Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review
Abstract
:1. Introduction
2. Main Components of Potato Peel and Their Bioactivities
2.1. Phenolic Compounds
2.2. Glycoalkaloids
2.3. Polysaccharides
2.4. Protein and Amino Acids
2.5. Vitamins and Minerals
3. Extraction Technologies for Bioactive Compound Recovery from Potato Peel
3.1. Conventional Methods
3.2. Emerging Extraction Methods
3.2.1. Ultrasound-Assisted Extraction
3.2.2. Microwave-Assisted Extraction
3.2.3. Supercritical Fluid Extraction
3.2.4. Pressurized Liquid Extraction
3.2.5. Sequential Hydrothermal Extraction
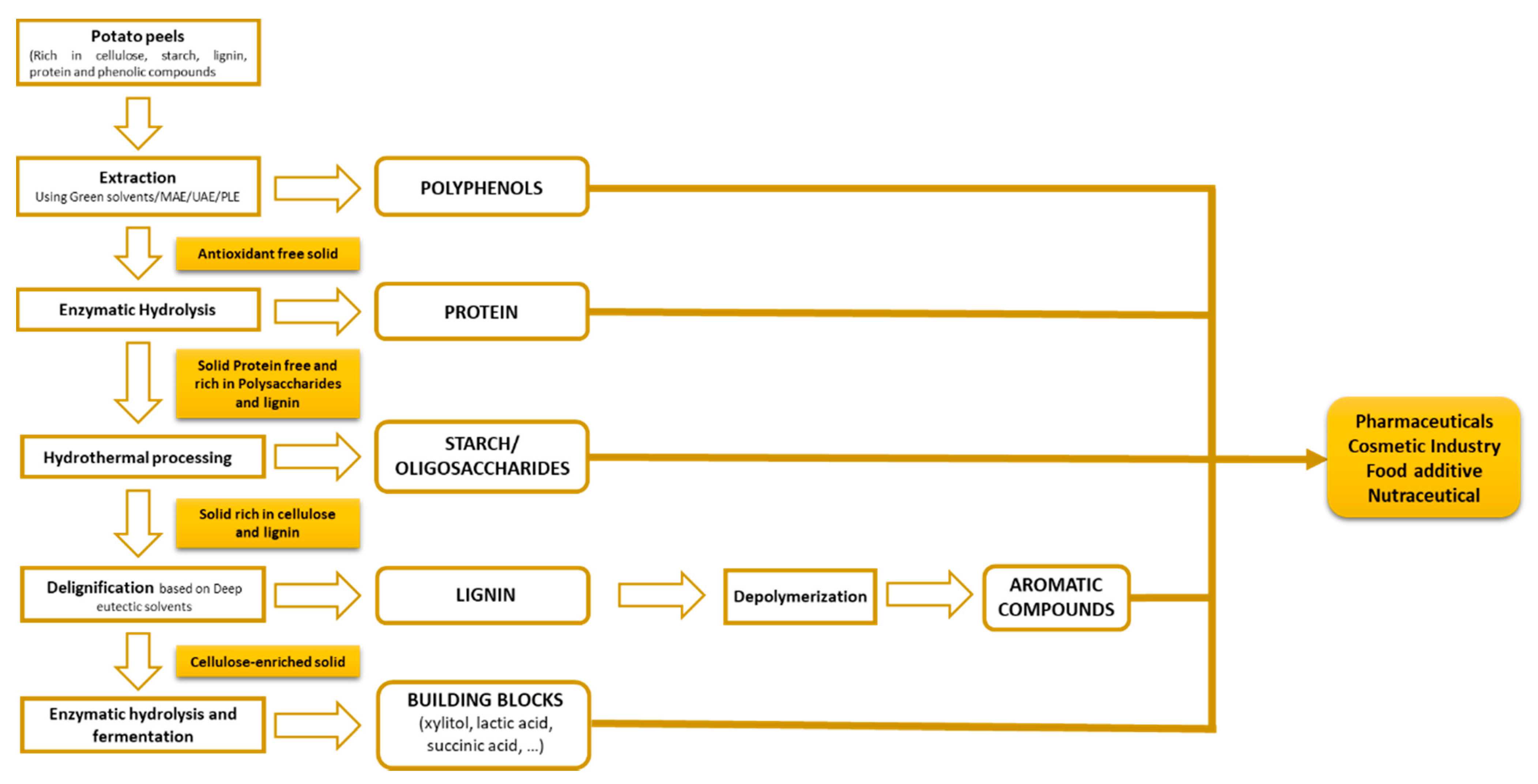
4. A Biorefinery Strategy for Integral Use of Potato Peel
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Herrera, R.; Labidi, J.; Gullón, P. Yerba Mate Waste: A Sustainable Resource of Antioxidant Compounds. Ind. Crops Prod. 2018, 113, 398–405. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of Conventional and Non-Conventional Processing on Prickly Pear (Opuntia spp.) and Their Derived Products: From Preservation of Beverages to Valorization of by-Products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Romaní, A.; Rocchetti, G.; Lorenzo, J.M. Smart Advanced Solvents for Bioactive Compounds Recovery from Agri-Food by-Products: A Review. Trends Food Sci. Technol. 2020, 101, 182–197. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Grigorakis, S.; Lalas, S.; Makris, D.P. Highly Efficient Extraction of Antioxidant Polyphenols from Olea Europaea Leaves Using an Eco-Friendly Glycerol/Glycine Deep Eutectic Solvent. Waste Biomass Valorization 2018, 9, 1985–1992. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; del Río, P.G.; Pérez-Pérez, A.; Yáñez, R.; Garrote, G.; Gullón, B. Recent Advances to Recover Value-Added Compounds from Avocado by-Products Following a Biorefinery Approach. Curr. Opin. Green Sustain. Chem. 2021, 28, 100433. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Compounds in Potato (Solanum Tuberosum L.) Peel and Their Health-Promoting Activities. Int. J. Food Sci. Technol. 2020, 55, 2273–2281. [Google Scholar] [CrossRef]
- Benkeblia, N. Potato Glycoalkaloids: Occurrence, Biological Activities and Extraction for Biovalorisation—A Review. Int. J. Food Sci. Technol. 2020, 55, 2305–2313. [Google Scholar] [CrossRef]
- Burlingame, B.; Mouillé, B.; Charrondière, R. Nutrients, Bioactive Non-Nutrients and Anti-Nutrients in Potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT-Producción Agrícola. Available online: https://www.fao.org/faostat/en/#data (accessed on 20 May 2021).
- Pathak, P.D.; Mandavgane, S.A.; Puranik, N.M.; Jambhulkar, S.J.; Kulkarni, B.D. Valorization of Potato Peel: A Biorefinery Approach. Crit. Rev. Biotechnol. 2018, 38, 218–230. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Chemical and Thermal Characterization of Potato Peel Waste and Its Fermentation Residue as Potential Resources for Biofuel and Bioproducts Production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef]
- Sepelev, I.; Galoburda, R. Industrial Potato Peel Waste Application in Food Production: A Review. Res. Rural Dev. 2015, 1, 130–136. [Google Scholar]
- Dos Santos, R.G.; Ventura, P.; Bordado, J.C.; Mateus, M.M. Valorizing Potato Peel Waste: An Overview of the Latest Publications. Rev. Environ. Sci. Biotechnol. 2016, 15, 585–592. [Google Scholar] [CrossRef]
