Sex Differences in the Effect of Alcohol Drinking on Myelinated Axons in the Anterior Cingulate Cortex of Adolescent Rats
Abstract
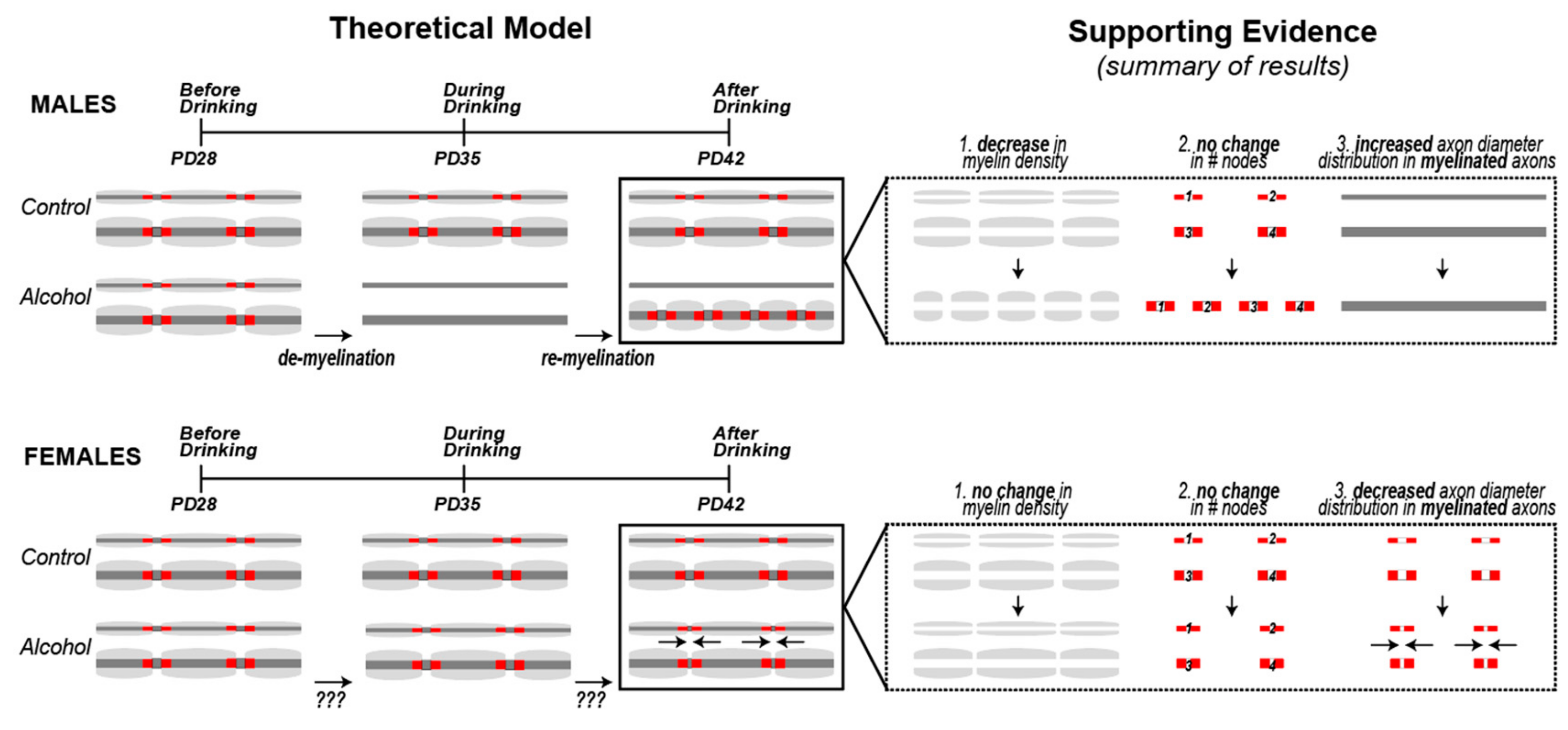
1. Introduction
2. Materials and Methods
2.1. Animals
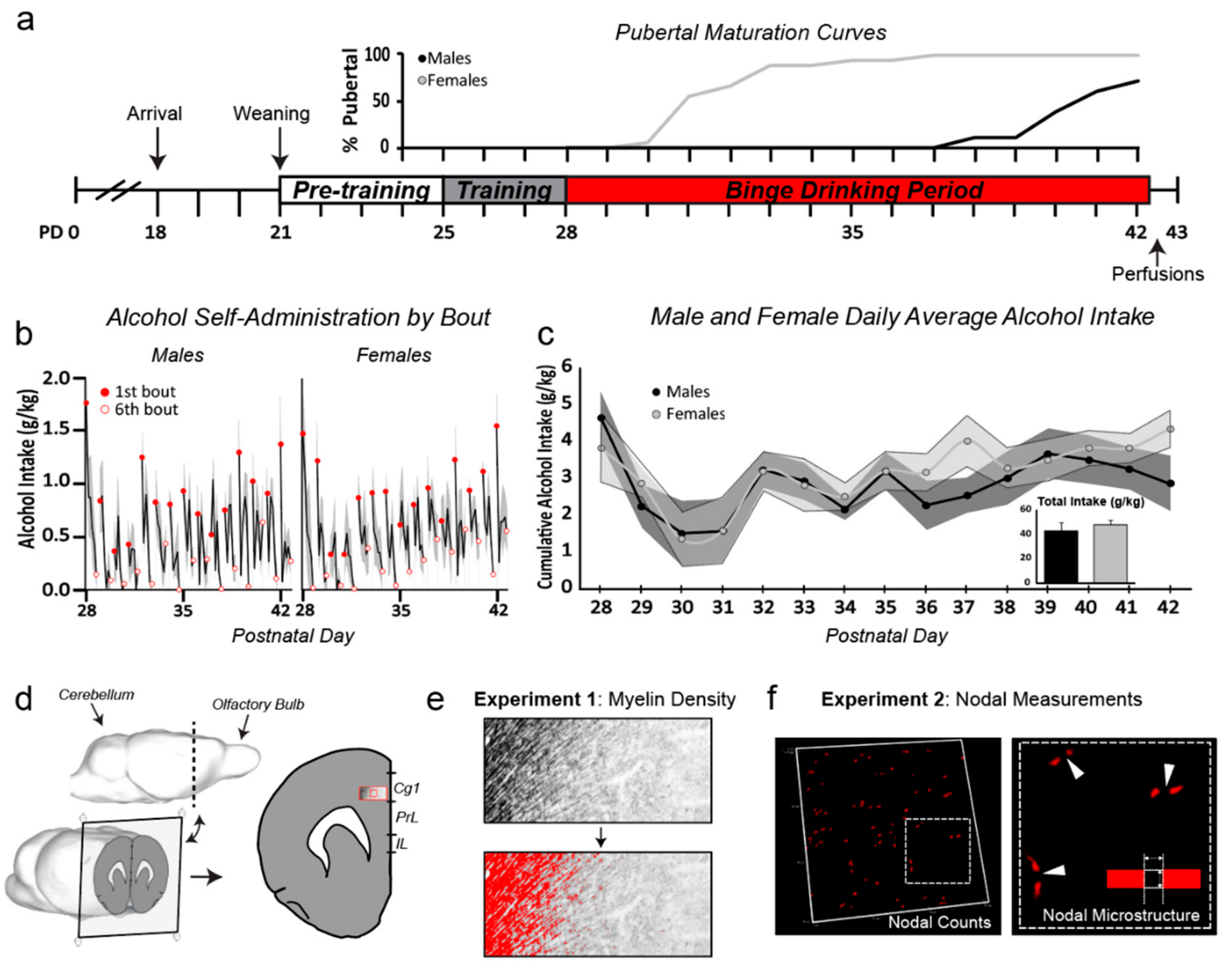
2.2. Experimental Design
2.3. Operant Training and Adolescent Alcohol Exposure
2.4. Perfusions and Brain Sectioning
2.5. Experiment 1: Myelin Labeling and Microscopic Analyses of Myelin Density in the Cg1
2.6. Experiment 2: Labeling and Confocal Analysis of the Node of Ranvier in the Cg1
2.6.1. Immunofluorescent Labeling
2.6.2. Confocal Imaging Acquisition and Nodal Analyses
2.7. Statistical Analyses
3. Results
3.1. Sex Differences in Pubertal Maturation
3.2. Drinking Patterns Were Similar in Males and Females
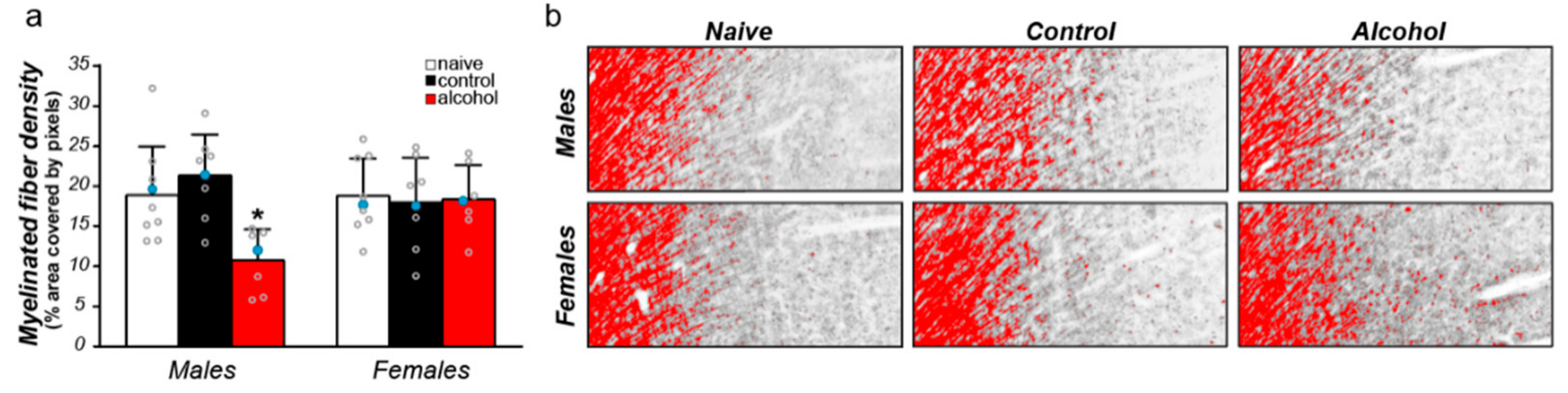
3.3. Sex Differences in the Effect of Alcohol on Myelinated Fiber Density in the Cg1
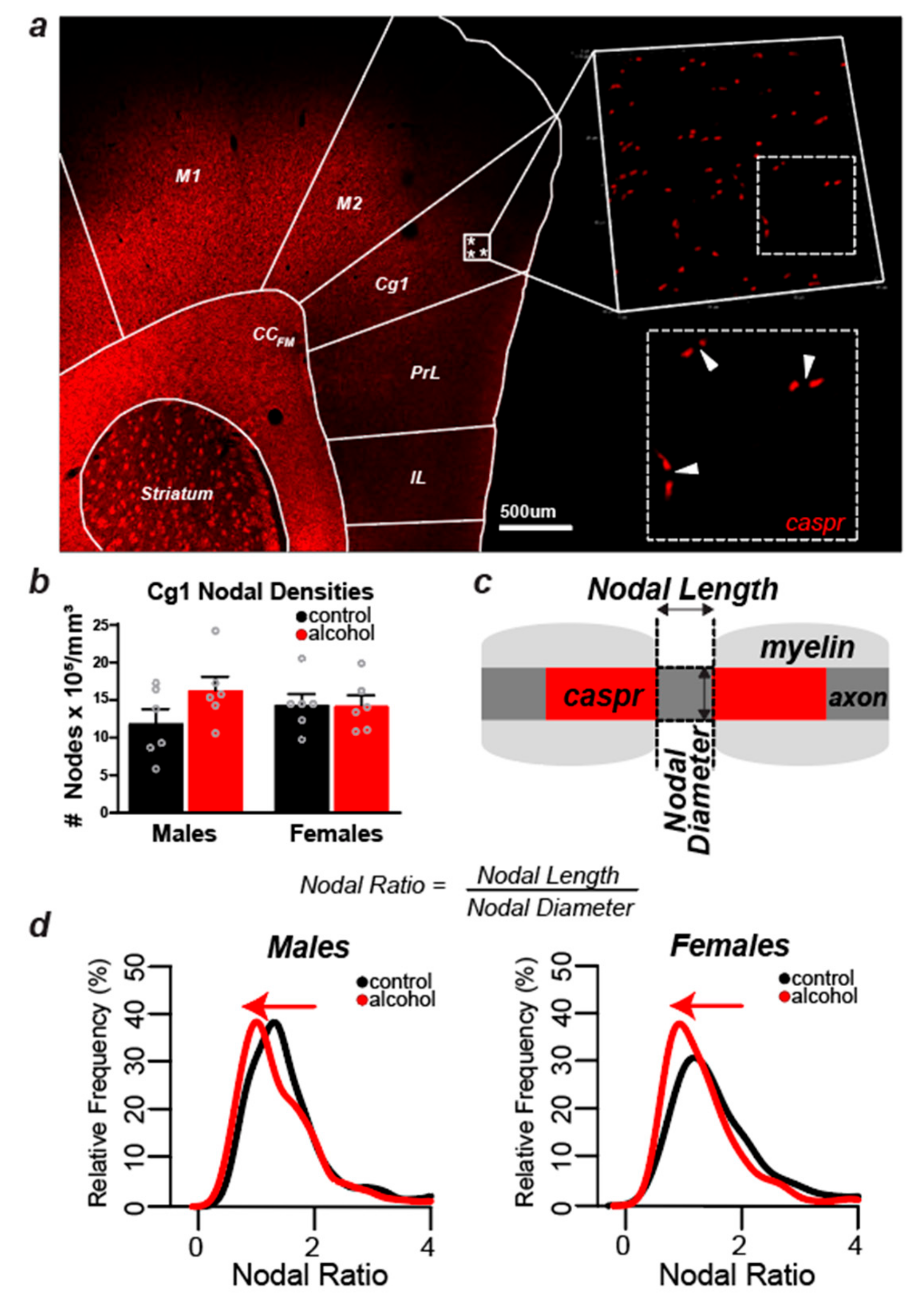
3.4. Alcohol Did Not Cause Measurable Changes in the Number of Nodes of Ranvier in Layers II/III of the Cg1 of Male and Female Rats
3.5. Nodal Ratios Were Reduced by Alcohol in Layers II/III of the Cg1 of Male and Female Rats
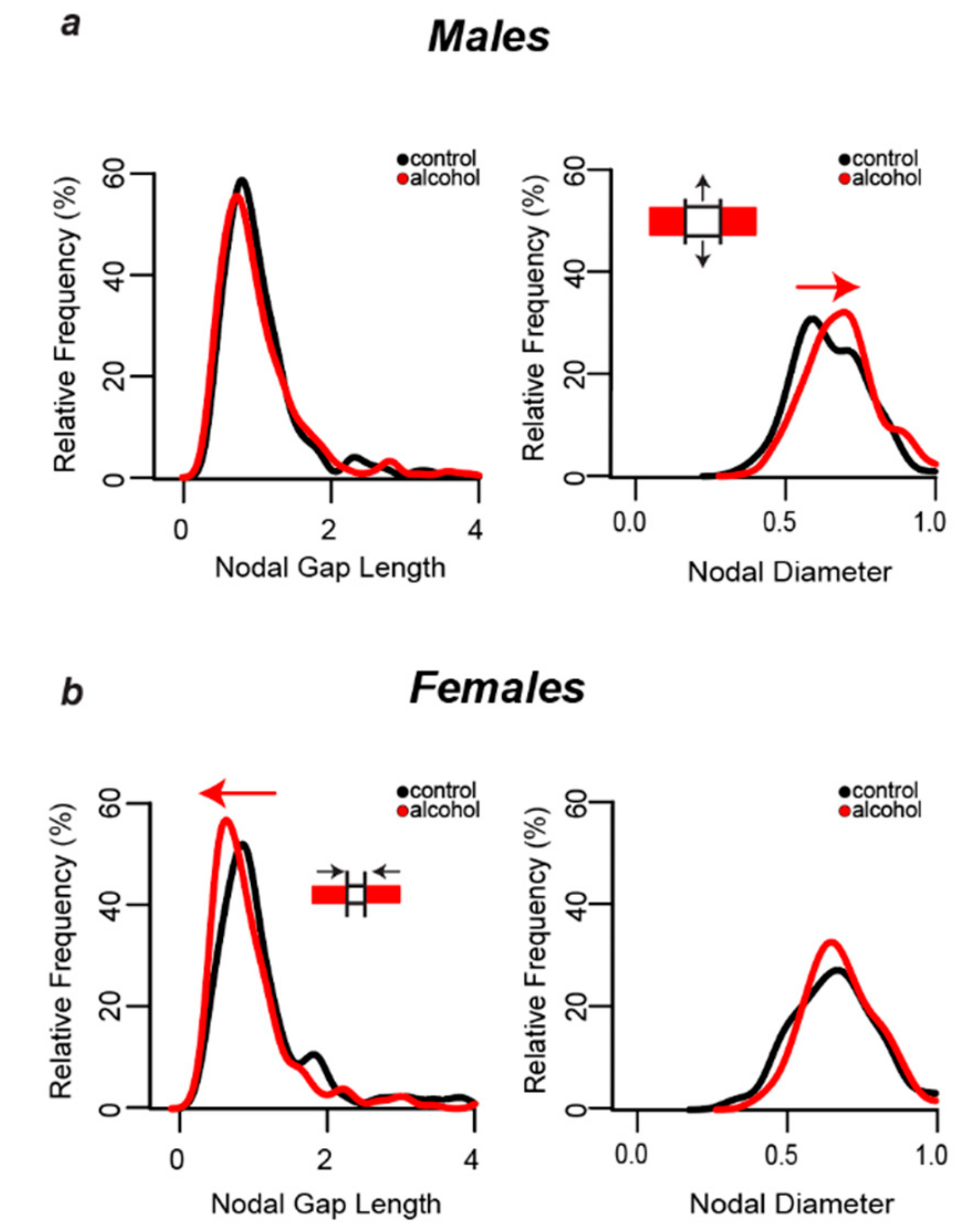
3.6. Alcohol-Induced Decreases in Nodal Ratios Were Due to Larger Nodal Diameters in Males and Shorter Nodal Lengths in Females
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). The Scope of the Problem. Alcohol Res. Heal. 2005, 28, 111–120. [Google Scholar]
