Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Design
2.2. Sampling
2.3. Biochemical Analysis
2.4. Protein Analyses
2.5. Statistical Analysis
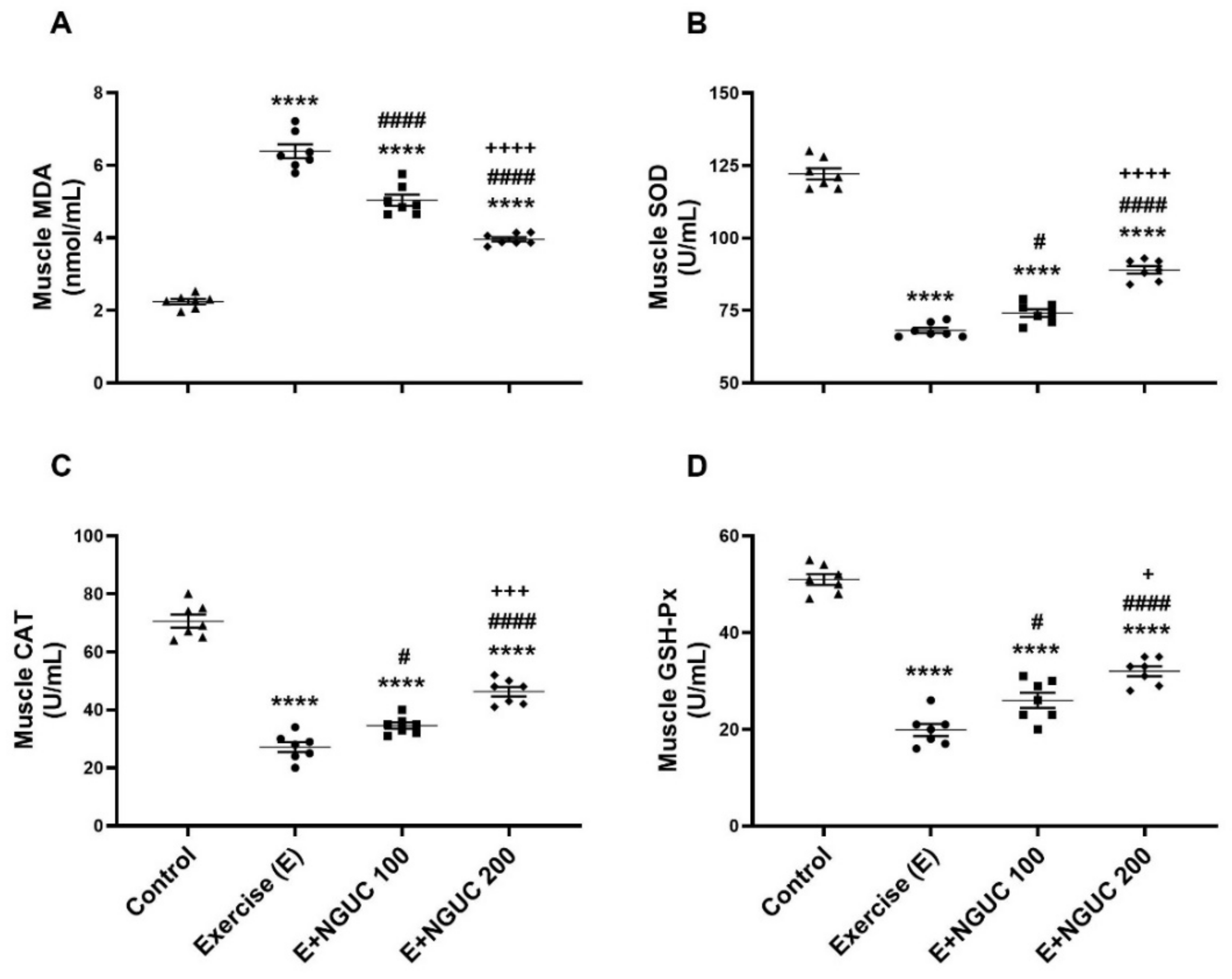
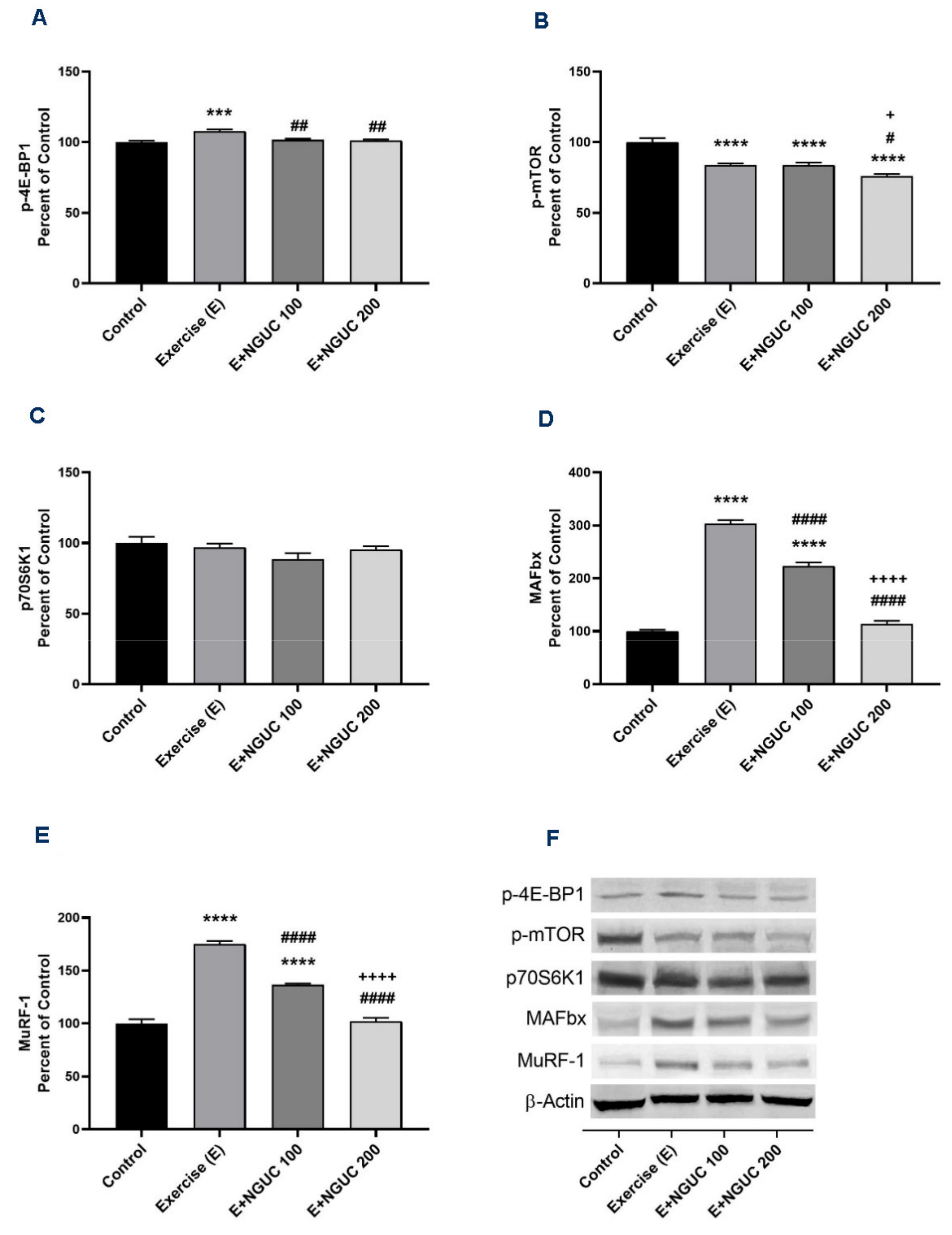
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muñoz, V.R.; Gaspar, R.C.; Esteca, M.V.; Baptista, I.L.; Vieira, R.F.L.; da Silva, A.S.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; Pauli, J.R. Physical exercise increases ROCK activity in the skeletal muscle of middle-aged rats. Mech. Ageing Dev. 2020, 186, 111213. [Google Scholar] [CrossRef]
- Yang, D.K.; Lee, S.-J.; Adam, G.O.; Kim, S.-J. Aralia continentalis kitagawa Extract Attenuates the Fatigue Induced by Exhaustive Exercise through Inhibition of Oxidative Stress. Antioxidants 2020, 9, 379. [Google Scholar] [CrossRef]
- Kemmler, W.; Von Stengel, S.; Engelke, K.; Kalender, W.A. Exercise decreases the risk of metabolic syndrome in elderly females. Med. Sci. Sports Exerc. 2009, 41, 297–305. [Google Scholar] [CrossRef]
- Silva, E., Jr.; Borges, L.; Mendes-da-Silva, C.; Hirabara, S.; Lambertucci, R. l-Arginine supplementation improves rats’ antioxidant system and exercise performance. Free Radic. Res. 2017, 51, 281–293. [Google Scholar] [CrossRef]
- Tominaga, T.; Ikemura, T.; Yada, K.; Kanda, K.; Sugama, K.; Ma, S.; Choi, W.; Araya, M.; Huang, J.; Nakamura, N.; et al. The Effects of Beverage Intake after Exhaustive Exercise on Organ Damage, Inflammation and Oxidative Stress in Healthy Males. Antioxidants 2021, 10, 866. [Google Scholar] [CrossRef]
- Liu, L.; Wu, X.; Zhang, B.; Yang, W.; Li, D.; Dong, Y.; Yin, Y.; Chen, Q. Protective effects of tea polyphenols on exhaustive exercise-induced fatigue, inflammation and tissue damage. Food Nutr. Res. 2017, 61, 1333390. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Li, J.; Liu, Z.; Chuang, C.C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Basham, S.A.; Waldman, H.S.; Krings, B.M.; Lamberth, J.; Smith, J.W.; McAllister, M.J. Effect of Curcumin Supplementation on Exercise-Induced Oxidative Stress, Inflammation, Muscle Damage, and Muscle Soreness. J. Diet. Suppl. 2020, 17, 401–414. [Google Scholar] [CrossRef]
