MTHFR Gene Mutations Correlate with White Matter Disease Burden and Predict Cerebrovascular Disease and Dementia
Abstract
1. Introduction
2. MTHFR Genetic Variant
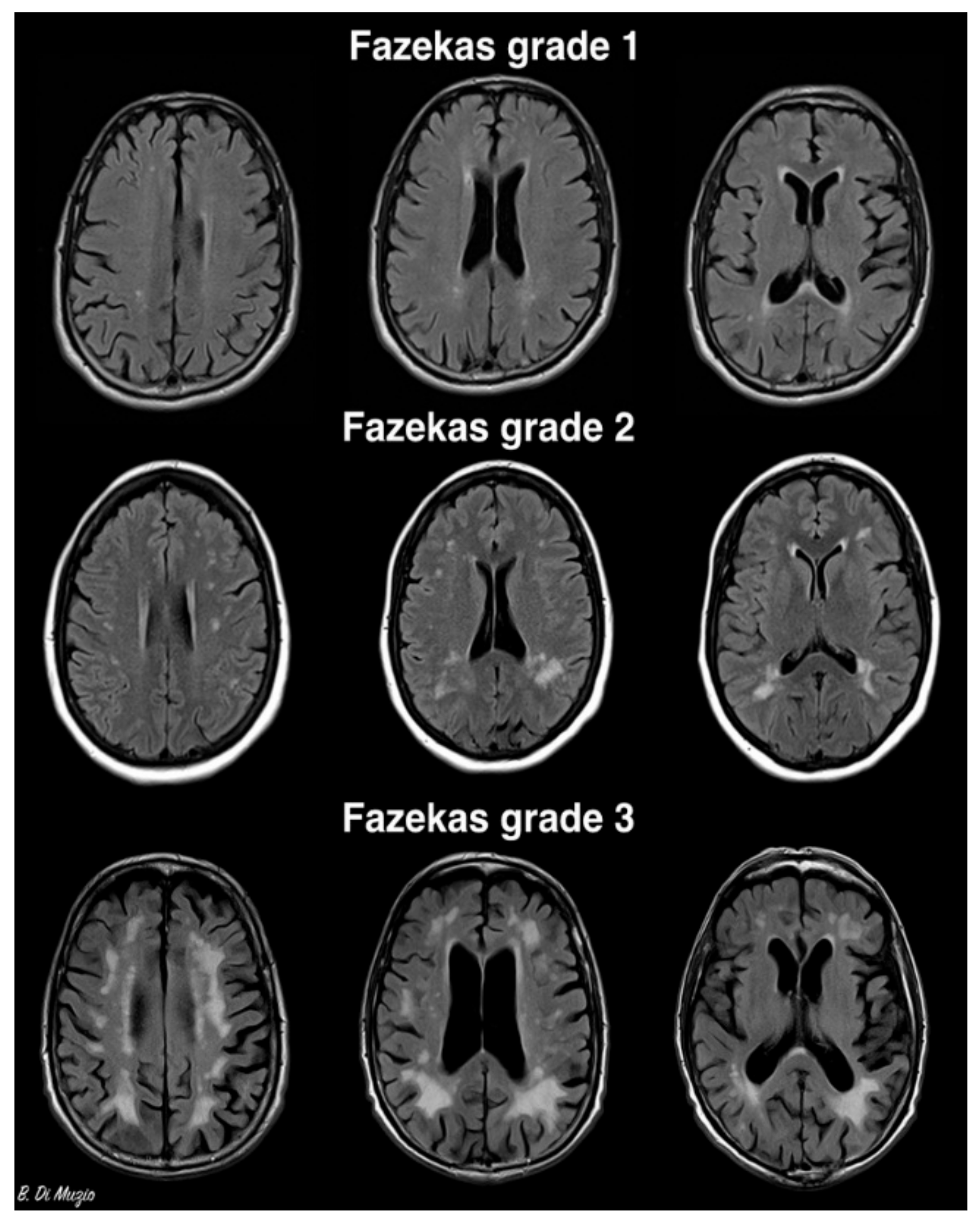
3. Epidemiology
4. Diagnosis
5. Susceptibility to Dementia
6. Small Vessel Disease and Stroke
7. Management
8. Conclusions
Conflicts of Interest
References
- World Alzheimer Report 2015. The Global Impact of Dementia; An Analysis of Prevalence, Incidence, Cost and Trends. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 10 October 2017).
- World Health Organization. Dementia: Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 10 October 2017).