- Singh, N.; Kamath, V.; Rajini, P.S. Protective Effect of Potato Peel Powder in Ameliorating Oxidative Stress in Streptozotocin Diabetic Rats. Plant Foods Hum. Nutr. 2005, 60, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering Valuable Bioactive Compounds from Potato Peels with Sequential Hydrothermal Extraction. Waste Biomass Valorization 2021, 12, 1465–1481. [Google Scholar] [CrossRef]
- Wu, Z.G.; Xu, H.Y.; Ma, Q.; Cao, Y.; Ma, J.N.; Ma, C.M. Isolation, Identification and Quantification of Unsaturated Fatty Acids, Amides, Phenolic Compounds and Glycoalkaloids from Potato Peel. Food Chem. 2012, 135, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Al-Weshahy, A.; Venket Rao, A. Isolation and Characterization of Functional Components from Peel Samples of Six Potatoes Varieties Growing in Ontario. Food Res. Int. 2009, 42, 1062–1066. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. Extraction of Phenolics and Changes in Antioxidant Activity of Red Wines during Vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Biochemistry, and Dietary Role of Potato Polyphenols. A Review. J. Agric. Food Chem. 1997, 45, 1523–1540. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic Content and Antioxidant Activities of Selected Potato Varieties and Their Processing By-Products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Rodriguez De Sotillo, D.; Hadley, M.; Wolf-Hall, C. Potato Peel Extract a Nonmutagenic Antioxidant with Potential Antimicrobial Activity. J. Food Sci. 1998, 63, 907–910. [Google Scholar] [CrossRef]
- Mäder, J.; Rawel, H.; Kroh, L.W. Composition of Phenolic Compounds and Glycoalkaloids α-Solanine and α-Chaconine during Commercial Potato Processing. J. Agric. Food Chem. 2009, 57, 6292–6297. [Google Scholar] [CrossRef] [PubMed]
- Akyol, H.; Riciputi, Y.; Capanoglu, E.; Caboni, M.F.; Verardo, V. Phenolic Compounds in the Potato and Its Byproducts: An Overview. Int. J. Mol. Sci. 2016, 17, 835. [Google Scholar] [CrossRef]
- Rodríguez De Sotillo, D.; Hadley, M.; Holm, E.T. Potato Peel Waste: Stability and Antioxidant Activity of a Freeze-Dried Extract. J. Food Sci. 1994, 59, 1031–1033. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of Ultrasound-Assisted Extraction of Phenolic Compounds from Industrial Potato by-Products Using Response Surface Methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Oertel, A.; Matros, A.; Hartmann, A.; Arapitsas, P.; Dehmer, K.J.; Martens, S.; Mock, H.P. Metabolite Profiling of Red and Blue Potatoes Revealed Cultivar and Tissue Specific Patterns for Anthocyanins and Other Polyphenols. Planta 2017, 246, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Deußer, H.; Guignard, C.; Hoffmann, L.; Evers, D. Polyphenol and Glycoalkaloid Contents in Potato Cultivars Grown in Luxembourg. Food Chem. 2012, 135, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Silva-Beltrán, N.P.; Chaidez-Quiroz, C.; López-Cuevas, O.; Ruiz-Cruz, S.; López-Mata, M.A.; Del-Toro-sánchez, C.L.; Marquez-Rios, E.; Ornelas-Paz, J.D.J. Phenolic Compounds of Potato Peel Extracts: Their Antioxidant Activity and Protection against Human Enteric Viruses. J. Microbiol. Biotechnol. 2017, 27, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Benavides-Guerrero, R.; Revelo-Cuarán, Y.A.; Arango-Bedoya, O.; Osorio-Mora, O. Ultrasound-Assisted Extraction of Phenolic Compounds from Two Varieties of an Andean Native Potato (Solanum Phureja) and Evaluation of Their Antioxidant Activity. Inf. Tecnol. 2020, 21, 43–50. [Google Scholar] [CrossRef]
