Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. The Preparation of Polyphenol Extract from Blumea laciniata (EBL)
2.3. C. elegans Strains and Maintenance
2.4. The Food Clearance Assay
2.5. Fertility Assay
2.6. Thermal Stress Assay
2.7. Assessment of Lifespan
2.8. Assessment of the Level of ROS
2.9. Assessment of the Activities of Antioxidant Enzymes and the Content of MDA
2.10. Visualization of the DAF-16 Transcription Factor
2.11. Statistics
3. Results
3.1. EBL Showed No Toxicity on C. elegans
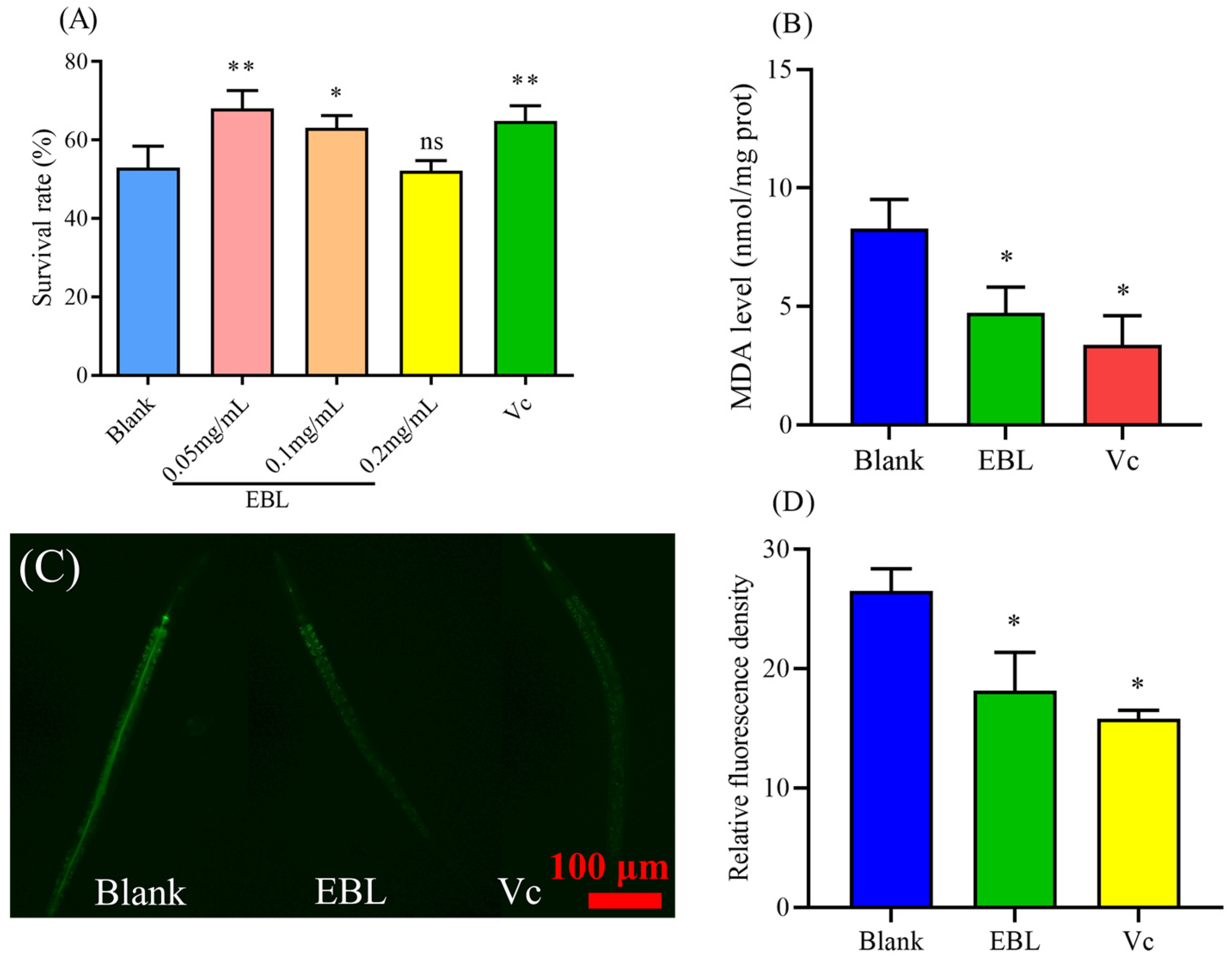
3.2. EBL Enhanced the Survival of C. elegans under Thermal Stress
3.3. EBL Reduced the Content of ROS and MDA in C. elegans under Thermal Stress
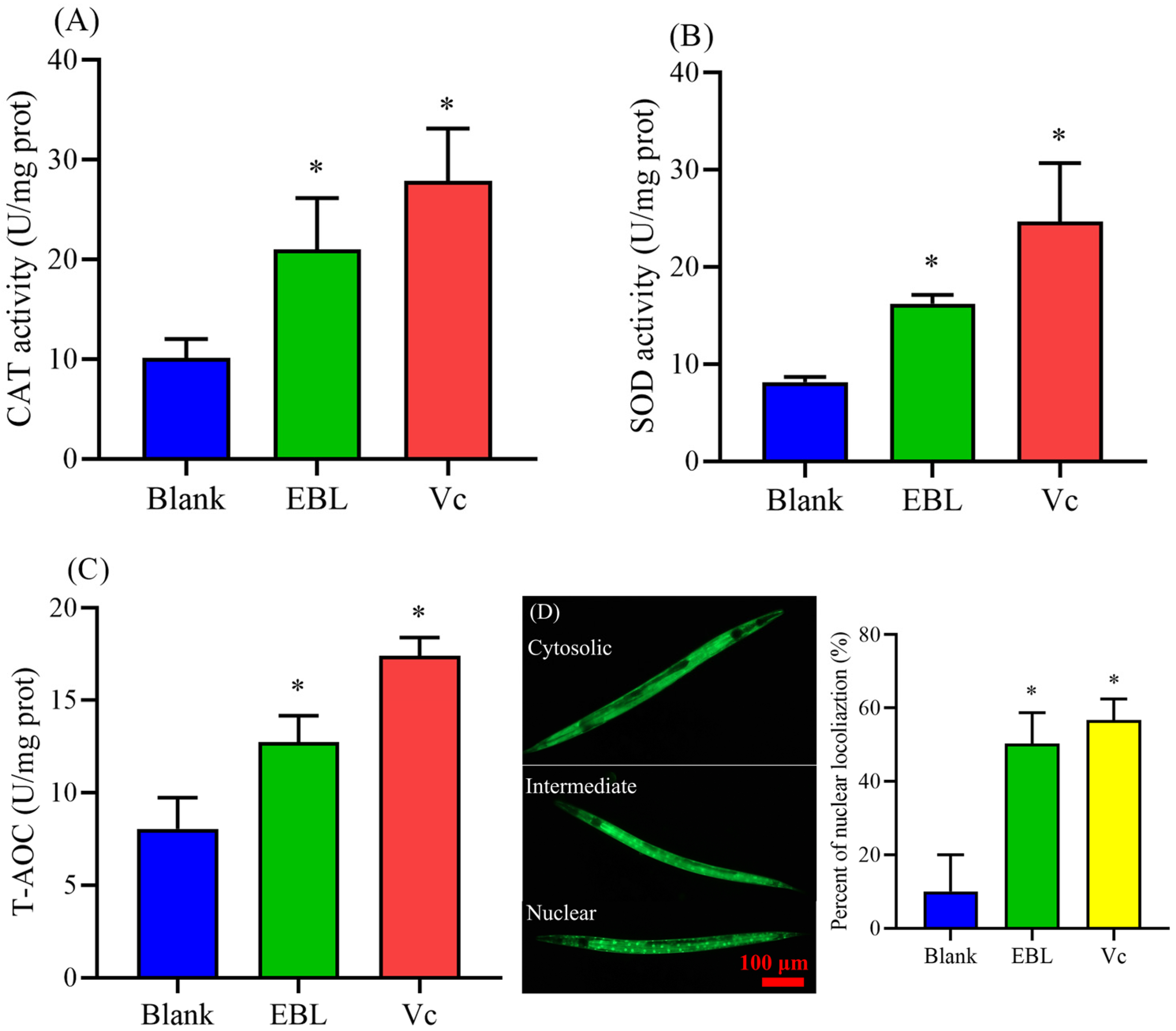
3.4. EBL Increased the Activities of Antioxidant Enzymes in C. elegans under Thermal Stress
3.5. EBL Promoted the Localization of DAF-16 to the Nucleus
3.6. EBL Failed to Increase the Survival of Mutants
3.7. EBL Extended the Lifespan of C. elegans
3.8. EBL Failed to Extend the Lifespan of Mutants
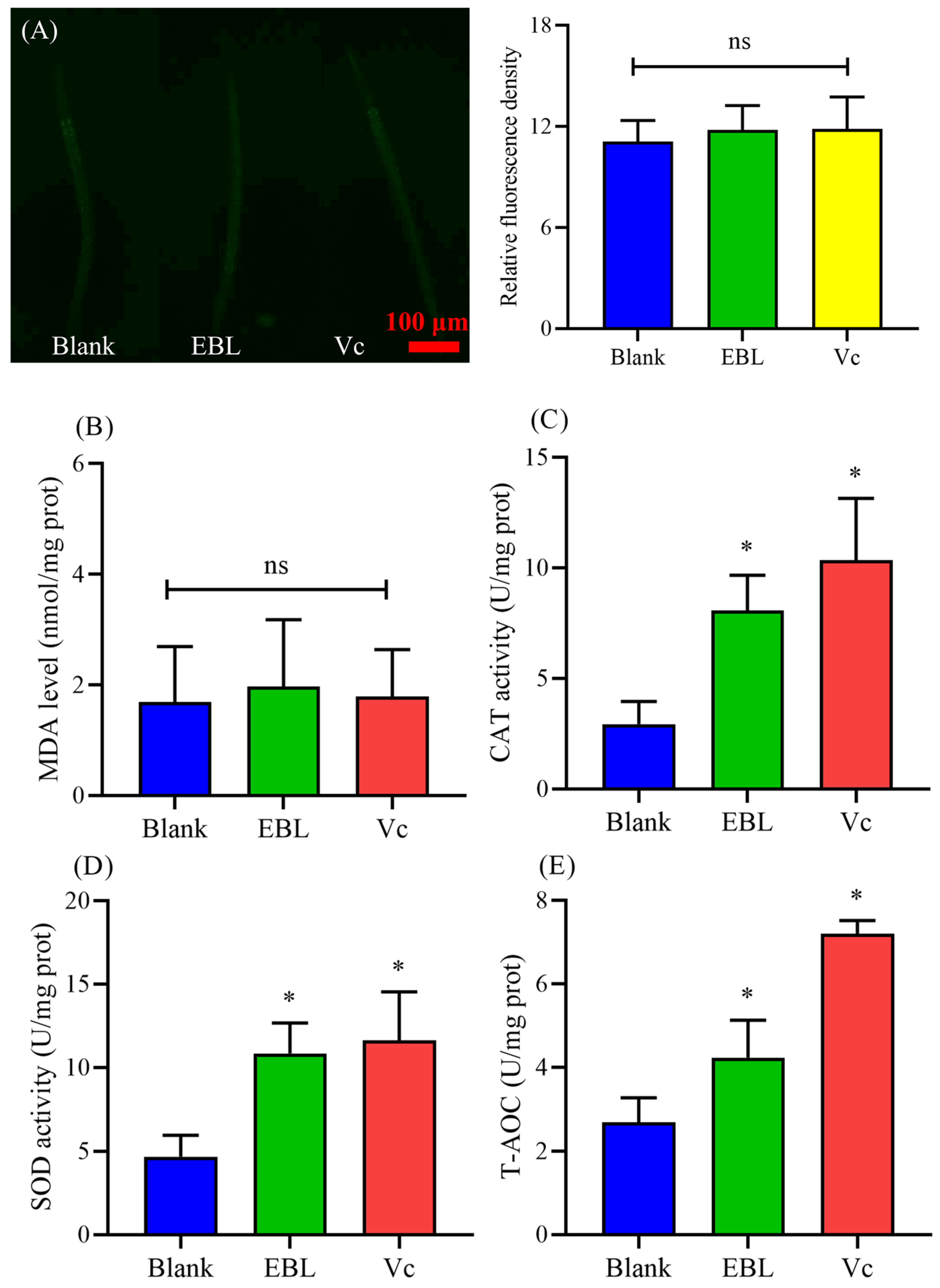
3.9. EBL Kept a Normal Level of ROS and MDA in C. elegans under Normal Conditions
3.10. EBL Increased the Antioxidant Ability of C. elegans under Normal Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eren, B.; Tanrıverdi, S.T.; Köse, F.A.; Özer, Ö. Antioxidant properties evaluation of topical astaxanthin formulations as anti-aging products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Bergamini, E.; Cavallini, G.; Donati, A.; Gori, Z. The role of macroautophagy in the ageing process, anti-ageing intervention and age-associated diseases. Int. J. Biochem. Cell Biol. 2004, 36, 2392–2404. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, X.; Nunomura, A.; Moreira, P.I.; Lee, H.; Perry, G.; Smith, M.A.; Zhu, X. Oxidative stress signaling in Alzheimer’s disease. Curr. Alzheimer Res. 2008, 5, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.K. The elderly, use of antioxidants and memory. Oxidative Stress Diet. Antioxid. Neurol. Dis. 2020, 12, 175–188. [Google Scholar] [CrossRef]