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Fujihara, R.; Uemura, N.; Kamada, M.; Yanase, K. Disturbance of Intracerebral Fluid Clearance and Blood-Brain Barrier in Vascular Cognitive Impairment. Int. J. Mol. Sci. 2019, 20, 2600. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino RBWilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Chen, S. Hyperhomocysteinemia and risk of incident cognitive outcomes: An updated dose-response meta-analysis of prospective cohort studies. Ageing Res. Rev. 2019, 51, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Mancera-Páez, O.; Bernal, C. Epigenetic Factors in Late-Onset Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 319. [Google Scholar] [CrossRef]
- Matthews, R.G. Methylenetetrahydrofolate reductase: A common human polymorphism and its biochemical implications. Chem. Rec. 2002, 2, 4–12. [Google Scholar] [CrossRef]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kåreholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Mühlmann, M.; Ertl-Wagner, B.; et al. Association of Vitamin B12, Folate, and Sulfur Amino Acids With Brain Magnetic Resonance Imaging Measures in Older Adults: A Longitudinal Population-Based Study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef]
- Shevchuk, S.V.; Postovitenko, K.P.; Iliuk, I.A.; Bezsmertna, H.V.; Bezsmertnyi, Y.O.; Kurylenko, I.V.; Biloshytska, A.V.; Baranova, I.V. The relationship between homocysteine level and vitamins B12, B9 and B6 status in patients with chronic kidney disease. Wiad. Lek. 2019, 72, 532–538. [Google Scholar]
- Can, M.; Açikgöz, S.; Mungan, G.; Bayraktaroğlu, T.; Koçak, E.; Güven, B.; Demirtas, S. Serum cardiovascular risk factors in obstructive sleep apnea. Chest 2006, 129, 233–237. [Google Scholar] [CrossRef]
- Dean, L.; Pratt, V.; McLeod, H.; Rubinstein, W.; Malheiro, A. Methylenetetrahydrofolate Reductase Deficiency; Medical Genetics Summaries [Internet], National Center for Biotechnology Information: Bethesda, MD, USA, 2012. [Google Scholar]
- Román, G.C. MTHFR Gene Mutations: A Potential Marker of Late-Onset Alzheimer’s Disease? J. Alzheimers Dis. 2015, 47, 323–327. [Google Scholar] [CrossRef]
- Zhuo, J.M.; Praticò, D. Acceleration of brain amyloidosis in an Alzheimer’s disease mouse model by a folate, vitamin B6 and B12-deficient diet. Exp. Gerontol. 2010, 45, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Mutchinick, O.M.; Lopez, M.A.; Luna, L.; Waxman, J.; Babinsky, V.E. High prevalence of the thermolabile methylenetetrahydrofolate reductase variant in Mexico: A country with a very high prevalence of neural tube defects. Mol. Genet. Metab. 1999, 68, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Balderrabano-Saucedo, N.A.; Sanchez-Urbina, R.; Sierra-Ramirez, J.A.; Garcia-Hernandez, N.; Sanchez-Boiso, A.; Klunder-Klunder, M.; Arena-Aranda, D.; Bravo-Hernandez, G.; Noriega-Zapata, P.; Vizcaino-Alarcon, A.; et al. Polymorphism 677C-T MTHFR gene in Mexican mothers of children with complex congenital heart disease. Pediatr. Cardiol. 2013, 34, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Y.; Li, Y.; Fan, S.; Zhi, X.; Lu, X.; Wang, D.; Zheng, Q.; Wang, Y.; Wang, Y.; et al. Geographical distribution of MTHFR C677T, A1298C and MTRR A66G gene polymorphisms in China: Findings from 15357 adults of Han nationality. PLoS ONE 2013, 8, e57917. [Google Scholar] [CrossRef] [PubMed]
- Nexo, E.; Hoffmann-Lücke, E. Holotranscobalamin, a marker of vitamin B-12 status: Analytical aspects and clinical utility. Am. J. Clin. Nutr. 2011, 94, 359S–365S. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wei, S.; Song, B.; Qin, J.; Fang, H.; Ji, Y.; Zhang, R.; Sun, S.; Xu, Y. Homocysteine Level Is Associated with White Matter Hyperintensity Locations in Patients with Acute Ischemic Stroke. PLoS ONE 2015, 10, e0144431. [Google Scholar] [CrossRef]
- Tran, T.; Cotlarciuc, I.; Yadav, S.; Hasan, N.; Bentley, P.; Levi, C.; Worrall, B.; Meschia, J.; Rost, N.; Sharma, P. Candidate-gene analysis of white matter hypertintensities on neuroimaging. J. Neurol. Neurosurg. Psychiatry 2016, 87, 260–266. [Google Scholar] [CrossRef]
- Ford, A.H.; Alemeida, O.P. Effect of Vitamin B Supplementation on Cognitive Function in the Elderly: A systematic Review and Meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef]
- Liu, H.; Yang, M.; Li, G.M.; Qiu, Y.; Zheng, J.; Du, X.; Wang, J.L.; Liu, R.W. The MTHFR C677T polymorphism contributes to an increased risk for vascular dementia: A meta-analysis. J. Neurol. Sci. 2010, 294, 74–80. [Google Scholar] [CrossRef]