- Cerón-Lasso, M.; Alzate-Arbeláez, A.F.; Rojano, B.A.; Ñuztez-Lopez, C.E. Physicochemical Composition and Antioxidant Properties of Native Diploid Potato (Solanum Tuberosum Phureja Group). Inf. Tecnol. 2018, 29, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Wijngaard, H.H.; Ballay, M.; Brunton, N. The Optimisation of Extraction of Antioxidants from Potato Peel by Pressurised Liquids. Food Chem. 2012, 133, 1123–1130. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A Review on Extraction, Identification and Purification Methods, Biological Activities and Approaches to Enhance Its Bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Jin, Z.; Shinde, P.L.; Yang, Y.X.; Choi, J.Y.; Yoon, S.Y.; Hahn, T.W.; Lim, H.T.; Park, Y.K.; Hahm, K.S.; Joo, J.W.; et al. Use of Refined Potato (Solanum Tuberosum L. Cv. Gogu Valley) Protein as an Alternative to Antibiotics in Weanling Pigs. Livest. Sci. 2009, 124, 26–32. [Google Scholar] [CrossRef]
- Manrique-Carpintero, N.C.; Tokuhisa, J.G.; Ginzberg, I.; Holliday, J.A.; Veilleux, R.E. Sequence Diversity in Coding Regions of Candidate Genes in the Glycoalkaloid Biosynthetic Pathway of Wild Potato Species. G3 Genes Genomes Genet. 2013, 3, 1467–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, K.K.; Kirk, H.G.; Olsson, K.; Labouriau, R.; Christiansen, J. A Major QTL and an SSR Marker Associated with Glycoalkaloid Content in Potato Tubers from Solanum Tuberosum x S. Sparsipilum Located on Chromosome I. Theor. Appl. Genet. 2008, 117, 1–9. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rawson, A.; Aguiló-Aguayo, I.; Brunton, N.P.; Rai, D.K. Recovery of Steroidal Alkaloids from Potato Peels Using Pressurized Liquid Extraction. Molecules 2015, 20, 8560–8573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Raigond, P.; Barwal, S.; Mehta, A.; Chopra, S.; Joshi, A.; Dutt, S. Glycoalkaloids in Peels of Indian Potatoes. Potato J. 2016, 43, 86–92. [Google Scholar]
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional Value of Potato (Solanum Tuberosum) in Hot Climates: Anthocyanins, Carotenoids, and Steroidal Glycoalkaloids. Planta 2019, 249, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Alves-Filho, E.G.; Sousa, V.M.; Ribeiro, P.R.V.; Rodrigues, S.; de Brito, E.S.; Tiwari, B.K.; Fernandes, F.A.N. Single-Stage Ultrasound-Assisted Process to Extract and Convert α-Solanine and α-Chaconine from Potato Peels into β-Solanine and β-Chaconine. Biomass Convers. Biorefinery 2018, 8, 689–697. [Google Scholar] [CrossRef]
- Distl, M.; Wink, M. Identification and Quantification of Steroidal Alkaloids from Wild Tuber-Bearing Solanum Species by HPLC and LC-ESI-MS. Potato Res. 2009, 52, 79–104. [Google Scholar] [CrossRef]
- Rytel, E.; Tajner-Czopek, A.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Hamouz, K. Content of Anthocyanins and Glycoalkaloids in Blue-Fleshed Potatoes and Changes in the Content of α-Solanine and α-Chaconine during Manufacture of Fried and Dried Products. Int. J. Food Sci. Technol. 2018, 53, 719–727. [Google Scholar] [CrossRef]