- Marchi, S.; Giorgi, C.; Suski, J.M.; Agnoletto, C.; Bononi, A.; Bonora, M.; Marchi, E.D.; Missiroli, S.; Patergnani, S.; Poletti, F.; et al. Mitochondria-Ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2011, 2012, 329635. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.B.; Shin, G.H.; Kim, J.M.; Kim, Y.H.; Lee, J.H.; Lee, J.S.; Song, H.J.; Choe, S.Y.; Park, I.J.; Cho, J.H.; et al. Antioxidant and anti-ageing activities of citrus-based juice mixture. Food Chem. 2016, 1, 920–927. [Google Scholar] [CrossRef]
- Zeng, R.; Farooq, M.U.; Zhang, G.; Tang, Z.; Zheng, T.; Su, Y.; Hussain, S.; Liang, Y.; Ye, X.; Jia, X.; et al. Dissecting the potential of selenoproteins extracted from selenium-enriched rice on physiological, biochemical and anti-ageing effects in vivo. Biol. Trace Elem. Res. 2019, 196, 119–130. [Google Scholar] [CrossRef]
- Stavropoulou, M.-I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. Anal. 2021, 194, 113814. [Google Scholar] [CrossRef]
- Carine, S. Natural antioxidants in prevention of accelerated ageing: A departure from conventional paradigms required. J. Physiol. Biochem. 2018, 74, 549–558. [Google Scholar] [CrossRef]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.-R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef]
- Petrascheck, M.; Ye, X.; Buck, L.B. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 2007, 450, 553–556. [Google Scholar] [CrossRef]
- Kimura, K.D.; Tissenbaum, H.A.; Liu, Y.; Ruvkun, G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 1997, 277, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.; Sternberg, P.; Durbin, R.; Mieg, J.T.; Spieth, J. WormBase: Network access to the genome and biology of Caenorhabditis elegans. Nucleic Acids Res. 2001, 29, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Phu, H.T.; Thuan, D.T.B.; Nguyen, T.H.D.; Posadino, A.M.; Eid, A.H.; Pintus, G. Herbal medicine for slowing aging and aging-associated conditions: Efficacy, mechanisms, and safety. Curr. Vasc. Pharmacol. 2020, 18, 369–393. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Li, T.; Liu, R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018, 9, 5273–5282. [Google Scholar] [CrossRef]
