Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Regents
2.2. Cell Viability with the MTT Assay
2.3. Quantitative RT-PCR Analysis of mRNA Expression
2.4. Western Blotting Analysis of Protein Expression
2.5. Detection of Cellular ROS and Apoptosis by Flow Cytometry
2.6. The Assays for Total, Reduced, and Oxidized Glutathione Levels
2.7. Assays for ROS-Scavenging Activities of Superoxide Dismutase and Catalase
2.8. ARE-Luciferase Reporter Assays
2.9. Statistical Analysis
3. Results
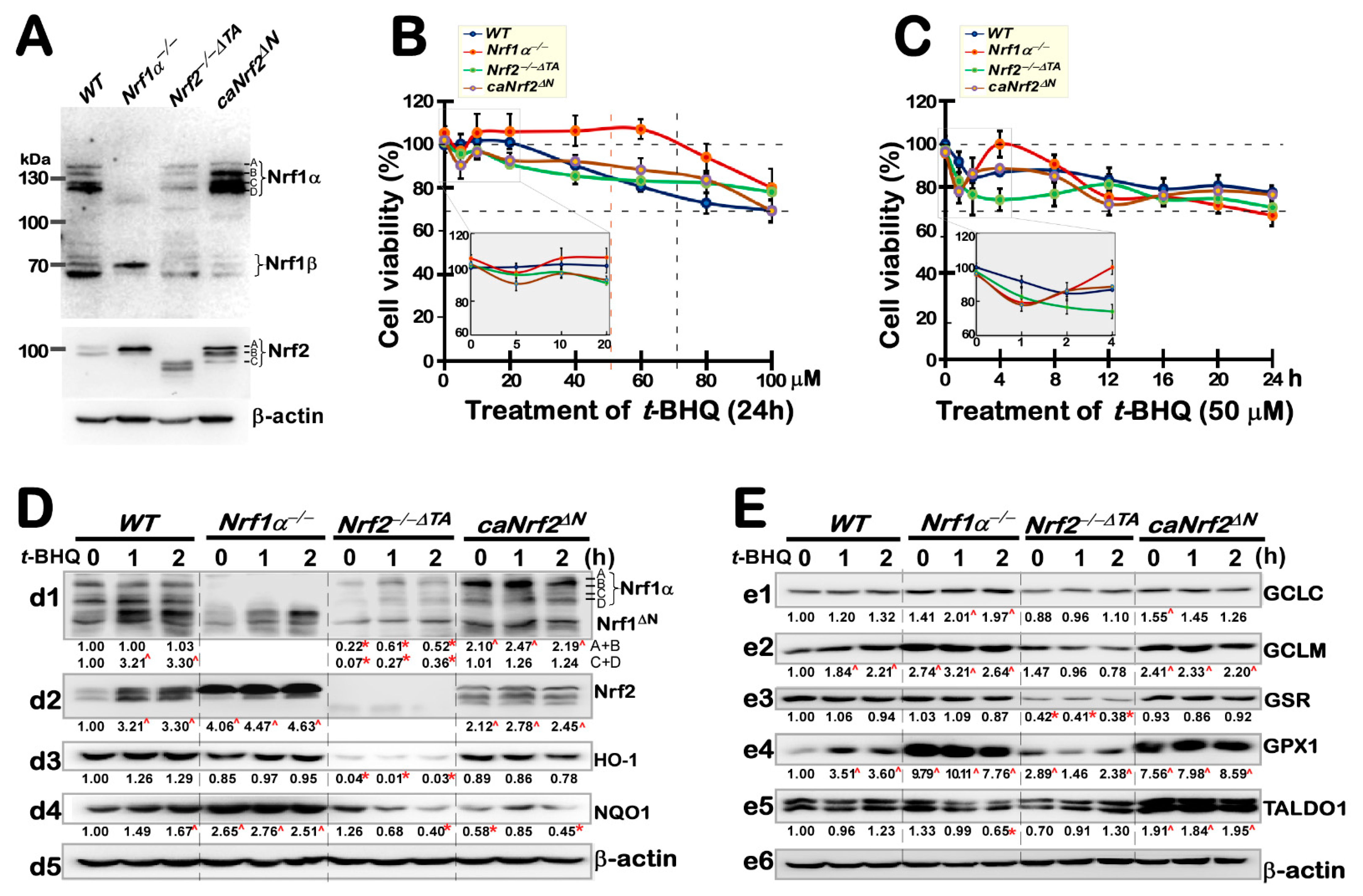
3.1. Different Effects of Nrf1 and Nrf2 on Cell Growth during tBHQ Intervention
3.2. Short-Term Intervening Effects of tBHQ on Nrf1, Nrf2, and AREs-Driven Genes in Distinct Genotypic Cells
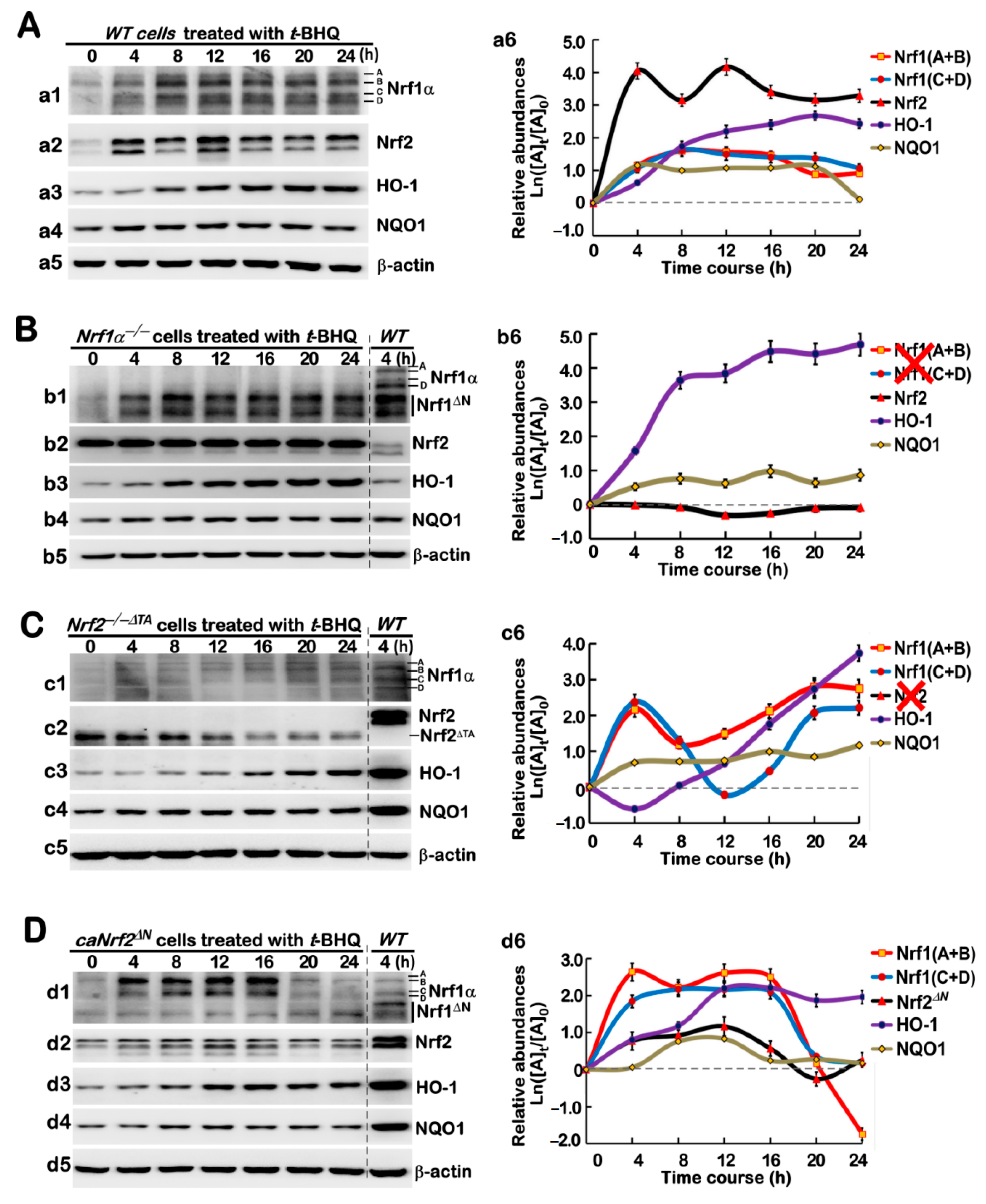
3.3. Long-Term Stimulating Effects of tBHQ on Nrf1, Nrf2, and Downstream Targets in Distinct Genotypic Cells
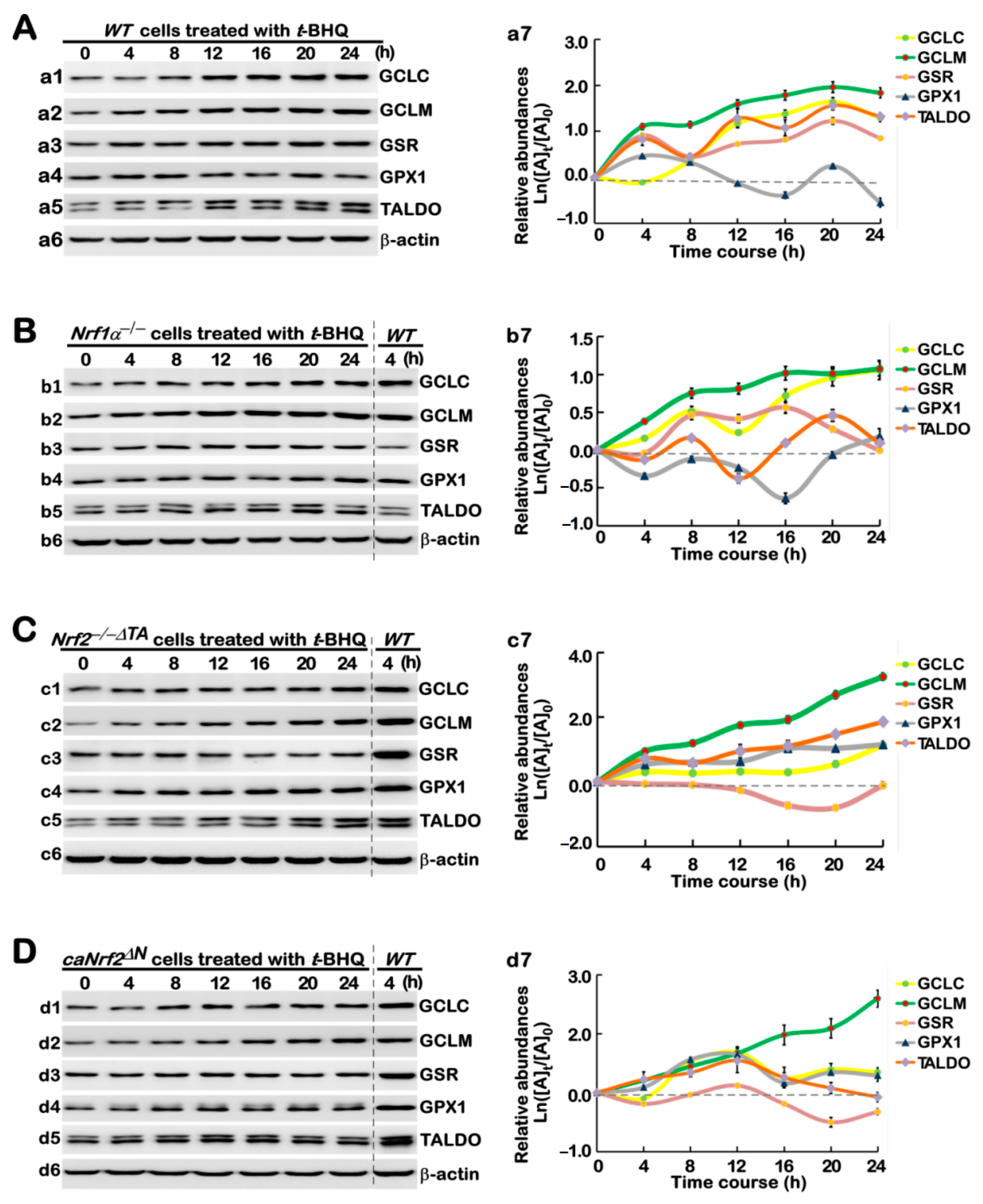
3.4. Long-Term Stimulation of Human Antioxidant and Detoxification Genes by tBHQ in Distinct Genotypic Cells
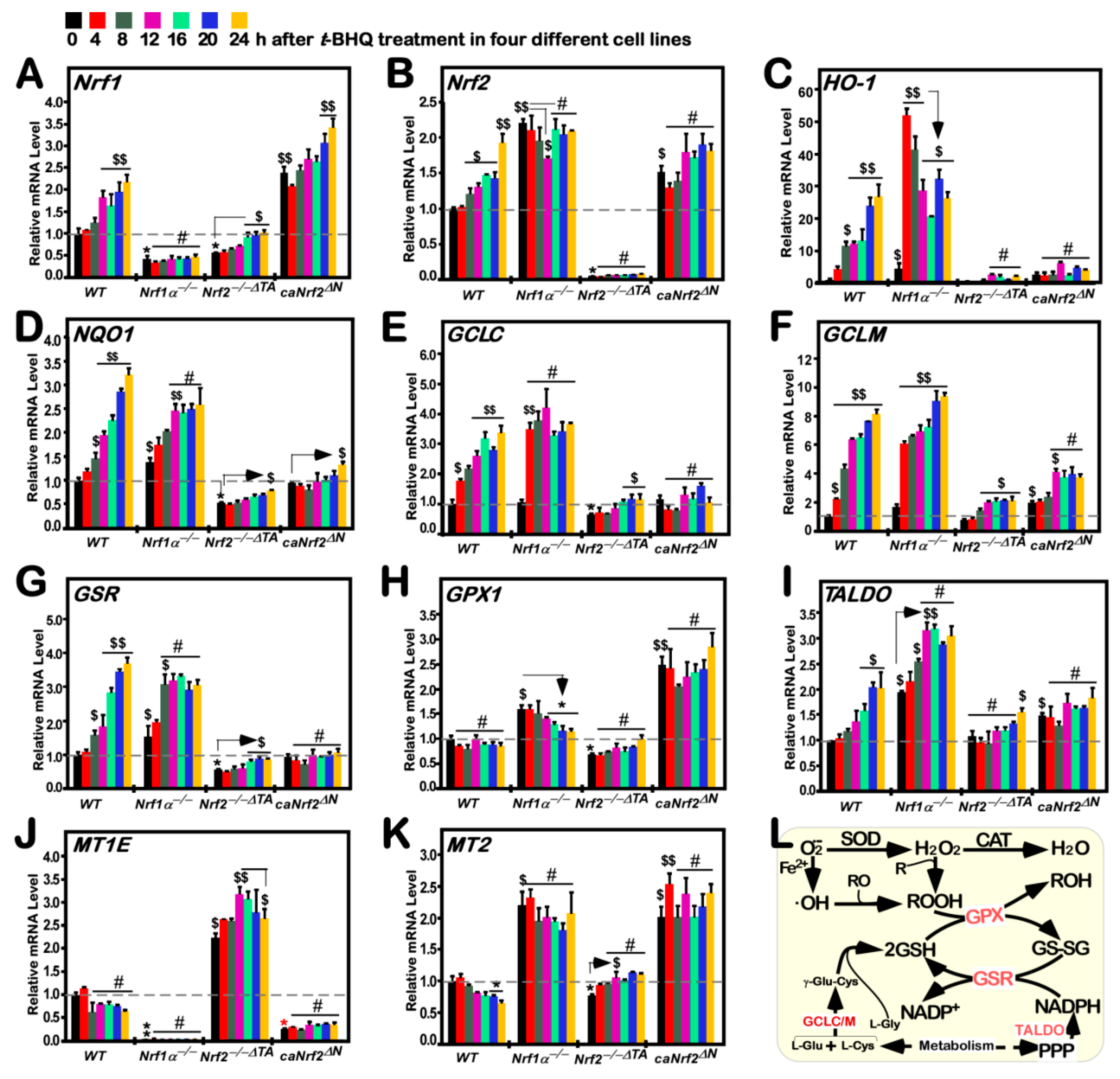
3.5. Distinct Time-Dependent Effects of tBHQ on Nrf1, Nrf2, and Target Gene Expression in Different Cell Lines
3.6. Different Time-Dependent Effects of tBHQ on Antioxidant Cytoprotective Gene Expression in Distinct Cell Lines
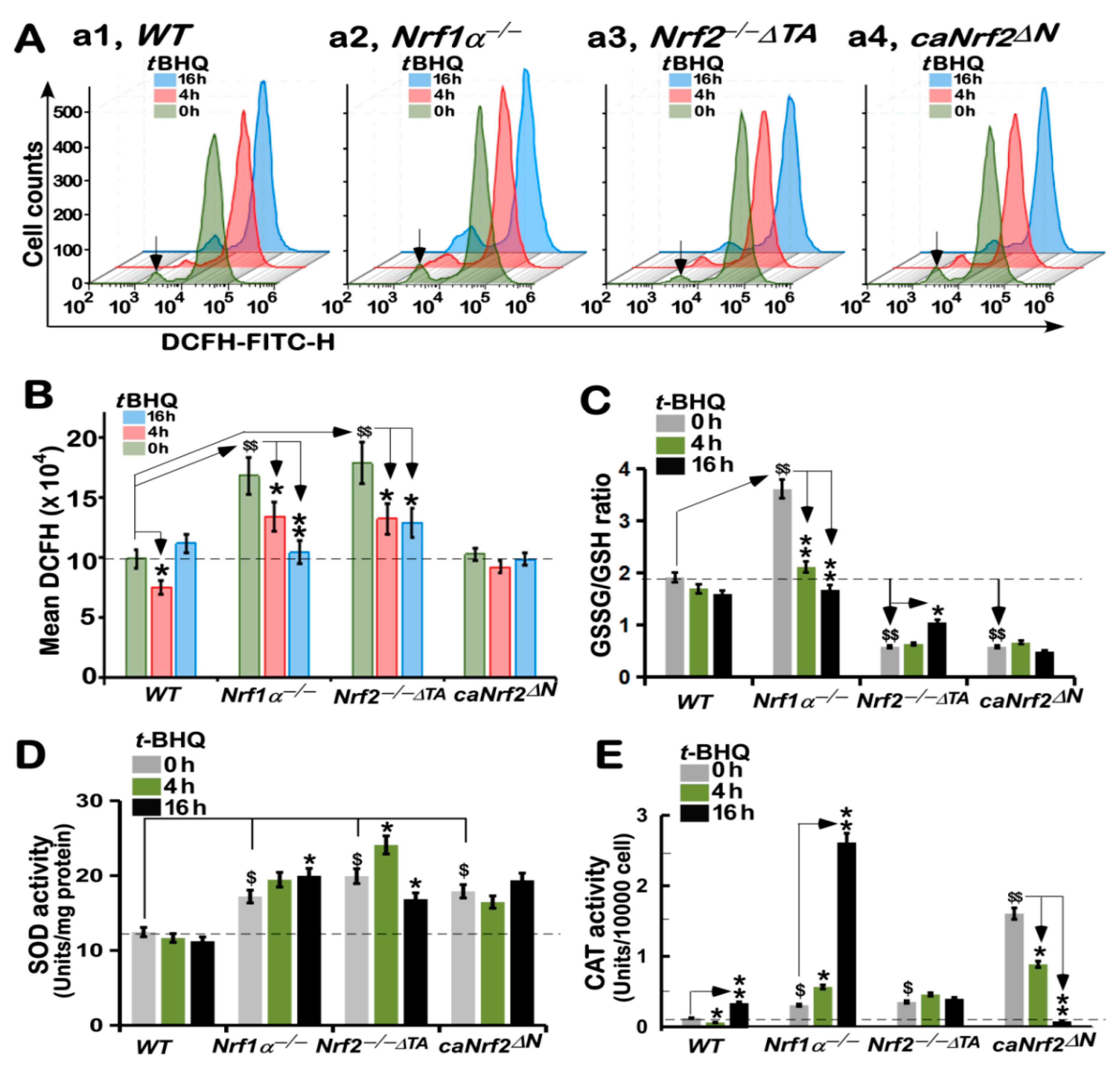
3.7. Different Antioxidant Responses of Four Distinct Genotypic Cell Lines to tBHQ as a Pro-Oxidative Stressor
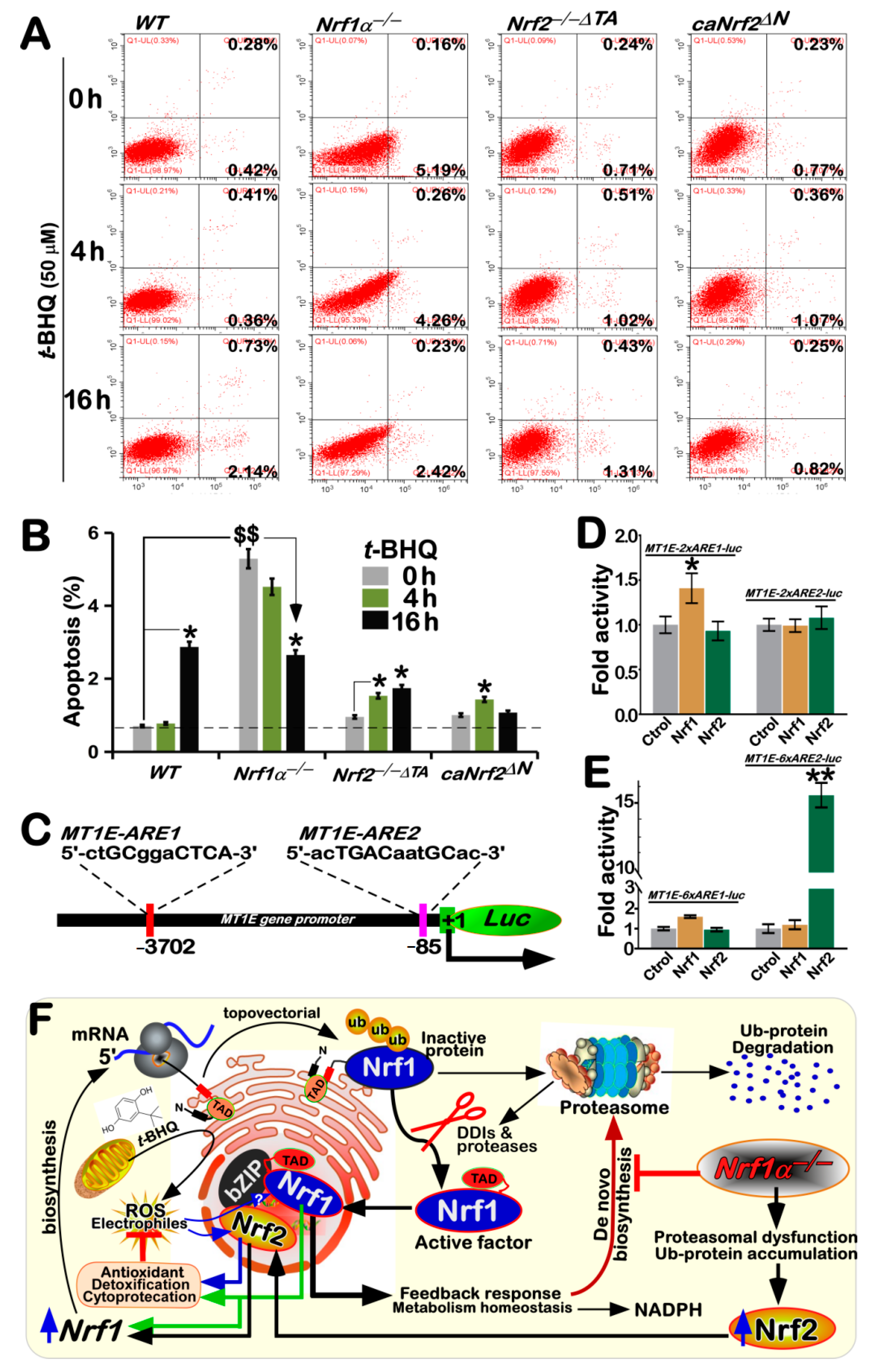
3.8. Distinct Roles of Nrf1 and Nrf2 in Different Cell Apoptosis Induced by tBHQ as a Pro-Oxidative Stressor
4. Discussion
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahidi, F. Antioxidants in food and food antioxidants. Food Nahr. 2000, 44, 158–163. [Google Scholar] [CrossRef]