- Rytel, E.; Tajner-Czopek, A.; Aniolowska, M.; Hamouz, K. The Influence of Dehydrated Potatoes Processing on the Glycoalkaloids Content in Coloured-Fleshed Potato. Food Chem. 2013, 141, 2495–2500. [Google Scholar] [CrossRef]
- Friedman, M.; Kozukue, N.; Kim, H.J.; Choi, S.H.; Mizuno, M. Glycoalkaloid, Phenolic, and Flavonoid Content and Antioxidative Activities of Conventional Nonorganic and Organic Potato Peel Powders from Commercial Gold, Red, and Russet Potatoes. J. Food Compos. Anal. 2017, 62, 69–75. [Google Scholar] [CrossRef]
- Elkahoui, S.; Bartley, G.E.; Yokoyama, W.H.; Friedman, M. Dietary Supplementation of Potato Peel Powders Prepared from Conventional and Organic Russet and Non-Organic Gold and Red Potatoes Reduces Weight Gain in Mice on a High-Fat Diet. J. Agric. Food Chem. 2018, 66, 6064–6072. [Google Scholar] [CrossRef]
- Friedman, M.; Huang, V.; Quiambao, Q.; Noritake, S.; Liu, J.; Kwon, O.; Chintalapati, S.; Young, J.; Levin, C.E.; Tam, C.; et al. Potato Peels and Their Bioactive Glycoalkaloids and Phenolic Compounds Inhibit the Growth of Pathogenic Trichomonads. J. Agric. Food Chem. 2018, 66, 7942–7947. [Google Scholar] [CrossRef]
- Thorne, H.V.; Clarke, G.F.; Skuce, R. The Inactivation of Herpes Simplex Virus by Some Solanaceae Glycoalkaloids. Antiviral Res. 1985, 5, 335–343. [Google Scholar] [CrossRef]
- Friedman, M. Potato Glycoalkaloids and Metabolites: Roles in the Plant and in the Diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Y.; Liao, A.M.; Huang, J.H.; Zhang, J.G.; Thakur, K.; Wei, Z.J. The Rheological Properties of Differentially Extracted Polysaccharides from Potatoes Peels. Int. J. Biol. Macromol. 2019, 137, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeddou, K.B.; Chaari, F.; Maktouf, S.; Nouri-Ellouz, O.; Helbert, C.B.; Ghorbel, R.E. Structural, Functional, and Antioxidant Properties of Water-Soluble Polysaccharides from Potatoes Peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato Peel Phenolics as Additives for Developing Active Starch-Based Films with Potential to Pack Smoked Fish Fillets. Food Packag. Shelf Life 2021, 28. [Google Scholar] [CrossRef]
- Rommi, K.; Rahikainen, J.; Vartiainen, J.; Holopainen, U.; Lahtinen, P.; Honkapää, K.; Lantto, R. Potato Peeling Costreams as Raw Materials for Biopolymer Film Preparation. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jeddou, K.B.; Bouaziz, F.; Helbert, C.B.; Nouri-Ellouz, O.; Maktouf, S.; Ellouz-Chaabouni, S.; Ellouz-Ghorbel, R. Structural, Functional, and Biological Properties of Potato Peel Oligosaccharides. Int. J. Biol. Macromol. 2018, 112, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Darwish, A.M.G.; El-Sohaimy, S.A. Physicochemical, Structural and Functional Properties of Water-Soluble Polysaccharides Extracted from Egyptian Agricultural by-Products. Ann. Agric. Sci. 2020, 65, 21–27. [Google Scholar] [CrossRef]
- Sani, I.K.; Geshlaghi, S.P.; Pirsa, S.; Asdagh, A. Composite Film Based on Potato Starch/Apple Peel Pectin/ZrO2 Nanoparticles/ Microencapsulated Zataria Multiflora Essential Oil; Investigation of Physicochemical Properties and Use in Quail Meat Packaging. Food Hydrocoll. 2021, 117, 106719. [Google Scholar] [CrossRef]
