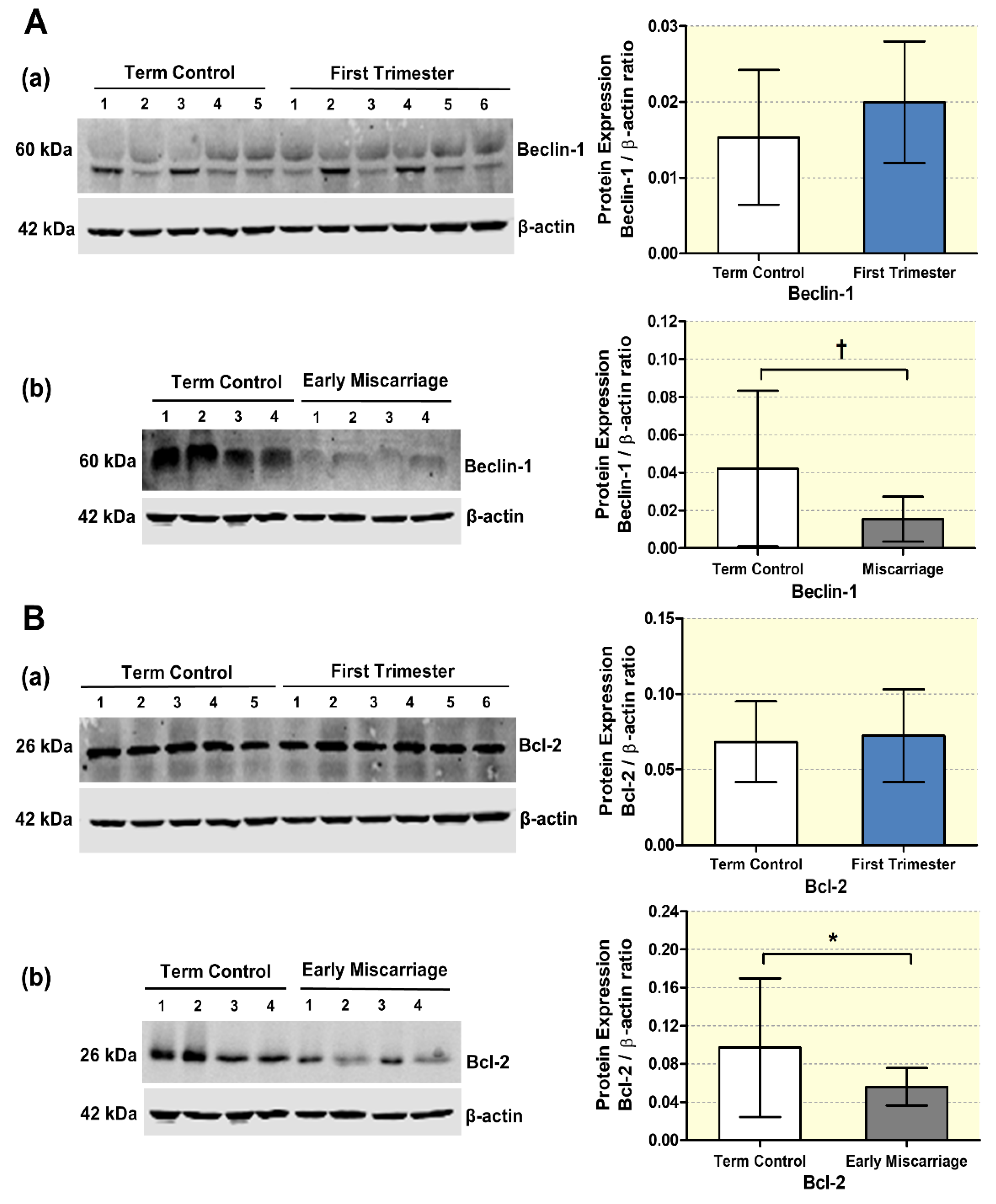
2.5. Assessment of Autophagy and ABC Transporters by Immunoblotting
2.6. Placental Tissue Homogenates for Biochemical Assays
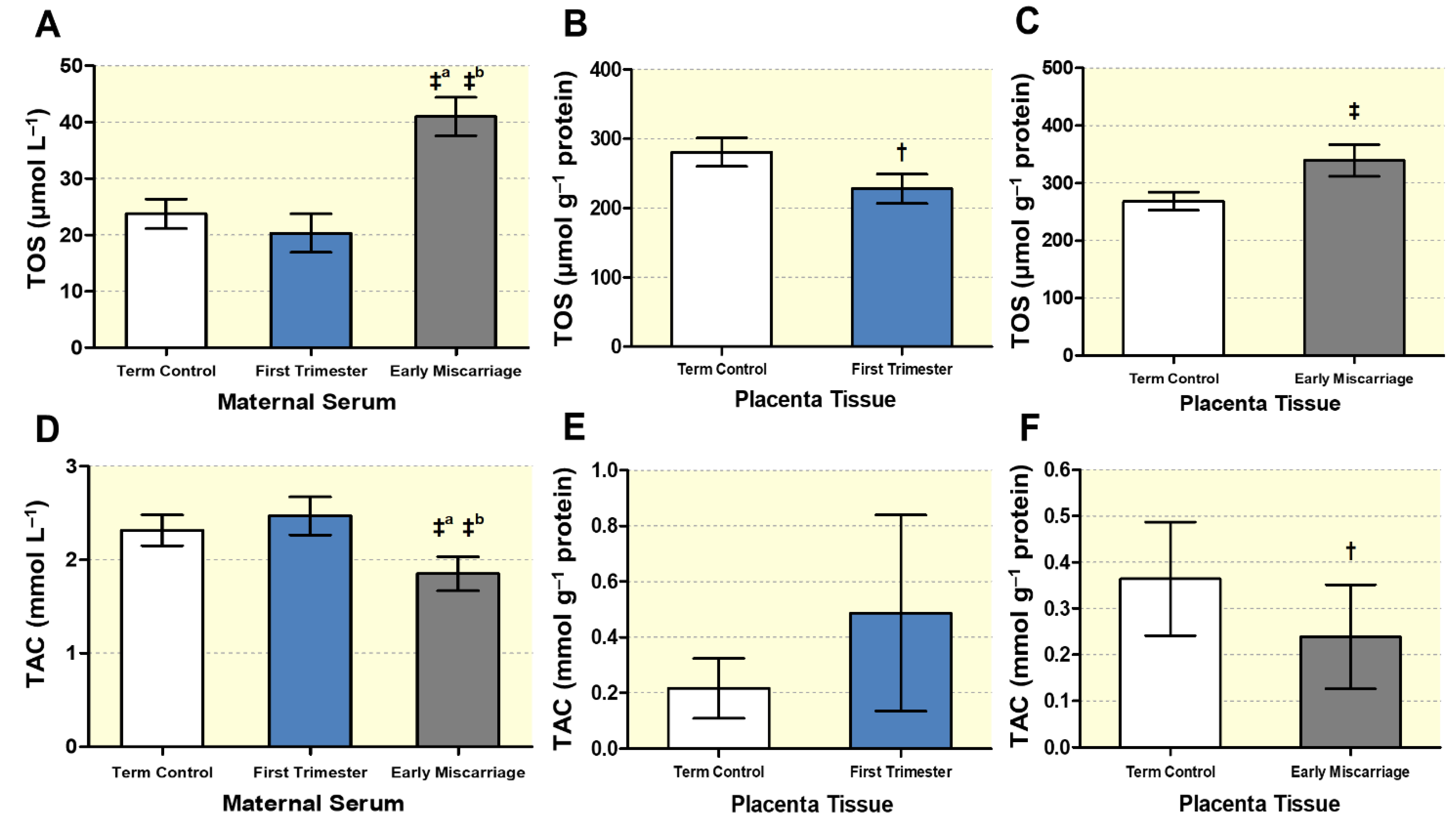
2.7. Total Oxidative Stress Assay (TOS)
2.8. Total Antioxidant Capacity Assay (TAC)
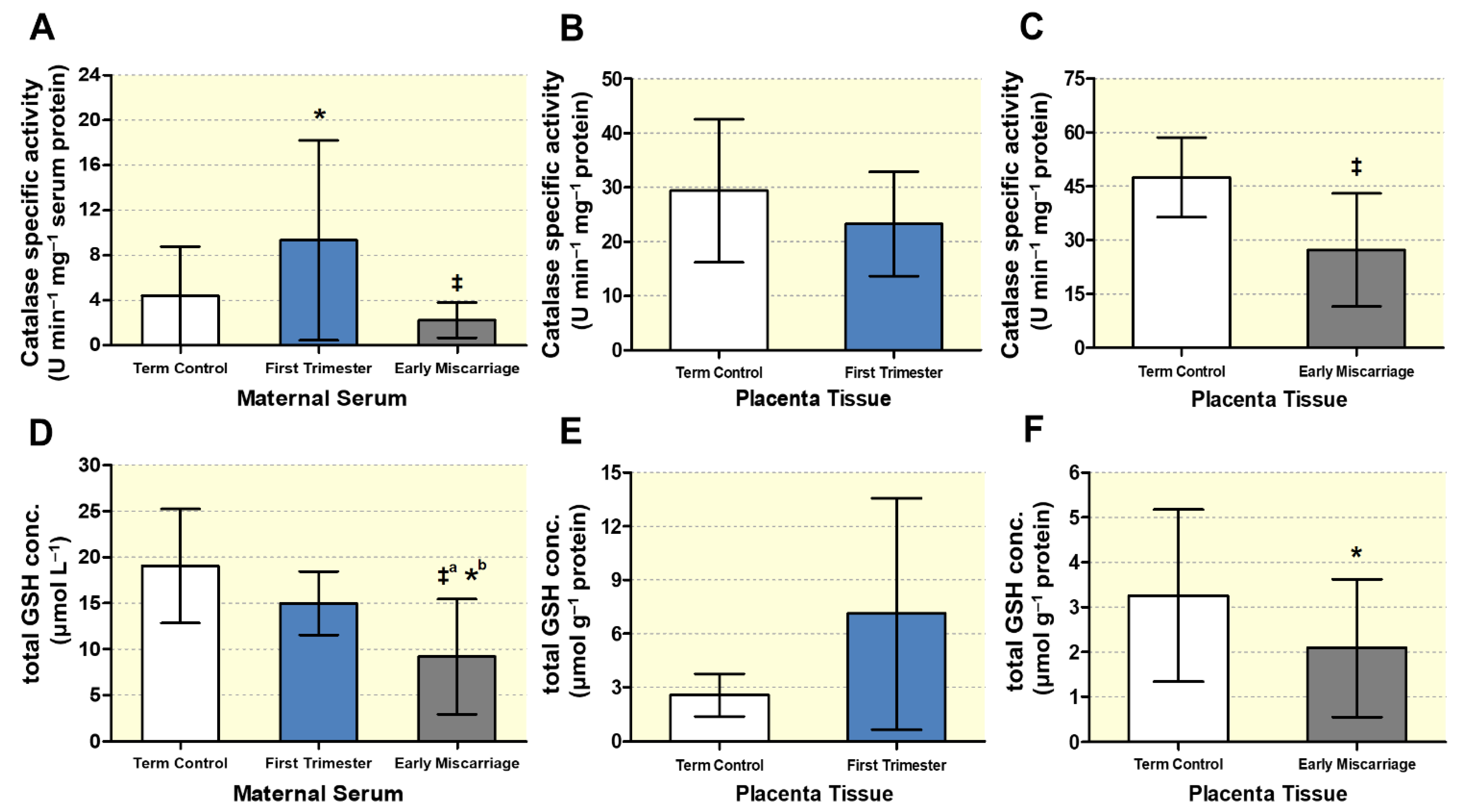
2.9. Enzymatic Antioxidant Assay by Measuring Catalase Activity
2.10. Measurement of Total Glutathione Levels
2.11. Measurement of the DNA Oxidative Stress Marker 8-OHdG
2.12. Measurement of Lipid Peroxidation Products (TBARS)
2.13. Measurement of Protein Carbonylation
2.14. Statistical Analysis
3. Results
3.1. Maternal Lipid Profile and Clinical Parameters of EMC Patients
3.2. mRNA and Protein Expression of Autophagy Markers in EMC
3.3. Altered Maternal Systemic and Placental Oxidative Stress
3.4. Biochemical Markers of DNA, Lipid, and Protein Oxidative Damage
3.5. Altered Expression of ABC Transporters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifert, S.; Altman, K. Miscarriage and Recurrent Pregnancy Loss. In The Johns Hopkins Manual of Gynecology and Obstetrics; Johnson, C.T., Hallock, J.L., Bienstock, J.L., Fox, H.E., Wallach, E.E., Eds.; Wolters Kluwer: Baltimore, MD, USA, 2015; pp. 485–496. ISBN 9781451188806. [Google Scholar]
- Prager, S.; Dalton, V.K.; Allen, R.H. ACOG Practice Bulletin No. 200: Early Pregnancy Loss. Obstet. Gynecol. 2018, 132, e197–e207. [Google Scholar] [CrossRef]
- Greenwold, N.; Jauniaux, E. Collection of villous tissue under ultrasound guidance to improve the cytogenetic study of early pregnancy failure. Hum. Reprod. 2002, 17, 452–456. [Google Scholar] [CrossRef]
- Ali, S.; Majid, S.; Niamat Ali, M.; Taing, S.; El-Serehy, H.A.; Al-Misned, F.A. Evaluation of etiology and pregnancy outcome in recurrent miscarriage patients. Saudi J. Biol. Sci. 2020, 27, 2809–2817. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Emr, S.D. Autophagy as a regulated pathway of cellular degradation. Science 2000, 290, 1717–1721. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Avagliano, L.; Terraneo, L.; Virgili, E.; Martinelli, C.; Doi, P.; Samaja, M.; Bulfamante, G.P.; Marconi, A.M. Autophagy in Normal and Abnormal Early Human Pregnancies. Reprod. Sci. 2015, 22, 1–7. [Google Scholar] [CrossRef]