- Oswell, N.J.; Thippareddi, H.; Pegg, R.B. Practical use of natural antioxidants in meat products in the U.S.: A review. Meat Sci. 2018, 145, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Conning, D.M.; Phillips, J.C. Comparative metabolism of BHA, BHT and other phenolic antioxidants and its toxicological relevance. Food Chem. Toxicol. 1986, 24, 1145–1148. [Google Scholar] [CrossRef]
- van Esch, G. Toxicology of tert-butylhydroquinone (TBHQ). Food Chem. Toxicol. 1986, 24, 1063–1065. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kumagai, T.; Yoshida, C.; Naito, Y.; Miyamoto, M.; Ohigashi, H.; Osawa, T.; Uchida, K. Pivotal role of electrophilicity in glutathione S-transferase induction by tert-butylhydroquinone. Biochemistry 2003, 42, 4300–4309. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, N.; Haggarty, S.; El-Kadi, A.S. Chemoprotective and carcinogenic effects of tert-butylhydroquinone and its metabolites. Curr. Drug Metab. 2007, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, N.; El-Kadi, A.O.S. tert-butylhydroquinone is a novel aryl hydrocarbon receptor ligand. Drug Metab. Dispos. 2004, 33, 365–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Lu, F.; Stewart, D.; Zhang, Y. Mechanisms underlying chemopreventive effects of flavonoids via multiple signaling nodes within Nrf2-ARE and AhR-XRE gene regulatory networks. Curr. Chem. Biol. 2013, 7, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharmacal Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Deneke, S.M. Thiol-based antioxidants. Curr. Top Cell Regul. 2000, 36, 151–180. [Google Scholar]
- Yamamoto, M.; Kensler, T.; Motohashi, H. The KEAP1-NRF2 System: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xiang, Y. Molecular and cellular basis for the unique functioning of Nrf1, an indispensable transcription factor for maintaining cell homoeostasis and organ integrity. Biochem. J. 2016, 473, 961–1000. [Google Scholar] [CrossRef]