- Rehm, J.; Taylor, B.; Room, R. Global burden of disease from alcohol, illicit drugs and tobacco. Drug Alcohol Rev. 2006, 25, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Schuckit, M.A.; Smith, T.L.; Hesselbrock, V.; Bucholz, K.K.; Bierut, L.; Edenberg, H.; Kramer, J.; Longacre, E.; Fukukura, T.; Kalmijn, J.; et al. Clinical Implications of Tolerance to Alcohol in Nondependent Young Drinkers. Am. J. Drug Alcohol Abus. 2008, 34, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N. Structural Magnetic Resonance Imaging of the Adolescent Brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Gee, M.; Camicioli, R.; Wieler, M.; Martin, W.; Beaulieu, C. Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 2012, 60, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Jacobus, J.; McQueeny, T.; Bava, S.; Schweinsburg, B.C.; Frank, L.; Yang, T.T.; Tapert, S.F. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology Teratol. 2009, 31, 349–355. [Google Scholar] [CrossRef] [PubMed]
- McQueeny, T.; Schweinsburg, B.C.; Schweinsburg, A.D.; Jacobus, J.; Bava, S.; Frank, L.R.; Tapert, S.F. Altered White Matter Integrity in Adolescent Binge Drinkers. Alcohol. Clin. Exp. Res. 2009, 33, 1278–1285. [Google Scholar] [CrossRef]
- Bava, S.; Jacobus, J.; Thayer, R.E.; Tapert, S.F. Longitudinal Changes in White Matter Integrity Among Adolescent Substance Users. Alcohol. Clin. Exp. Res. 2013, 37, 181–189. [Google Scholar] [CrossRef]
- Hingson, R.W.; Heeren, T.; Winter, M.R. Age at Drinking Onset and Alcohol Dependence. Arch. Pediatr. Adolesc. Med. 2006, 160, 739. [Google Scholar] [CrossRef]
- Marshall, E.J. Adolescent Alcohol Use: Risks and Consequences. Alcohol Alcohol. 2014, 49, 160–164. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Mahmood, T.; Harris, G.M.; Abbas, S.; Miles, M.F. Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC. Front. Mol. Neurosci. 