- Hamidon, N.H.; Zaidel, D.N.A. Effect of Extraction Conditions on Pectin Yield Extracted from Sweet Potato Peels Residues Using Hydrochloric Acid. Chem. Eng. Trans. 2017, 56, 979–984. [Google Scholar] [CrossRef]
- Byg, I.; Diaz, J.; Øgendal, L.H.; Harholt, J.; Jørgensen, B.; Rolin, C.; Svava, R.; Ulvskov, P. Large-Scale Extraction of Rhamnogalacturonan i from Industrial Potato Waste. Food Chem. 2012, 131, 1207–1216. [Google Scholar] [CrossRef]
- Peterson, R.B.; Rankin, S.A.; Ikeda, S. Short Communication: Stabilization of Milk Proteins at PH 5.5 Using Pectic Polysaccharides Derived from Potato Tubers. J. Dairy Sci. 2019, 102, 8691–8695. [Google Scholar] [CrossRef]
- Martínez-Fernández, J.S.; Gu, X.; Chen, S. Techno-Economic Assessment of Bioactive Compound Recovery from Potato Peels with Sequential Hydrothermal Extraction. J. Clean. Prod. 2021, 282, 1465–1481. [Google Scholar] [CrossRef]
- Bogucka, B.; Elżbieta, T. Effect of Nitrogen and Potassium Fertilization on Mineral and Amino Acid Content of Colored Flesh Potato Cultivar Blue Congo. J. Plant Nutr. 2018, 41, 856–866. [Google Scholar] [CrossRef]
- Talley, E.A.; Toma, R.B.; Orr, P.H. Composition of Raw and Cooked Potato Peel and Flesh: Amino Acid Content. J. Food Sci. 1983, 48, 1360–1361. [Google Scholar] [CrossRef]
- Mushinskiy, A.A.; Aminova, E.V.; Fedotova, L.S.; Dergileva, T.T. Evaluation of Potato Tubers of Nevsky Variety and Selection Hybrids by Amino Acid Composition. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012155. [Google Scholar] [CrossRef]
- Xu, D.; Liu, H.; Jin, C.Y.; Cao, C.M.; Li, W.G.; Zeng, F.K.; Zhao, Y.C.; Liu, G. A New Potato Variety Grown in China Suitable for Raw Eating. Eur. Food Res. Technol. 2018, 244, 851–860. [Google Scholar] [CrossRef]
- Choi, S.H.; Kozukue, N.; Kim, H.J.; Friedman, M. Analysis of Protein Amino Acids, Non-Protein Amino Acids and Metabolites, Dietary Protein, Glucose, Fructose, Sucrose, Phenolic, and Flavonoid Content and Antioxidative Properties of Potato Tubers, Peels, and Cortexes (Pulps). J. Food Compos. Anal. 2016, 50, 77–87. [Google Scholar] [CrossRef]
- Tako, E. Dietary Trace Minerals. Nutrients 2019, 11, 2823. [Google Scholar] [CrossRef] [Green Version]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of Phenolic Compounds and Other Polar Compounds in the Tuber of Solanum Tuberosum L. by HPLC-DAD-q-TOF and Study of Their Antioxidant Activity. J. Food Compos. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Zhang, Z.; Poojary, M.M.; Choudhary, A.; Rai, D.K.; Lund, M.N.; Tiwari, B.K. Ultrasound Processing of Coffee Silver Skin, Brewer’s Spent Grain and Potato Peel Wastes for Phenolic Compounds and Amino Acids: A Comparative Study. J. Food Sci. Technol. 2021, 58, 2273–2282. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, Ultrasound-Assisted, and Accelerated-Solvent Extractions of Anthocyanins from Purple Sweet Potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-Assisted Extraction of Polyphenols from Potato Peels: Profiling and Kinetic Modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Scharf, R.; Wang, R.; Maycock, J.; Ho, P.; Chen, S.; Orfila, C. Correction to: Valorisation of Potato (Solanum Tuberosum) Peel Waste: Extraction of Fibre, Monosaccharides and Uronic Acids. Waste Biomass Valorization 2020, 11, 4571. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Sabally, K.; Kubow, S.; Donnelly, D.J.; Gariepy, Y.; Orsat, V.; Raghavan, G.S.V. Microwave-Assisted Extraction of Phenolic Antioxidants from Potato Peels. Molecules 2011, 16, 2218–2232. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.F.; Tu, Z.C.; Zhang, L.; Wang, H.; Wen, Q.H.; Huang, T. Antioxidant Activities and Polyphenols of Sweet Potato (Ipomoea Batatas L.) Leaves Extracted with Solvents of Various Polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Panić, M.; Andlar, M.; Tišma, M.; Rezić, T.; Šibalić, D.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Natural Deep Eutectic Solvent as a Unique Solvent for Valorisation of Orange Peel Waste by the Integrated Biorefinery Approach. Waste Manag. 2021, 120, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Karbuz, P.; Tugrul, N. Microwave and Ultrasound Assisted Extraction of Pectin from Various Fruits Peel. J. Food Sci. Technol. 2021, 58, 641–650. [Google Scholar] [CrossRef]
- Joly, N.; Souidi, K.; Depraetere, D.; Wils, D.; Martin, P. Potato By-Products as a Source of Natural Chlorogenic Acids and Phenolic Compounds: Extraction, Characterization, and Antioxidant Capacity. Molecules 2020, 26, 177. [Google Scholar] [CrossRef]
- Glisic, S.B.; Ristic, M.; Skala, D.U. The Combined Extraction of Sage (Salvia Officinalis L.): Ultrasound Followed by Supercritical CO2 Extraction. Ultrason. Sonochem. 2011, 18, 318–326. [Google Scholar] [CrossRef]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef] [Green Version]
- Páramos, P.R.S.; Granjo, J.F.O.; Corazza, M.L.; Matos, H.A. Extraction of High Value Products from Avocado Waste Biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Bouazzaoui, N.; Drici, W.; Bouazzaoui, W.; Lemerini, W.; Arrar, Z.; Bendiabdellah, D.; Mulengi, J.K. Fatty Acids and Mineral Composition of Melon (Cucumis Melo L. Inodorus) Seeds from West Algeria. Mediterr. J. Chem. 2016, 5, 340–346. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Optimization of Extraction of Functional Protein Hydrolysates from Chicken Egg Shell Membrane (ESM) by Ultrasonic Assisted Extraction (UAE) and Enzymatic Hydrolysis. LWT—Food Sci. Technol. 2016, 69, 295–302. [Google Scholar] [CrossRef]
- Kamal, H.; Le, C.F.; Salter, A.M.; Ali, A. Extraction of Protein from Food Waste: An Overview of Current Status and Opportunities. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2455–2475. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Alonso, J.L.; Parajó, J.C.; Yáñez, R. Valorization of Peanut Shells: Manufacture of Bioactive Oligosaccharides. Carbohydr. Polym. 2018, 183, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rico, X.; Gullón, B.; Yáñez, R. Environmentally Friendly Hydrothermal Processing of Melon By-Products for the Recovery of Bioactive Pectic-Oligosaccharides. Foods 2020, 9, 1702. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Eibes, G.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal Treatment of Chestnut Shells (Castanea Sativa) to Produce Oligosaccharides and Antioxidant Compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of Suitability of Vine Shoots for Hemicellulosic Oligosaccharides Production through Aqueous Processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
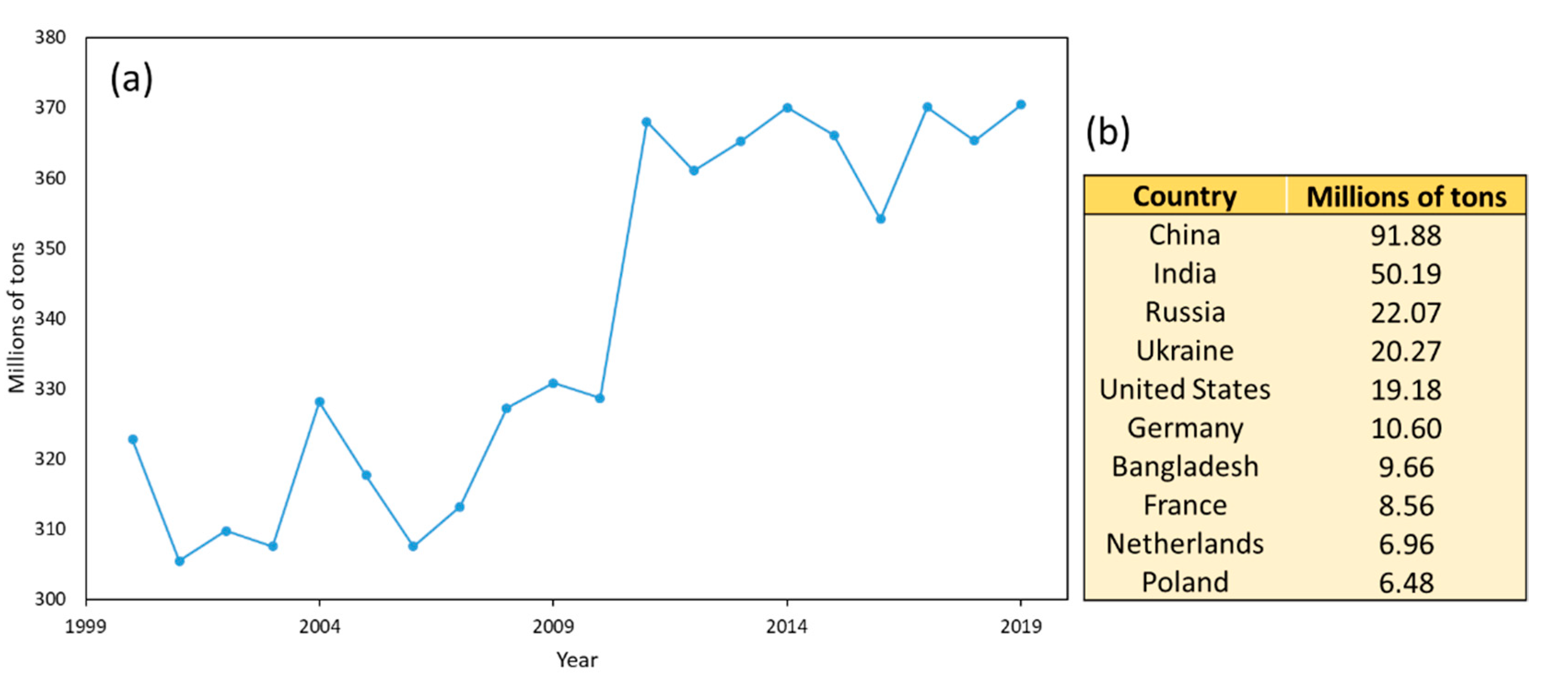