- Tan, H.X.; Yang, S.L.; Li, M.Q.; Wang, H.Y. Autophagy suppression of trophoblast cells induces pregnancy loss by activating decidual NK cytotoxicity and inhibiting trophoblast invasion. Cell Commun. Signal. 2020, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.-J.; Lin, Y. Oxidative Stress in Placenta: Health and Diseases. Biomed Res. Int. 2015, 2015, 293271. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Oh, S.Y.; Roh, C.R. Autophagy in the placenta. Obstet. Gynecol. Sci. 2017, 60, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.-A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Jauniaux, E.; Greenwold, N.; Hempstock, J.; Burton, G.J. Comparison of ultrasonographic and Doppler mapping of the intervillous circulation in normal and abnormal early pregnancies. Fertil. Steril. 2003, 79, 100–106. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Aouache, R.; Biquard, L.; Vaiman, D.; Miralles, F. Oxidative Stress in Preeclampsia and Placental Diseases. Int. J. Mol. Sci. 2018, 19, 1496. [Google Scholar] [CrossRef]
- Şimşek, M.; Naziroǧlu, M.; Şimşek, H.; Çay, M.; Aksakal, M.; Kumru, S. Blood plasma levels of lipoperoxides, glutathione peroxidase, beta carotene, vitamin A and E in women with habitual abortion. Cell Biochem. Funct. 1998, 16, 227–231. [Google Scholar] [CrossRef]
- NICOL, C.J.; ZIELENSKI, J.; TSUI, L.-C.; WELLS, P.G. An embryoprotective role for glucose-6-phosphate dehydrogenase in developmental oxidative stress and chemical teratogenesis. FASEB J. 2000, 14, 111–127. [Google Scholar] [CrossRef]
- Vural, P.; Akgül, C.; Yildirim, A.; Canbaz, M. Antioxidant defence in recurrent abortion. Clin. Chim. Acta 2000, 295, 169–177. [Google Scholar] [CrossRef]
- Chifenti, B.; Locci, M.T.; Lazzeri, G.; Guagnozzi, M.; Dinucci, D.; Chiellini, F.; Filice, M.E.; Salerno, M.G.; Battini, L. Autophagy-related protein LC3 and Beclin-1 in the first trimester of pregnancy. Clin. Exp. Reprod. Med. 2013, 40, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, X.; An, P.; Dang, H.; Liu, Y.; Liu, R. Effects of resveratrol on autophagy and the expression of inflammasomes in a placental trophoblast oxidative stress model. Life Sci. 2020, 256, 117890. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Singh, A.T.; Keelan, J.A. Transport of lipids by ABC proteins: Interactions and implications for cellular toxicity, viability and function. Chem. Biol. Interact. 2009, 180, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Kaminski, W.E. ABC transporters and cholesterol metabolism. Front. Biosci. 2001, 6. [Google Scholar] [CrossRef]