2017, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Yaxley, R.; Paniagua, B.; Crews, F.T. Diffusion tensor imaging reveals adolescent binge ethanol-induced brain structural integrity alterations in adult rats that correlate with behavioral dysfunction. Addict. Biol. 2016, 21, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Koss, W.; Sadowski, R.; Sherrill, L.; Gulley, J.; Juraska, J. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012, 1466, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.; Pascual, M.; Pla, A.; Maldonado, C.; Rodriguez-Arias, M.; Miñarro, J.; Guerri, C. TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain Behav. Immun. 2015, 45, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Karanikas, C.A.; Richardson, H.N. Adolescent Binge Drinking Leads to Changes in Alcohol Drinking, Anxiety, and Amygdalar Corticotropin Releasing Factor Cells in Adulthood in Male Rats. PLoS ONE 2012, 7, e31466. [Google Scholar] [CrossRef]
- Alfonso-Loeches, S.; Pascual, M.; Gomez-Pinedo, U.; Pascual-Lucas, M.; Renau-Piqueras, J.; Guerri, C. Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia 2012, 60, 948–964. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sullivan, E.V.; Stankovic, R.K.; Harper, C.G.; Pfefferbaum, A. Interaction of Thiamine Deficiency and Voluntary Alcohol Consumption Disrupts Rat Corpus Callosum Ultrastructure. Neuropsychopharmacology 2007, 32, 2207–2216. [Google Scholar] [CrossRef]
- Vargas, W.M.; Bengston, L.; Gilpin, N.W.; Whitcomb, B.W.; Richardson, H.N. Alcohol Binge Drinking during Adolescence or Dependence during Adulthood Reduces Prefrontal Myelin in Male Rats. J. Neurosci. 2014, 34, 14777–14782. [Google Scholar] [CrossRef]