| Potato Variety | Concentration (mg/g db) | Antioxidant Assay | Ref | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | I | II | III | IV | V | ||
| Siècle | 2.79 | 0.52 | - | - | - | - | - | - | - | 1.07 | 0.55 | 90 | - | - | [19] |
| Purple majesty | 2.19 | 0.72 | - | - | - | - | - | - | - | 0.72 | 0.50 | 88 | - | - | |
| Dakota pearl | 0.78 | 0.47 | - | - | - | - | - | - | - | 0.65 | 0.38 | 87 | - | - | |
| FL 1533 | 1.33 | 0.44 | - | - | - | - | - | - | - | 0.50 | 0.35 | 85 | - | - | |
| Vivaldi | 1.54 | 0.26 | - | - | - | - | - | - | - | 0.50 | 0.35 | 85 | - | - | |
| Yukon gold | 0.86 | 0.41 | - | - | - | - | - | - | - | 0.43 | 0.28 | 82 | - | - | |
| Ratona morada | 0.38 | - | - | - | - | - | - | - | 1.77 * | - | - | - | 39.81 ** | 0.410 | [32] |
| Curiquinga | 0.44 | - | - | - | - | - | - | - | 1.91 * | - | - | - | 49.98 ** | 0.412 | |
| Russet Burbank | - | - | - | - | - | - | - | - | 7.0 | - | - | - | 21–42 stage 1 33–65 stage 2 | - | [17] |
| Mixed peels | - | - | - | - | - | - | - | - | 8.0 | - | - | - | 15–34 stage 1 21–42 stage 2 | - | |
| Bintje | 1.97 | 0.24 | 0.06 | - | - | - | - | - | 3.23 | - | - | - | 28.25 *** | 4059.05 3 | [28] |
| Challenger | 1.27 | 0.22 | 0.05 | - | - | - | - | - | 2.48 | - | - | - | 21.04 *** | 3179.92 3 | |
| Daisy | 4.10 | 0.16 | 0.12 | - | - | - | - | - | 7.23 | - | - | - | 42.30 *** | 5745.18 3 | |
| Innovator | 2.52 | 0.30 | 0.06 | - | - | - | - | - | 5.04 | - | - | - | 39.88 *** | 5138.86 3 | |
| Fontane | 3.04 | 1.22 | 0.12 | - | - | - | - | - | 6.15 | - | - | - | 46.40 *** | 6037.12 3 | |
| Fianna | 3.46 | 3.33 | 0.03 | 2.33 | 0.05 | 0.11 | - | - | - | - | - | - | 0.38 **** | 1.4 **** | [31] |
| Innovator | 1.29 | 1.09 | 0.85 | - | - | - | 5.30 | 0.002 | 11.5 * | 1907.26 *** | 1.86 | - | 32.75 *** | - | [23] |
| Russet | 1.35 | 0.99 | 0.57 | - | - | - | 0.09 | 0.004 | 9.32 * | 1533.88 *** | 1.10 | - | 28.01 *** | - | |
| Yellow | 0.17 | 0.30 | 0.13 | - | - | - | 0.03 | 0.003 | 4.54 * | 560.93 *** | 0.70 | - | 13.88 *** | - | |
| Purple | 3.65 | 0.92 | 0.07 | - | - | - | 0.07 | 0.068 | 13.9 * | 1179.76 *** | 2.14 | - | 17.10 *** | - | |
| Potato Variety | Concentration (mg/100 g db) | Identification Method | Ref | ||
|---|---|---|---|---|---|
| α-Solanine | α-Chaconine | Total SGA | |||
| n.d. | 429 | 328 | 1012 | UPLC-MS/MS | [41] |
| Desireé | 5294 * | 11,881 * | 17,175 * | UPLC–Triple Quadrupole–MS | [43] |
| n.d. | 1.6 | 15.40 | - | UHPLC-qTOF-MS | [44] |
| Conventional Gold | 25.3 | 67 | 92 | HPLC | [48] |
| Conventional Red | 41.2 | 12.97 | 17.09 | ||
| Conventional Russet | 21.5 | 42.4 | 63.9 | ||
| Organic Gold | 75 | 28.3 | 35.8 | ||
| Organic Red | 23.9 | 61 | 85 | ||
| Organic Russet | 37.4 | 11.80 | 15.50 | ||
| S. acaule ssp. acaule | traces | traces | 121.05 | LC-ESI-MS and HPLC-DAD | [45] |
| S. ajanhuiri | traces | 25.86 | 1449.17 | ||
| S. alandiae | 918.17 | 1743.17 | 2664.57 | ||
| S. bulbocastanum ssp. bulbocastanum | 10.66 | 34.93 | 45.59 | ||
| S. chaucha | 61.82 | 116.93 | 829.47 | ||
| S. chomatophilum | 2.99 | 16.98 | 64.07 | ||
| S. curtilobum | 37.68 | 106.82 | 144.50 | ||
| S. demissum | traces | 9.13 | 889.58 | ||
| S. maglia | 13.13 | 31.51 | 227.34 | ||
| S. microdontum | 33.68 | 50.01 | 1870.31 | ||
| S. pascoense | 42.83 | 15.26 | 822.41 | ||
| S. phureja ssp. Phureja | 425.61 | 974.70 | 1453.26 | ||