- Aye, I.L.M.H.; Waddell, B.J.; Mark, P.J.; Keelan, J.A. Placental ABCA1 and ABCG1 transporters efflux cholesterol and protect trophoblasts from oxysterol induced toxicity. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2010, 1801, 1013–1024. [Google Scholar] [CrossRef]
- Nikitina, L.; Wenger, F.; Baumann, M.; Surbek, D.; Körner, M.; Albrecht, C. Expression and localization pattern of ABCA1 in diverse human placental primary cells and tissues. Placenta 2011, 32, 420–430. [Google Scholar] [CrossRef]
- Stefulj, J.; Panzenboeck, U.; Becker, T.; Hirschmugl, B.; Schweinzer, C.; Lang, I.; Marsche, G.; Sadjak, A.; Lang, U.; Desoye, G.; et al. Human endothelial cells of the placental barrier efficiently deliver cholesterol to the fetal circulation via ABCA1 and ABCG1. Circ. Res. 2009, 104, 600–608. [Google Scholar] [CrossRef]
- Ming, J.E.; Roessler, E.; Muenke, M. Human developmental disorders and the Sonic hedgehog pathway. Mol. Med. Today 1998, 4, 343–349. [Google Scholar] [CrossRef]
- Palinski, W. Maternal–Fetal Cholesterol Transport in the Placenta: Good, bad, and target for modulation. Circ. Res. 2009, 104, 569–571. [Google Scholar] [CrossRef]
- Woollett, L.A. Maternal cholesterol in fetal development: Transport of cholesterol from the maternal to the fetal circulation. Am. J. Clin. Nutr. 2005, 82, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Keelan, J.A. Placental ABC transporters, cellular toxicity and stress in pregnancy. Chem. Biol. Interact. 2013, 203, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Maliepaard, M.; Scheffer, G.L.; Faneyte, I.F.; van Gastelen, M.A.; Pijnenborg, A.C.L.M.; Schinkel, A.H.; van de Vijver, M.J.; Scheper, R.J.; Schellens, J.H.M. Subcellular Localization and Distribution of the Breast Cancer Resistance Protein Transporter in Normal Human Tissues. Cancer Res. 2001, 61, 3458. [Google Scholar] [PubMed]