- Sant, K.; Hansen, J.M.; Williams, L.; Tran, N.L.; Goldstone, J.; Stegeman, J.J.; Hahn, M.; Timme-Laragy, A. The role of Nrf1 and Nrf2 in the regulation of glutathione and redox dynamics in the developing zebrafish embryo. Redox Biol. 2017, 13, 207–218. [Google Scholar] [CrossRef]
- Leung, L.; Kwong, M.; Hou, S.; Lee, C.; Chan, J.Y.; Jenkins-Kruchten, A.E.; Bennaars-Eiden, A.; Ross, J.R.; Shen, W.-J.; Kraemer, F.B.; et al. Deficiency of the Nrf1 and Nrf2 Transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 2003, 278, 48021–48029. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Magilnick, N.; Lee, C.; Kalmaz, D.; Ou, X.; Chan, J.Y.; Lu, S.C. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NFƘB and AP-1. Mol. Cell. Biol. 2005, 25, 5933–5946. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuji, M.; Katsuoka, F.; Kobayashi, A.; Aburatani, H.; Hayes, J.; Yamamoto, M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J. Biol. Chem. 2008, 283, 33554–33562. [Google Scholar] [CrossRef] [Green Version]
- Tusi, S.K.; Nouhi, F.; Abdi, A.; Khodagholi, F. Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid beta-injected rat. J. Biotechnol. 2010, 150, 455. [Google Scholar] [CrossRef]
- Lau, A.; Whitman, S.A.; Jaramillo, M.C.; Zhang, N.D. Arsenic-mediated activation of the Nrf2-Keap1 antioxidant pathway. J. Biochem. Mol. Toxicol. 2012, 27, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Parastan, R.H.; Christopher, M.; Torrys, Y.S.; Mahadewa, T.G.B. Combined therapy potential of apocynin and tert-butylhydroquinone as a therapeutic agent to prevent secondary progression to traumatic brain injury. Asian J. Neurosurg. 