| S. polyadenium | 6.14 | 47.06 | 53.20 | ||
| S. raphanifolium | 1.68 | 27.19 | 28.87 | ||
| S. sparsipilum | 563.86 | 948.10 | 1520.31 | ||
| S. tarijense | traces | 46.77 | 2369.52 | ||
| S. tuberosum ssp. andigena (white) | 208.63 | 52.45 | 355.79 | ||
| S. tuberosum ssp. andigena (violet) | 8.91 | 64.43 | 80.18 | ||
| S. tuberosum ssp. Andig | traces | 11.12 | 572.20 | ||
| Rote Emma | 5.12 | 10.12 | 15.33 | HPLC | [47] |
| Rosemarie | 7.17 | 12.20 | 19.37 | ||
| Blaue Annelise | 8.79 | 15.66 | 24.45 | ||
| Blaue St. Galler | 6.09 | 11.96 | 18.05 | ||
| Valfi | 24.9 | 58.2 | 83.1 | HPLC | [46] |
| Blaue Elise | 21.2 | 45.5 | 66.6 | ||
| Red potato | 57.2 | 160.4 | - | n.d. | [49] |
| Gold potato | 63.6 | 130.1 | - | ||
| Organic Russet | 26.8 | 59.3 | - | ||
| Non-organic Russet | 34.7 | 78.1 | - | ||
| Potato Variety | Extraction Conditions | Phenolic Compounds | Antioxidant Activity | Ref | ||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | I | II | III | |||
| Conventional Extraction | ||||||||
| Ratona Morada | 70% methanol or acetone (v/v), room temperature, 15 min, 5% (p/v) | 6.29–6.50 | - | - | - | - | - | [32] |
| Curiquinga | 8.03–8.78 | - | - | - | - | - | ||
| Lady Rosetta | 80% methanol, 23 °C, 15 h | 3.28 | 237.36 | 85.08 | 3.51 | 6.27 | - | [73] |
| Lady Claire | 2.17 | 2.16 | 68.19 | 1.75 | 3.45 | - | ||
| n.d. | 50% methanol, 1:20 S-L, 25 °C, 60 min | 6.26 | - | - | - | - | - | [74] |
| Lady Claire | Methanol and 75% ethanol, 80 °C, 22 min | 1.26–3.94 | - | - | 2.00 | 3.52 | - | [34] |
| Ultrasound-Assited Extraction (UAE) | ||||||||
| Ratona Morada | 70% acetone, 5% (p/v); 50 min, 50 °C | 17.70 | 380.0 | - | 39.81 * | - | 0.410 | [32] |
| Curiquinga | 19.10 | 440.0 | - | 49.98 * | - | 0.412 | ||
| Lady Rosetta | 80% methanol, 1:10 (w/v), 30–45 °C, 30–900 min, 42 and 33 kHz | 7.67 | 267.4 | 129.05 | 5.86 | 22.21 | - | [73] |
| Lady Claire | 3.8–4.24 | 5.98–8.69 | 118.28–120.83 | 3.16–3.86 | 5.64–5.85 | - | ||
| Indirect Ultrasound-Assited Extraction (IUAE) | ||||||||
| n.d. | 50% Methanol, 1:20 S-L, 25 °C, 30 min | 9.09 | - | - | - | - | - | [74] |
| Direct Ultrasound-Assited Extraction (DUAE) | ||||||||
| n.d. | 50% Methanol, 1:20 S-L, 25 °C, 30 min | 9.33 | - | - | 54.2 ** | - | - | [74] |
| Microwave-Assited Extraction (MAE) | ||||||||
| Russett Burbank | 67.33% Methanol, 15 min, 1:20 S-L, 14.67% level power and room temperature | 3.94 | - | - | 74 ** | - | - | [75] |
| Sequential Hydrothermal Extraction (SeqHTE) | ||||||||
| Mixture | water stage 1: 170 °C, 1 MPa, 20 min | 17.01 *** | - | - | - | - | - | [63] |
| water stage 2: 220 °C, 2.5 MPa, 20 min | 3.72 *** | - | - | - | - | - | ||
| Pressurized Liquid Extraction (PLE) | ||||||||
| Lady Claire | 70% ethanol and 125 °C | 3.68 | - | - | 3.39 | - | - | [34] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review. Antioxidants 2021, 10, 1630. https://doi.org/10.3390/antiox10101630
Rodríguez-Martínez B, Gullón B, Yáñez R. Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review. Antioxidants. 2021; 10(10):1630. https://doi.org/10.3390/antiox10101630
Chicago/Turabian StyleRodríguez-Martínez, Beatriz, Beatriz Gullón, and Remedios Yáñez. 2021. "Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review" Antioxidants 10, no. 10: 1630. https://doi.org/10.3390/antiox10101630