- Ding, R.; Jin, S.; Pabon, K.; Scotto, K.W. A role for ABCG2 beyond drug transport: Regulation of autophagy. Autophagy 2016, 12, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. The Xenobiotic Transporter ABCG2 Plays a Novel Role in Differentiation of Trophoblast-like BeWo Cells. Placenta 2007, 28, S116–S120. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.A.; Murthi, P.; Paxton, J.W.; Reid, G.; Emerald, B.S.; Mohankumar, K.M.; Lobie, P.E.; Brennecke, S.P.; Kalionis, B.; Keelan, J.A. The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 2007, 21, 3592–3605. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Abele, R. An inventory of lysosomal ABC transporters. FEBS Lett. 2020, 594, 3965–3985. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Körner, M.; Huang, X.; Wenger, F.; Surbek, D.; Albrecht, C. Placental ABCA1 and ABCG1 expression in gestational disease: Pre-eclampsia affects ABCA1 levels in syncytiotrophoblasts. Placenta 2013, 34, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Huang, X.; Anderle, P.; Hostettler, L.; Baumann, M.U.; Surbek, D.V.; Ontsouka, E.C.; Albrecht, C. Identification of placental nutrient transporters associated with intrauterine growth restriction and pre-eclampsia. BMC Genomics 2018, 19, 173. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Potter, T.M.; Neun, B.W.; Stern, S.T. Assay to detect lipid peroxidation upon exposure to nanoparticles. Methods Mol. Biol. 2011, 697, 181–189. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, F.; Levine, B. The role of autophagy in mammalian development: Cell makeover rather than cell death. Dev. Cell 2008, 15, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.H.; Hsieh, T.T.A.; Chen, S.F.; Li, M.J.; Yeh, Y.L. Autophagy in the human placenta throughout gestation. PLoS One 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Signorelli, P.; Avagliano, L.; Virgili, E.; Gagliostro, V.; Doi, P.; Braidotti, P.; Bulfamante, G.P.; Ghidoni, R.; Marconi, A.M. Autophagy in term normal human placentas. Placenta 2011, 32, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Tian, F.-J.; Lin, Y.; Xu, W.-M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Korolchuk, V.I.; Renna, M.; Imarisio, S.; Fleming, A.; Williams, A.; Garcia-Arencibia, M.; Rose, C.; Luo, S.; Underwood, B.R.; et al. Complex inhibitory effects of nitric oxide on autophagy. Mol. Cell 2011, 43, 19–32. [Google Scholar] [CrossRef]
- Luo, S.; Rubinsztein, D.C. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: An effect rescued by Bcl-xL. Cell Death Differ. 2010, 17, 268–277. [Google Scholar] [CrossRef]