- Roussotte, F.F.; Hua, X.; Narr, K.L.; Small, G.W.; Thompson, P.M.; Initiative AsDN. The C677T variant in MTHFR modulates associations between brain integrity, mood, and cognitive functioning in old age. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.; Taleski, G.; Qian, H.; Wasek, B.; Arning, E.; Bottiglieri, T.; Sontag, J.M.; Sontag, E. Methylenetetrahydrofolate Reductase Deficiency Deregulates a Regional Brain Amyloid-B Protein Precursor Expression and Phosphorylation Levels. J. Alzheimers Dis. 2018, 64, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.T.; Hsu, S.W.; Tsai, S.J.; Chang, Y.T.; Huang, C.W.; Liu, M.E.; Chen, N.C.; Chang, W.N.; Hsu, J.L.; Lee, C.C.; et al. Genetic effect of MTHFR C655T polymorphism on the structural covariance network and white-matter integrity in Alzheimer’s disease. Hum. Brain Mapp. 2017, 38, 3039–3051. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, X.; Kong, W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Attems, J.; Jellinger, K.A. The overlap between vascular disease and Alzheimer’s disease—Lessons from pathology. BMC Med. 2014, 12, 206. [Google Scholar] [CrossRef]
- Irizarry, M.C.; Gurol, M.E.; Raju, S.; Diaz-Arrastia, R.; Locascio, J.J.; Tennis, M.; Hyman, B.T.; Growdon, J.H.; Greenberg, S.M.; Bottiglieri, T.; et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology 2005, 65, 1402–1408. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef]
- Harris, S.; Rasyid, A.; Kurniawan, M.; Mesiano, T.; Hidayat, R. Association of High Blood Homocysteine and Risk of Increased Severity of Ischemic Stroke Events. Int. J. Angiol. 2019, 28, 34–38. [Google Scholar]
- Larsson, S.C.; Traylor, M.; Markus, H.S. Homocysteine and small vessel stroke: A mendelian randomization analysis. Ann. Neurol. 2019, 85, 495–501. [Google Scholar] [CrossRef]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Kleinert, R.; Offenbacher, H.; Schmidt, R.; Kleinert, G.; Payer, F. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 1993, 43, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Griffanti, L.; Jenkinson, M.; Suri, S.; Zsoldos, E.; Mahmood, A.; Filippini, N. Classification and characterization of periventricular and deep white matter hyperintensities on MRI: A study in older adults. Neuroimage 2018, 170, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Rudilosso, S.; San Román, L.; Blasco, J.; Hernández-Pérez, M.; Urra, X.; Chamorro, Á. Evaluation of white matter hypodensities on computed tomography in stroke patients using the Fazekas score. Clin. Imaging 2017, 46, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D.; Yi, Q.; Hankey, G.J. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Hooshmand, B.; Solomon, A.; Kåreholt, I.; Leiviskä, J.; Rusanen, M.; Ahtiluoto, S. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: A longitudinal study. Neurology 2010, 75, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Solomon, A.; Kåreholt, I.; Rusanen, M.; Hänninen, T.; Leiviskä, J. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: A longitudinal study. J. Intern. Med. 2012, 271, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Faux, N.G.; Ellis, K.A.; Porter, L.; Fowler, C.J.; Laws, S.M.; Martins, R.N. Homocysteine, vitamin B12, and folic acid levels in Alzheimer’s disease, mild cognitive impairment, and healthy elderly: Baseline characteristics in subjects of the Australian Imaging Biomarker Lifestyle study. J. Alzheimers Dis. 2011, 27, 909–922. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cajavilca, C.E.; Gadhia, R.R.; Román, G.C. MTHFR Gene Mutations Correlate with White Matter Disease Burden and Predict Cerebrovascular Disease and Dementia. Brain Sci. 2019, 9, 211. https://doi.org/10.3390/brainsci9090211
Cajavilca CE, Gadhia RR, Román GC. MTHFR Gene Mutations Correlate with White Matter Disease Burden and Predict Cerebrovascular Disease and Dementia. Brain Sciences. 2019; 9(9):211. https://doi.org/10.3390/brainsci9090211
Chicago/Turabian StyleCajavilca, Christian E., Rajan R. Gadhia, and Gustavo C. Román. 2019. "MTHFR Gene Mutations Correlate with White Matter Disease Burden and Predict Cerebrovascular Disease and Dementia" Brain Sciences 9, no. 9: 211. https://doi.org/10.3390/brainsci9090211
APA StyleCajavilca, C. E., Gadhia, R. R., & Román, G. C. (2019). MTHFR Gene Mutations Correlate with White Matter Disease Burden and Predict Cerebrovascular Disease and Dementia. Brain Sciences, 9(9), 211. https://doi.org/10.3390/brainsci9090211