- Rangsinth, P.; Prasansuklab, A.; Duangjan, C.; Gu, X.; Meemon, K.; Wink, M.; Tencomnao, T. Leaf extract of Caesalpinia mimosoides enhances oxidative stress resistance and prolongs lifespan in Caenorhabditis elegans. BMC Complement. Altern. Med. 2019, 19, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, G.D.; Brunetti, G.; Scuto, M.; Salinaro, A.T.; Calabrese, E.J.; Crea, R.; Schmitz-Linneweber, C.; Calabrese, V.; Saul, N. Healthspan enhancement by olive polyphenols in C. elegans wild type and Parkinson’s models. Int. J. Mol. Sci. 2020, 21, 3893. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Amin, M.A.; Zhao, C.; An, F.; Wang, Y.; Huang, Q.; Teng, H.; Song, H. Catechinic acid, a natural polyphenol compound, extends the lifespan of Caenorhabditis elegans via mitophagy pathways. Food Funct. 2020, 11, 5621–5634. [Google Scholar] [CrossRef]
- Dhanjal, D.S.; Bhardwaj, S.; Sharma, R.; Bhardwaj, K.; Kumar, D.; Chopra, C.; Nepovimova, E.; Singh, R.; Kuca, K. Plant fortification of the diet for anti-ageing effects: A review. Nutrients 2020, 12, 3008. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, S.; Li, J.; Zhou, Y.; Chen, T.; Feng, S.; Ding, C. Simultaneous optimization of extraction and antioxidant activity from Blumea laciniata and the protective effect on Hela cells against oxidative damage. Arab. J. Chem. 2020, 13, 9231–9242. [Google Scholar] [CrossRef]
- Luo, S.; Jiang, X.; Jia, L.; Tan, C.; Li, M.; Yang, Q.; Du, Y.; Ding, C. In vivo and in vitro antioxidant activities of methanol extracts from olive leaves on Caenorhabditis elegans. Molecules 2019, 24, 704. [Google Scholar] [CrossRef] [Green Version]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiou, C.D.; Grintzalis, K.; Zervoudakis, G.; Papapostolou, I. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 391, 391–403. [Google Scholar] [CrossRef]
- Ma, X.; Li, J.; Cui, X.; Li, C.; Wang, Z. Dietary supplementation with peptides from sesame cake alleviates Parkinson’s associated pathologies in Caenorhabditis elegans. J. Funct. Foods 2019, 65, 103737. [Google Scholar] [CrossRef]