- Kokawa, K.; Shikone, T.; Nakano, R. Apoptosis in human chorionic villi and decidua during normal embryonic development and spontaneous abortion in the first trimester. Placenta 1998, 19, 21–26. [Google Scholar] [CrossRef]
- Qumsiyeh, M.B.; Kim, K.R.; Ahmed, M.N.; Bradford, W. Cytogenetics and mechanisms of spontaneous abortions: Increased apoptosis and decreased cell proliferation in chromosomally abnormal villi. Cytogenet. Cell Genet. 2000, 88, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka-Tatematsu, M.; Nakashima, A.; Fujita, N.; Shima, T.; Yoshimori, T.; Saito, S. Autophagy Induced by HIF1α Overexpression Supports Trophoblast Invasion by Supplying Cellular Energy. PLoS ONE 2013, 8, e76605. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, H.-J.; Yang, T.-H.; Kim, G.J. Effects of hypoxia inducible factors-1α on autophagy and invasion of trophoblasts. Clin. Exp. Reprod. Med. 2012, 39, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of Oxidative Stress on Pregnancy. Oxid. Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.R.; Jo, Y.H.; Kim, J.; Shin, Y.; Kim, S.S.; Choi, T.G. Roles of Autophagy in Oxidative Stress. Int. J. Mol. Sci. 2020, 21, 3289. [Google Scholar] [CrossRef]
- Lee, Y.J.; Chen, J.C.; Amoscato, A.A.; Bennouna, J.; Spitz, D.R.; Suntharalingam, M.; Rhee, J.G. Protective role of Bcl2 in metabolic oxidative stress-induced cell death. J. Cell Sci. 2001, 114. [Google Scholar] [CrossRef] [PubMed]
- Hockenbery, D.M.; Oltvai, Z.N.; Yin, X.M.; Milliman, C.L.; Korsmeyer, S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993, 75, 241–251. [Google Scholar] [CrossRef]
- Kane, D.J.; Sarafian, T.A.; Anton, R.; Hahn, H.; Gralla, E.B.; Valentine, J.S.; Ord, T.; Bredesen, D.E. Bcl-2 inhibition of neural death: Decreased generation of reactive oxygen species. Science 1993, 262, 1274–1277. [Google Scholar] [CrossRef]
- Susnow, N.; Zeng, L.; Margineantu, D.; Hockenbery, D.M. Bcl-2 family proteins as regulators of oxidative stress. Semin. Cancer Biol. 2009, 19, 42–49. [Google Scholar] [CrossRef]
- Yiyenoğlu, Ö.B.; Uğur, M.G.; Özcan, H.Ç.; Can, G.; Öztürk, E.; Balat, Ö.; Erel, Ö. Assessment of oxidative stress markers in recurrent pregnancy loss: A prospective study. Arch. Gynecol. Obstet. 2014, 289. [Google Scholar] [CrossRef] [PubMed]