2020, 15, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kwong, M.; Kan, Y.W.; Chan, J.Y. The CNC basic leucine zipper factor, Nrf1, Is essential for cell survival in response to oxidative stress-inducing agents. J. Biol. Chem. 1999, 274, 37491–37498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Kwong, M.; Lu, R.; Ginzinger, D.; Lee, C.; Leung, L.; Chan, J.Y. Nrf1 Is critical for redox balance and survival of liver cells during development. Mol. Cell. Biol. 2003, 23, 4673–4686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Chen, L.; Leung, L.; Yen, T.S.B.; Lee, C.; Chan, J.Y. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc. Natl. Acad. Sci. USA 2005, 102, 4120–4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40. [Google Scholar] [CrossRef]
- Cuadrado, A.I.; Rojo, A.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.; Lu, R.; Chang, J.C.; Kan, Y.W. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 1996, 93, 13943–13948. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Huang, M.-T.; Shen, G.; Yuan, X.; Lin, W.; Khor, T.O.; Conney, A.H.; Kong, A.-N.T. Inhibition of 7,12-Dimethylbenz(a)anthracene-Induced Skin Tumorigenesis in C57BL/6 Mice by sulforaphane is mediated by nuclear factor e2–related factor. Cancer Res. 2006, 66, 8293–8296. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Hayes, J.D.; McMahon, M.; Chowdhry, S.; Dinkova-Kostova, A. Cancer Chemoprevention mechanisms mediated through the Keap1–Nrf2 Pathway. Antioxid. Redox Signal. 2010, 13, 1713–1748. [Google Scholar] [CrossRef] [PubMed]
- De La Vega, M.R.; Chapman, E.; Zhang, D.D. NRF2 and the hallmarks of cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Wang, M.; Hu, S.; Ru, X.; Ren, Y.; Zhang, Z.; Yu, S.; Zhang, Y. Oncogenic Activation of Nrf2, though as a master antioxidant transcription factor, liberated by specific knockout of the full-length Nrf1α that acts as a dominant tumor repressor. Cancers 2018, 10, 520. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhang, S.; Zhang, Y. Nrf1 is paved as a new strategic avenue to prevent and treat cancer, neurodegenerative and other diseases. Toxicol. Appl. Pharmacol. 