- Salucci, S.; Falcieri, E. Polyphenols and their potential role in preventing skeletal muscle atrophy. Nutr. Res. 2020, 74, 10–22. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [Green Version]
- Yabas, M.; Orhan, C.; Er, B.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Bhanuse, P.; Morde, A.A.; Padigaru, M.; et al. A Next Generation Formulation of Curcumin Ameliorates Experimentally Induced Osteoarthritis in Rats via Regulation of Inflammatory Mediators. Front. Immunol. 2021, 12, 157. [Google Scholar] [CrossRef]
- Sahin, K.; Pala, R.; Tuzcu, M.; Ozdemir, O.; Orhan, C.; Sahin, N.; Juturu, V. Curcumin prevents muscle damage by regulating NF-κB and Nrf2 pathways and improves performance: An in vivo model. J. Inflamm. Res. 2016, 9, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Ono, T.; Takada, S.; Kinugawa, S.; Tsutsui, H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp. Physiol. 2015, 100, 1052–1063. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Li, Y.-P. Curcumin prevents lipopolysaccharide-induced atrogin-1/MAFbx upregulation and muscle mass loss. J. Cell Biochem. 2007, 100, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [Green Version]
- Tønnesen, H.H.; Karlsen, J. Studies on curcumin and curcuminoids. Z. Lebensm. Unters. Forsch. 1985, 180, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Tuzcu, Z.; Orhan, C.; Sahin, N.; Juturu, V.; Sahin, K. Cinnamon Polyphenol Extract Inhibits Hyperlipidemia and Inflammation by Modulation of Transcription Factors in High-Fat Diet-Fed Rats. Oxid. Med. Cell. Longev. 2017, 2017, 1583098. [Google Scholar] [CrossRef]
- Amalraj, A.; Divya, C.; Gopi, S. The Effects of Bioavailable Curcumin (Cureit) on Delayed Onset Muscle Soreness Induced by Eccentric Continuous Exercise: A Randomized, Placebo-Controlled, Double-Blind Clinical Study. J. Med. Food 2020, 23, 545–553. [Google Scholar] [CrossRef]