- Dues, D.J.; Andrews, E.K.; Schaar, C.E.; Bergsma, A.L.; Senchuk, M.M.; Raamsdonk, J.M.V. Aging causes decreased resistance to multiple stresses and a failure to activate specific stress response pathways. Aging 2016, 8, 777–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Yu, Y.; Lin, H.; Liao, D.; Cui, X.; Wang, H. Lonicera japonica extends lifespan and healthspan in Caenorhabditis elegans. Free Radic. Biol. Med. 2018, 129, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zheng, B.; Li, T.; Liu, R.H. Raspberry extract promoted longevity and stress tolerance via the insulin/IGF signaling pathway and DAF-16 in Caenorhabditis elegans. Food Funct. 2020, 11, 3598–3609. [Google Scholar] [CrossRef]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Su, Z.; Luo, J.; Jiang, L.; Shen, S.; Zheng, W.; Gu, W.; Cao, Y.; Chen, Y. Polysaccharide extracted from the leaves of Cyclocarya paliurus (Batal.) Iljinskaja enhanced stress resistance in Caenorhabditis elegans via skn-1 and hsf-1. Int. J. Biol. Macromol. 2019, 143, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 target genes that control C. elegans life-span and metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandarakis, E.D.; Dattilo, M.; Macut, D.; Duntas, L.; Gonos, E.S.; Goulis, D.G.; Gantenbein, C.K.; Kapetanou, M.; Koukkou, E.; Lambrinoudaki, I.; et al. Mechanisms in endocrinology: Aging and anti-aging: A Combo-Endocrinology overview. Eur. J. Endocrinol. 2017, 176, 283–308. [Google Scholar] [CrossRef]
- Durán, B.A.; Manzano, S.G.; Sánchez, I.G.; Arribas, M.V.M.; Bartolomé, B.; Buenhombre, M.S.; Guadarrama, A.; Buelga, C.S.; Paramás, A.M.G. Antioxidant characterization and biological effects of grape pomace Extracts supplementation in Caenorhabditis elegans. Foods 2019, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Wink, M.; Tencomnao, T. Lifespan extending and oxidative stress resistance properties of a leaf extracts from Anacardium occidentale L. in Caenorhabditis elegans. Oxid. Med. Cell. Longev. 2019, 2019, 9012396. [Google Scholar] [CrossRef] [Green Version]