- Qanungo, S.; Mukherjea, M. Ontogenic profile of some antioxidants and lipid peroxidation in human placental and fetal tissues. Mol. Cell. Biochem. 2000, 215, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Fowler, P.A.; Garrel, C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int. J. Biochem. Cell Biol. 2010, 42, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Sane, A.S.; Chokshi, S.A.; Mishra, V.V.; Barad, D.P.; Shah, V.C.; Nagpal, S. Serum lipoperoxides in induced and spontaneous abortions. Gynecol. Obstet. Investig. 1991, 31, 172–175. [Google Scholar] [CrossRef]
- Biri, A.; Kavutcu, M.; Bozkurt, N.; Devrim, E.; Nurlu, N.; Durak, I. Investigation of free radical scavenging enzyme activities and lipid peroxidation in human placental tissues with miscarriage. J. Soc. Gynecol. Investig. 2006, 13, 384–388. [Google Scholar] [CrossRef]
- Fujimaki, A.; Watanabe, K.; Mori, T.; Kimura, C.; Shinohara, K.; Wakatsuki, A. Placental oxidative DNA damage and its repair in preeclamptic women with fetal growth restriction. Placenta 2011, 32, 367–372. [Google Scholar] [CrossRef]
- Takagi, Y.; Nikaido, T.; Toki, T.; Kita, N.; Kanai, M.; Ashida, T.; Ohira, S.; Konishi, I. Levels of oxidative stress and redox-related molecules in the placenta in preeclampsia and fetal growth restriction. Virchows Arch. 2004, 444, 49–55. [Google Scholar] [CrossRef]
- Jenkins, C.; Wilson, R.; Roberts, J.; Miller, H.; McKillop, J.H.; Walker, J.J. Antioxidants: Their Role in Pregnancy and Miscarriage. Antioxid. Redox Signal. 2000, 2, 623–628. [Google Scholar] [CrossRef]
- Woollett, L.A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32, S218–S221. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Soumian, S.; Tetlow, N.; Patel, P.; Sullivan, M.H.F.; Lakasing, L.; Nicolaides, K.; Williamson, C. Placental ABCA1 expression is reduced in primary antiphospholipid syndrome compared to pre-eclampsia and controls. Placenta 2007, 28, 701–708. [Google Scholar] [CrossRef]
- Chigusa, Y.; Kondoh, E.; Mogami, H.; Nishimura, F.; Ujita, M.; Kawasaki, K.; Fujita, K.; Tatsumi, K.; Konishi, I. ATP-binding cassette transporter A1 expression is decreased in preeclamptic placentas. Reprod. Sci. 2013, 20, 891–898. [Google Scholar] [CrossRef][Green Version]