- Mallard, A.R.; Briskey, D.; Richards, B.A.; Rao, A. Curcumin Improves Delayed Onset Muscle Soreness and Postexercise Lactate Accumulation. J. Diet Suppl. 2020, 18, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Chiu, W.C.; Chiu, Y.S.; Li, T.; Sung, H.C.; Hsiao, C.Y. Supplementation of nano-bubble curcumin extract improves gut microbiota composition and exercise performance in mice. Food Funct. 2020, 11, 3574–3584. [Google Scholar] [CrossRef]
- Campbell, M.S.; Carlini, N.A.; Fleenor, B.S. Influence of curcumin on performance and post-exercise recovery. Crit. Rev. Food Sci. Nutr. 2021, 61, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef] [Green Version]
- Vainshtein, A.; Tryon, L.D.; Pauly, M.; Hood, D.A. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef] [Green Version]
- Brandt, N.; Dethlefsen, M.M.; Bangsbo, J.; Pilegaard, H. PGC-1α and exercise intensity dependent adaptations in mouse skeletal muscle. PLoS ONE 2017, 12, e0185993. [Google Scholar] [CrossRef] [Green Version]
- Hamidie, R.D.R.; Shibaguchi, T.; Yamada, T.; Koma, R.; Ishizawa, R.; Saito, Y.; Hosoi, T.; Masuda, K. Curcumin induces mitochondrial biogenesis by increasing cyclic AMP levels via phosphodiesterase 4A inhibition in skeletal muscle. Br. J. Nutr. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Hamidie, R.D.R.; Yamada, T.; Ishizawa, R.; Saito, Y.; Masuda, K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 2015, 64, 1334–1347. [Google Scholar] [CrossRef]
- Kang, C.; O’Moore, K.M.; Dickman, J.R.; Ji, L.L. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic. Biol. Med. 2009, 47, 1394–1400. [Google Scholar] [CrossRef]
- Khani, M.; Motamedi, P.; Dehkhoda, M.R.; Dabagh Nikukheslat, S.; Karimi, P. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J. Int. Soc. Sports Nutr. 2017, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Kang, C.; Ji, L.L. Role of PGC-1α signaling in skeletal muscle health and disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Marabita, M.; Baraldo, M.; Solagna, F.; Ceelen, J.J.M.; Sartori, R.; Nolte, H.; Nemazanyy, I.; Pyronnet, S.; Kruger, M.; Pende, M.; et al. S6K1 Is Required for Increasing Skeletal Muscle Force during Hypertrophy. Cell Rep. 2016, 17, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Iemitsu, M.; Naito, H.; Kakigi, R.; Kakehashi, C.; Maeda, S.; Akema, T. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem. Biophys. Res. Commun. 2011, 414, 756–760. [Google Scholar] [CrossRef]