- Karanikas, C.A.; Lu, Y.-L.; Richardson, H.N. Adolescent drinking targets corticotropin-releasing factor (CRF) peptide labeled cells in the central amygdala of male and female rats. Neuroscience 2013, 249, 98–105. [Google Scholar] [CrossRef]
- Ojeda, S.R.; Wheaton, J.E.; Jameson, H.E.; McCann, S.M. The onset of puberty in the female rat: Changes in plasma prolactin, gonadotropins, luteinizing hormone-releasing hormone (LHRH), and hypothalamic LHRH content. Endocrinology 1976, 98, 630–638. [Google Scholar] [CrossRef]
- Sisk, C.L.; Richardson, H.N.; Chappell, P.E.; Levine, J.E. In Vivo Gonadotropin-Releasing Hormone Secretion in Female Rats during Peripubertal Development and on Proestrus 1. Endocrinology 2001, 142, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Korenbrot, C.C. Preputial Separation as an External Sign of Pubertal Development in the Male Rat. Biol. Reprod. 1977, 17, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, D.R.; Ferragud-Agulló, J.; Pérez-Feito, R.; García-Manrique, J.A.; Canals, S.; Moratal, D. A Tangible Educative 3D Printed Atlas of the Rat Brain. Materials 2018, 11, 1531. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.; Bowyer, J.; Cozart, M.; Heard, D.; Binienda, Z.; Paule, M. Introducing Black-Gold II, a highly soluble gold phosphate complex with several unique advantages for the histochemical localization of myelin. Brain Res. 2008, 1229, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Cunningham, M.G.; Bhattacharyya, S.; Benes, F.M. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002, 453, 116–130. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. Available online: https://www.R-project.org/ (accessed on 1 January 2019).
- Vetter-O’Hagen, C.S.; Spear, L.P. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev. Psychobiol. 2012, 54, 523–535. [Google Scholar] [CrossRef]