2018, 360, 273–283. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Xiang, Y.; Ru, X.; Ren, Y.; Liu, X.; Qiu, L.; Zhang, Y. Nrf1 Is Endowed with a Dominant Tumor-Repressing Effect onto the Wnt/ß-Catenin-Dependent and Wnt/ß-catenin-independent signaling networks in the human liver cancer. Oxid. Med. Cell. Longev. 2020, 2020, 5138539. [Google Scholar] [CrossRef]
- Farmer, S.C.; Sun, C.W.E.; Winnier, G.; Hogan, B.L.; Townes, T.M. The bZIP transcription factor LCR-F1 is essential for mesoderm formation in mouse development. Genes Dev. 1997, 11, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.Y.; Kwong, M.; Lu, R.; Chang, J.; Wang, B.; Yen, T.S.; Kan, Y.W. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. Embo J. 1998, 17, 1779–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsujita, T.; Peirce, V.; Baird, L.; Matsuyama, Y.; Takaku, M.; Walsh, S.V.; Griffin, J.L.; Uruno, A.; Yamamoto, M.; Hayes, J.D. Transcription Factor Nrf1 Negatively Regulates the Cystine/Glutamate Transporter and Lipid-Metabolizing Enzymes. Mol. Cell. Biol. 2014, 34, 3800–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.; Fu, J.; Xue, P.; Zhao, R.; Dong, J.; Liu, D.; Yamamoto, M.; Tong, Q.; Teng, W.; Qu, W.; et al. CNC-bZIP protein Nrf1-dependent regulation of glucose-stimulated insulin secretion. Antioxid. Redox Signal. 2015, 22, 819–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, A.; Tsukide, T.; Miyasaka, T.; Morita, T.; Mizoroki, T.; Saito, Y.; Ihara, Y.; Takashima, A.; Noguchi, N.; Fukamizu, A.; et al. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Genes Cells 2011, 16, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Hu, T.; Nguyen, J.M.; Zhang, J.; Martin, M.V.; Vawter, M.P.; Huang, E.J.; Chan, J.Y. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 8408–8413. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.P.; Zheng, Z.; Hu, S.; Ru, X.; Fan, Z.; Qiu, L.; Zhang, Y. Unification of opposites between two antioxidant transcription factors Nrf1 and Nrf2 in mediating distinct cellular responses to the endoplasmic reticulum stressor tunicamycin. Antioxidants 2019, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Wang, M.; Hu, S.; Qiu, L.; Yang, F.; Zhang, Z.; Yu, S.; Pi, J.; Zhang, Y. Mechanisms controlling the multistage post-translational processing of endogenous Nrf1α/TCF11 proteins to yield distinct isoforms within the coupled positive and negative feedback circuits. Toxicol. Appl. Pharmacol. 2018, 360, 212–235. [Google Scholar] [CrossRef]
- Hanczko, R.; Fernandez, D.R.; Doherty, E.; Qian, Y.; Vas, G.; Niland, B.; Telarico, T.; Garba, A.; Banerjee, S.; Middleton, F.A.; et al. Prevention of hepatocarcinogenesis and increased suscepti-bility to acetaminophen-induced liver failure in transaldolase-deficient mice by N-acetylcysteine. J. Clin. Investig. 2009, 119, 1546–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavić, M.; Turčić, P.; Ljubojević, M. Forgotten partners and function regulators of inducible metallothioneins. Arch. Ind. Hyg. Toxicol. 2019, 70, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized dcfda and flow-cytometry. Microglia 2009, 594, 57–72. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Zheng, Z.; Xiang, Y.; Zhang, Y. Glucose Starvation-Induced Rapid Death of Nrf1α-Deficient, but Not Nrf2-Deficient, hepatoma cells results from its fatal defects in the redox metabolism reprogramming. Oxidative Med. Cell. Longev. 2020, 2020, 1–20. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Zhang, Y. Molecular and Cellular Control of the Nrf1 Transcription Factor: An Integral Membrane Glycoprotein, 1st ed.; Verlag Dr Müller Publishing House: Saarbrücken, Germany, 2009; pp. 1–264. [Google Scholar]