- Rivas, D.A.; Yaspelkis, B.B., 3rd; Hawley, J.A.; Lessard, S.J. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J. Endocrinol. 2009, 202, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Elfving, B.; Christensen, T.; Ratner, C.; Wienecke, J.; Klein, A.B. Transient activation of mTOR following forced treadmill exercise in rats. Synapse 2013, 67, 620–625. [Google Scholar] [CrossRef]
- Liu, H.T.; Pan, S.S. Late Exercise Preconditioning Promotes Autophagy against Exhaustive Exercise-Induced Myocardial Injury through the Activation of the AMPK-mTOR-ULK1 Pathway. BioMed Res. Int. 2019, 2019, 5697380. [Google Scholar] [CrossRef]
- Pazoki-Toroudi, H.; Amani, H.; Ajami, M.; Nabavi, S.F.; Braidy, N.; Kasi, P.D.; Nabavi, S.M. Targeting mTOR signaling by polyphenols: A new therapeutic target for ageing. Ageing Res. Rev. 2016, 31, 55–66. [Google Scholar] [CrossRef]
- Beevers, C.S.; Chen, L.; Liu, L.; Luo, Y.; Webster, N.J.; Huang, S. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009, 69, 1000–1008. [Google Scholar] [CrossRef] [Green Version]
- Sahin, K.; Orhan, C.; Tuzcu, M.; Muqbil, I.; Sahin, N.; Gencoglu, H.; Guler, O.; Padhye, S.B.; Sarkar, F.H.; Mohammad, R.M. Comparative In Vivo Evaluations of Curcumin and Its Analog Difluorinated Curcumin Against Cisplatin-Induced Nephrotoxicity. Biol. Trace Elem. Res. 2014, 157, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Rodgers, J.T.; Arlow, D.H.; Vazquez, F.; Mootha, V.K.; Puigserver, P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature 2007, 450, 736–740. [Google Scholar] [CrossRef]
- Ogasawara, R.; Fujita, S.; Hornberger, T.A.; Kitaoka, Y.; Makanae, Y.; Nakazato, K.; Naokata, I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci. Rep. 2016, 6, 31142. [Google Scholar] [CrossRef]