- Lancaster, F.E.; Brown, T.D.; Coker, K.L.; Elliott, J.A.; Wren, S.B. Sex Differences in Alcohol Preference and Drinking Patterns Emerge during the Early Postpubertal Period in Sprague-Dawley Rats. Alcohol. Clin. Exp. Res. 1996, 20, 1043–1049. [Google Scholar] [CrossRef]
- Almeida, O.F.; Shoaib, M.; Deicke, J.; Fischer, D.; Darwish, M.H.; Patchev, V.K. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J. Clin. Invest. 1998, 101, 2677–2685. [Google Scholar] [CrossRef]
- Juarez, J.; De Tomasi, E.B. Sex Differences in Alcohol Drinking Patterns During Forced and Voluntary Consumption in Rats. Alcohol 1999, 19, 15–22. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Ford, M.M.; Yu, C.-H.; Brown, L.L.; Finn, D.A.; Garland, T.; Crabbe, J.C.; Ford, M. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007, 6, 1–18. [Google Scholar] [CrossRef]
- Iii, W.C.G.; Lopez, M.F.; Becker, H.C.; Griffin, W.C. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol. Clin. Exp. Res. 2009, 33, 1893–1900. [Google Scholar]
- Crabbe, J.C.; Barkley-Levenson, A.M.; Barkley-Levenson, A.M.; Barkley-Levenson, A.M. Ethanol drinking microstructure of a High Drinking in the Dark selected mouse line. Alcohol. Clin. Exp. Res. 2012, 36, 1330–1339. [Google Scholar]
- Wilcox, M.V.; Cuzon Carlson, V.C.; Sherazee, N.; Sprow, G.M.; Bock, R.; Thiele, T.E.; Lovinger, D.M.; Alvarez, V.A. Repeated Binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 2014, 39, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, W.; Shi, Y.; Shi, R.; Cheng, J.