- Sykiotis, G.P.; Bohmann, D. Stress-Activated Cap’n’collar Transcription Factors in Aging and Human Disease. Sci. Signal. 2010, 3, re3. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.P.; Wang, M.; Xiang, Y.; Qiu, L.; Hu, S.; Zhang, Z.; Mattjus, P.; Zhu, X.; Zhang, Y. Nach Is a Novel Subgroup at an Early Evolutionary Stage of the CNC-bZIP Subfamily Transcription Factors from the Marine Bacteria to Humans. Int. J. Mol. Sci. 2018, 19, 2927. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Kerins, M.J.; Tian, W.; Neupane, D.; Zhang, D.D.; Ooi, A. Differential and overlapping targets of the transcriptional regulators NRF1, NRF2, and NRF3 in human cells. J. Biol. Chem. 2019, 294, 18131–18149. [Google Scholar] [CrossRef] [PubMed]
- Lehrbach, N.J.; Ruvkun, G. Endoplasmic reticulum-associated SKN-1A/Nrf1 mediates a cytoplasmic unfolded protein response and promotes longevity. ELife 2019, 8, e44425. [Google Scholar] [CrossRef]
- Karim, M.R.; Taniguchi, H.; Kobayashi, A. Constitutive activation of Drosophila CncC transcription factor reduces lipid formation in the fat body. Biochem. Biophys. Res. Commun. 2015, 463, 693–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Li, S.; Hayes, J. Transcription Factor Nrf1 Is Topologically Repartitioned across Membranes to Enable Target Gene Transactivation through Its Acidic Glucose-Responsive Domains. PLoS ONE 2014, 9, e93458. [Google Scholar] [CrossRef]
- Xiang, Y.; Halin, J.; Fan, Z.; Hu, S.; Wang, M.; Qiu, L.; Zhang, Z.; Mattjus, P.; Zhang, Y. Topovectorial mechanisms control the juxtamembrane proteolytic processing of Nrf1 to remove its N-terminal polypeptides during maturation of the CNC-bZIP factor. Toxicol. Appl. Pharmacol. 2018, 360, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, Y.; Hu, S.; Liu, K.; Qiu, L.; Zhang, Y. TCF11 Has a Potent Tumor-Repressing Effect Than Its Prototypic Nrf1α by definition of both similar yet different regulatory profiles, with a striking disparity from Nrf2. Front. Oncol. 2021, 11, 707032. [Google Scholar] [CrossRef] [PubMed]