- Kawanishi, N.; Kato, K.; Takahashi, M.; Mizokami, T.; Otsuka, Y.; Imaizumi, A.; Shiva, D.; Yano, H.; Suzuki, K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochem. Biophys. Res. Commun. 2013, 441, 573–578. [Google Scholar] [CrossRef]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Xie, H.; Wu, S. Dietary Supplementation of Curcumin Alleviates NF-κB-dependent Skeletal Muscle Wasting in Rat. Endocr. Metab. Immune Disord.-Drug Targets 2016, 16, 140–147. [Google Scholar] [CrossRef] [PubMed]




| Groups | p | ||||
|---|---|---|---|---|---|
| Control | Exercise (E) | E+NGUC100 | E+NGUC200 | ||
| Glucose, mg/dL | 109.86 ± 2.69 | 106.14 ± 2.69 | 105.14 ± 1.58 | 108.14 ± 3.33 | 0.603 |
| TG, mg/dL | 114.43 ± 3.71 | 113.86 ± 2.99 | 112.29 ± 3.80 | 110.86 ± 4.48 | 0.908 |
| TC, mg/dL | 133.71 ± 3.90 | 135.57 ± 2.95 | 132.29 ± 3.96 | 130.00 ± 3.41 | 0.732 |
| BUN, g/dL | 20.49 ± 0.66 | 20.54 ± 0.53 | 20.26 ± 0.32 | 20.13 ± 0.43 | 0.928 |
| ALT, U/L | 83.57 ± 2.57 | 87.86 ± 3.89 | 86.86 ± 3.38 | 83.57 ± 1.60 | 0.650 |
| AST, U/L | 105.43 ± 1.76 | 107.86 ± 4.73 | 106.57 ± 2.76 | 103.29 ± 3.96 | 0.819 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahin, E.; Orhan, C.; Erten, F.; Er, B.; Acharya, M.; Morde, A.A.; Padigaru, M.; Sahin, K. Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats. Antioxidants 2021, 10, 1692. https://doi.org/10.3390/antiox10111692
Sahin E, Orhan C, Erten F, Er B, Acharya M, Morde AA, Padigaru M, Sahin K. Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats. Antioxidants. 2021; 10(11):1692. https://doi.org/10.3390/antiox10111692
Chicago/Turabian StyleSahin, Emre, Cemal Orhan, Fusun Erten, Besir Er, Manutosh Acharya, Abhijeet A. Morde, Muralidhara Padigaru, and Kazim Sahin. 2021. "Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats" Antioxidants 10, no. 11: 1692. https://doi.org/10.3390/antiox10111692
APA StyleSahin, E., Orhan, C., Erten, F., Er, B., Acharya, M., Morde, A. A., Padigaru, M., & Sahin, K. (2021). Next-Generation Ultrasol Curcumin Boosts Muscle Endurance and Reduces Muscle Damage in Treadmill-Exhausted Rats. Antioxidants, 10(11), 1692. https://doi.org/10.3390/antiox10111692