-X. Glutamate Excitotoxicity Inflicts Paranodal Myelin Splitting and Retraction. PLoS ONE 2009, 4, e6705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arancibia-Cárcamo, I.L.; Ford, M.C.; Cossell, L.; Ishida, K.; Tohyama, K.; Attwell, D.; Nave, K.-A. Node of Ranvier length as a potential regulator of myelinated axon conduction speed. eLife 2017, 6, 521. [Google Scholar] [CrossRef]
- Johnston, L.D.; Miech, R.A.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E.; Patrick, M.E. Monitoring the Future National Survey Results on Drug Use, 1975–2018: Overview, Key Findings on Adolescent Drug Use; Institute for Social Research, The University of Michigan: Ann Arbor, MI, USA, 2019. [Google Scholar]
- McDougall, S.; Riad, W.V.; Silva-Gotay, A.; Tavares, E.R.; Harpalani, D.; Li, G.-L.; Richardson, H.N. Myelination of Axons Corresponds with Faster Transmission Speed in the Prefrontal Cortex of Developing Male Rats. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Mason, J.L.; Langaman, C.; Morell, P.; Matsushima, G.K.; Suzuki, K. Episodic demyelination and subsequent remyelination within the murine central nervous system: Changes in axonal calibre. Neuropathol. Appl. Neurobiol. 2001, 27, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jarjour, A.A.; Boyd, A.; Williams, A. Central nervous system remyelination in culture—A tool for multiple sclerosis research. Exp. Neurol. 2011, 230, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Lasiene, J.; Matsui, A.; Sawa, Y.; Wong, F.; Horner, P.J. Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell 2009, 8, 201–213. [Google Scholar] [CrossRef]
- Franklin, R.; Hinks, G. Understanding CNS remyelination: Clues from developmental and regeneration biology. J. Neurosci. Res. 1999, 58, 207–213. [Google Scholar] [CrossRef]