- Saier, C.; Gommlich, I.; Hiemann, V.; Baier, S.; Koch, K.; Horn, G.; Kowalewsky, T.; Bartelt, J.; Seemann, M.; Wätjen, W. Agrimonia procera Wallr. extract increases stress resistance and prolongs life span in Caenorhabditis elegans via transcription factor DAF-16 (FoxO Orthologue). Antioxidants 2018, 7, 192. [Google Scholar] [CrossRef] [Green Version]
- Lapierre, L.R.; Hansen, M. Lessons from C. elegans: Signaling pathways for longevity. Trends Endocrinol. Metab. 2012, 23, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tullet, J.M.A.; Green, J.W.; Au, C.; Benedetto, A.; Thompson, M.A.; Clark, E.; Gilliat, A.F.; Young, A.; Schmeisser, K.; Gems, D. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 2017, 16, 1191–1194. [Google Scholar] [CrossRef] [Green Version]
- Carranza, A.D.V.; Saragusti, A.; Chiabrando, G.A.; Carrari, F.; Asis, R. Effects of chlorogenic acid on thermal stress tolerance in C. elegans via HIF-1, HSF-1 and autophagy. Phytomedicine 2019, 66, 153132. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, X.; Zhuang, C.; Lin, Y.; Cao, Y.; Chen, Y. Healthspan improvements in Caenorhabditis elegans with traditional chinese herbal tea. Oxid. Med. Cell. Longev. 2020, 2020, 4057841. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central organelles for melatonin′s antioxidant and anti-aging actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Wang, Z. Which is the most reasonable anti-aging strategy: Meta-analysis. Adv. Exp. Med. Biol. 2018, 1086, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.W.; Cheon, S.; Nam, M.S.; Kim, K.K. Alpha-Casein and Beta-Lactoglobulin from cow milk exhibit antioxidant activity: A plausible link to antiaging effects. J. Food Sci. 2019, 84, 3083–3090. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Guan, Y.; Rui, X.; Zhang, Y.; Dong, M.; Ma, W. An aqueous polyphenol extract from Rosa rugosa tea has antiaging effects on Caenorhabditis elegans. J. Food Biochem. 2019, 43, e12796. [Google Scholar] [CrossRef]