- Cheng-Mao, X.; Yan, L.; Li, L.; Hua, J.; Xiao-Ju, W.; Jie-Wen, Z. Placental ABCA1 Expression Is Increased in Spontaneous Preterm Deliveries Compared with Iatrogenic Preterm Deliveries and Term Deliveries. Biomed Res. Int. 2017, 2017, 8248094. [Google Scholar] [CrossRef]
- Christiansen-Weber, T.A.; Voland, J.R.; Wu, Y.; Ngo, K.; Roland, B.L.; Nguyen, S.; Peterson, P.A.; Fung-Leung, W.P. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 2000, 157, 1017–1029. [Google Scholar] [CrossRef]
- Fuenzalida, B.; Cantin, C.; Kallol, S.; Carvajal, L.; Pastén, V.; Contreras-Duarte, S.; Albrecht, C.; Gutierrez, J.; Leiva, A. Cholesterol uptake and efflux are impaired in human trophoblast cells from pregnancies with maternal supraphysiological hypercholesterolemia. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Baardman, M.E.; Kerstjens-Frederikse, W.S.; Berger, R.M.F.; Bakker, M.K.; Hofstra, R.M.W.; Plösch, T. The Role of Maternal-Fetal Cholesterol Transport in Early Fetal Life: Current Insights1. Biol. Reprod. 2013, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.S.; Pitkin, R.M.; Connor, W.E. Placental transfer of cholesterol into the human fetus. Am. J. Obstet. Gynecol. 1977, 128, 735–739. [Google Scholar] [CrossRef]
- Edison, R.J.; Berg, K.; Remaley, A.; Kelley, R.; Rotimi, C.; Stevenson, R.E.; Muenke, M. Adverse birth outcome among mothers with low serum cholesterol. Pediatrics 2007, 120, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Greer, I.A.; Galloway, P.J.; Packard, C.J.; Shepherd, J.; Kelly, T.; Mathers, A. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J. Clin. Endocrinol. Metab. 1999, 84, 128–130. [Google Scholar] [CrossRef]
- Chiang, A.N.; Yang, M.L.; Hung, J.H.; Chou, P.; Shyn, S.K.; Ng, H.T. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci. 1995, 56, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Valbuena-Diez, A.C.; Blanco, F.J.; Oujo, B.; Langa, C.; Gonzalez-Nuñez, M.; Llano, E.; Pendas, A.M.; Díaz, M.; Castrillo, A.; Lopez-Novoa, J.M.; et al. Oxysterol-induced soluble endoglin release and its involvement in hypertension. Circulation 2012, 126, 2612–2624. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.A.; Paxton, J.W.; Keelan, J.A. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R1357–R1365. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Huang, Y.; Shi, M.; Qian, X.; Li, H.; Peng, C.; Kong, B.; Zou, X.; Shen, S. Protective role of ABCG2 against oxidative stress in colorectal cancer and its potential underlying mechanism. Oncol. Rep. 2018, 40, 2137–2146. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Term Control | First Trimester | EMC |