| ID | Name | Forward Primers (5′–3′) | Reverse Primers (5′–3′) |
|---|---|---|---|
| 60 | β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| 4776 | Nrf1 | GAAGCCCACCAAGACCGAA | GCCTCTTCCTGTACACTGACC |
| 4780 | Nrf2 | ATATTCCCGGTCACATCGAGA | ATGTCCTGTTGCATACCGTCT |
| 2729 | GCLC | TCAATGGGAAGGAAGGTGTGTT | TCAATGGGAAGGAAGGTGTGTT |
| 2730 | GCLM | TCAATGGGAAGGAAGGTGTGTT | CGCTTGAATGTCAGGAATGCTT |
| 2876 | Gpx1 | CAGTCGGTGTATGCCTTCTCG | GAGGGACGCCACATTCTCG |
| 2936 | GSR | CACGAGTGATCCCAAGCCC | CAATGTAACCTGCACCAACAATG |
| 6888 | TALDO | GGGCCGAGTATCCACAGAAG | GGCGAAGGAGAAGAGTAACG |
| 1728 | NQO1 | AAGAAGAAAGGATGGGAGGTGG | GAACAGACTCGGCAGGATACTG |
| 3162 | HO-1 | CAGAGCCTGGAAGACACCCTAA | AAACCACCCCAACCCTGCTAT |
| 4493 | MT1E | ATGGACCCCAACTGCTCTTGCGCCA | ACAGCAGCTGCACTTCTCCGATG |
| 4502 | MT2 | GTGGGCTGTGCCAAGTGT | CAAACGGTCACGGTCAGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wufuer, R.; Fan, Z.; Liu, K.; Zhang, Y. Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor. Antioxidants 2021, 10, 1610. https://doi.org/10.3390/antiox10101610
Wufuer R, Fan Z, Liu K, Zhang Y. Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor. Antioxidants. 2021; 10(10):1610. https://doi.org/10.3390/antiox10101610
Chicago/Turabian StyleWufuer, Reziyamu, Zhuo Fan, Keli Liu, and Yiguo Zhang. 2021. "Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor" Antioxidants 10, no. 10: 1610. https://doi.org/10.3390/antiox10101610
APA StyleWufuer, R., Fan, Z., Liu, K., & Zhang, Y. (2021). Differential Yet Integral Contributions of Nrf1 and Nrf2 in the Human HepG2 Cells on Antioxidant Cytoprotective Response against Tert-Butylhydroquinone as a Pro-Oxidative Stressor. Antioxidants, 10(10), 1610. https://doi.org/10.3390/antiox10101610