- Zheng, S.; Huang, X.; Xing, T.; Ding, A.; Wu, G.; Luo, H. Chlorogenic acid extends the lifespan of Caenorhabditis elegans via Insulin/IGF-1 signaling pathway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [Green Version]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.V.; Puntel, R.L.; Ávila, D.S. Resveratrol attenuates iron-induced toxicity in a chronic post-treatment paradigm in Caenorhabditis elegans. Free Radic. Res. 2018, 52, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Chen, W.; Jia, R.; Ye, X.; Li, Y.; Liu, Y.; Jiang, Y.; Zheng, X. Tetrastigma hemsleyanum leaves extract against acrylamide-induced toxicity in HepG2 cells and Caenorhabditis elegans. J. Hazard. Mater. 2020, 393, 122364. [Google Scholar] [CrossRef] [PubMed]



| Stains | Treatment | Numbers of Trials | Mean Lifespan (Days) | Significance |
|---|---|---|---|---|
| Wild-type | - | 158 | 16.33 ± 0.58 | |
| 0.05 mg/mL of EBL | 161 | 17.00 ± 1.00 | ns | |
| 0.10 mg/mL of EBL | 139 | 19.17 ± 1.61 | * | |
| 0.20 mg/mL of EBL | 126 | 16.50 ± 0.87 | ns | |
| TJ1052 age-1 (hx546) | - | 84 | 23.33 ± 1.16 | |
| 0.10 mg/mL of EBL | 104 | 23.33 ± 0.76 | ns | |
| CF1038 daf-16(mu86) | - | 141 | 12.67 ± 0.58 | |
| 0.10 mg/mL of EBL | 157 | 12.67 ± 0.43 | ns | |
| CB1370 daf-2 (e1370) | - | 187 | 23.33 ± 1.15 | |
| 0.10 mg/mL of EBL | 197 | 23.67 ± 0.58 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Luo, S.; Wang, X.; Zhou, Y.; Dai, Y.; Zhou, L.; Feng, S.; Yuan, M.; Ding, C. Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants 2021, 10, 1744. https://doi.org/10.3390/antiox10111744
Chen T, Luo S, Wang X, Zhou Y, Dai Y, Zhou L, Feng S, Yuan M, Ding C. Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants. 2021; 10(11):1744. https://doi.org/10.3390/antiox10111744
Chicago/Turabian StyleChen, Tao, Siyuan Luo, Xiaoju Wang, Yiling Zhou, Yali Dai, Lijun Zhou, Shiling Feng, Ming Yuan, and Chunbang Ding. 2021. "Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway" Antioxidants 10, no. 11: 1744. https://doi.org/10.3390/antiox10111744
APA StyleChen, T., Luo, S., Wang, X., Zhou, Y., Dai, Y., Zhou, L., Feng, S., Yuan, M., & Ding, C. (2021). Polyphenols from Blumea laciniata Extended the Lifespan and Enhanced Resistance to Stress in Caenorhabditis elegans via the Insulin Signaling Pathway. Antioxidants, 10(11), 1744. https://doi.org/10.3390/antiox10111744