|---|---|---|---|
| Number of individuals | 19 | 20 | 20 |
| Delivery mode/type | Cesarean section (17) Vaginal delivery (2) | Incomplete (EMC) | |
| Gestational age (weeks) | 37.52 ± 2.1 | 10.11 ± 1.9 ‡ | 10.15 ± 1.9 ‡ |
| Parity | 2.68 ± 1.77 | 1.00 ± 0.79 † | 1.35 ± 1.73 † |
| Maternal age (years) | 28.8 ± 4.0 (23–35) | 28.3 ± 4.8 (20–35) | 28.0 ± 4.999 (20–35) |
| Height (cm) | 163.42 ± 1.81 | 164.54 ± 1.24 | 163.86 ± 2.41 |
| Weight pregravid (kg) | 65.11 ± 7.5 | 62.19 ± 5.7 | 62.78 ± 7.2 |
| BMI pregravid (kg m−2) | 24.4 ± 2.8 | 23.0 ± 2.0 | 23.4 ± 2.7 |
| Weight (kg) peripartum | 78.3 ± 6.9 | 65.6 ± 5.8 ‡ | 66.28 ± 7.4 ‡ |
| BMI (kg m−2) peripartum | 29.3 ± 2.6 | 24.2 ± 2 ‡ | 24.7 ± 2.7 ‡ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahnawaz, S.; Nawaz, U.S.; Zaugg, J.; Hussain, G.; Malik, N.; Dogar, M.Z.-u.-H.; Malik, S.A.; Albrecht, C. Dysregulated Autophagy Leads to Oxidative Stress and Aberrant Expression of ABC Transporters in Women with Early Miscarriage. Antioxidants 2021, 10, 1742. https://doi.org/10.3390/antiox10111742
Shahnawaz S, Nawaz US, Zaugg J, Hussain G, Malik N, Dogar MZ-u-H, Malik SA, Albrecht C. Dysregulated Autophagy Leads to Oxidative Stress and Aberrant Expression of ABC Transporters in Women with Early Miscarriage. Antioxidants. 2021; 10(11):1742. https://doi.org/10.3390/antiox10111742
Chicago/Turabian StyleShahnawaz, Saira, Usman Shah Nawaz, Jonas Zaugg, Ghulam Hussain, Nadia Malik, Muhammad Zahoor-ul-Hassan Dogar, Shoaib Ahmad Malik, and Christiane Albrecht. 2021. "Dysregulated Autophagy Leads to Oxidative Stress and Aberrant Expression of ABC Transporters in Women with Early Miscarriage" Antioxidants 10, no. 11: 1742. https://doi.org/10.3390/antiox10111742
APA StyleShahnawaz, S., Nawaz, U. S., Zaugg, J., Hussain, G., Malik, N., Dogar, M. Z.-u.-H., Malik, S. A., & Albrecht, C. (2021). Dysregulated Autophagy Leads to Oxidative Stress and Aberrant Expression of ABC Transporters in Women with Early Miscarriage. Antioxidants, 10(11), 1742. https://doi.org/10.3390/antiox10111742