- Scurfield, A.; Latimer, D.C. A computational study of the impact of inhomogeneous internodal lengths on conduction velocity in myelinated neurons. PLoS ONE 2018, 13, e0191106. [Google Scholar] [CrossRef] [PubMed]
- Duncan, I.D.; Marik, R.L.; Broman, A.T.; Heidari, M. Thin myelin sheaths as the hallmark of remyelination persist over time and preserve axon function. Proc. Natl. Acad. Sci. USA 2017, 114, E9685–E9691. [Google Scholar] [CrossRef] [PubMed]
- Villalón, E.; Barry, D.M.; Byers, N.; Frizzi, K.; Jones, M.R.; Landayan, D.S.; Dale, J.M.; Downer, N.L.; Calcutt, N.A.; Garcia, M.L. Internode length is reduced during myelination and remyelination by neurofilament medium phosphorylation in motor axons. Exp. Neurol. 2018, 306, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.L.; McQueeny, T.; Nagel, B.J.; Hanson, K.L.; Schweinsburg, A.D.; Tapert, S.F. Prefrontal cortex volumes in adolescents with alcohol use disorders: Unique gender effects. Alcohol. Clin. Exp. Res. 2008, 32, 386–394. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Narasimhan, A.; Thatcher, D.L.; Keshavan, M.S.; Soloff, P.; Clark, D.B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 2005, 29, 1590–1600. [Google Scholar] [CrossRef]
- Blakemore, W.F. Pattern of remyelination in the CNS. Nature 1974, 249, 577–578. [Google Scholar] [CrossRef]
- Ludwin, S.K.; Maitland, M. Long-term remyelination fails to reconstitute normal thickness of central myelin sheaths. J. Neurol. Sci. 1984, 64, 193–198. [Google Scholar] [CrossRef]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef]
- Babbs, C.F.; Shi, R. Subtle Paranodal Injury Slows Impulse Conduction in a Mathematical Model of Myelinated Axons. PLoS ONE 2013, 8, e67767. [Google Scholar] [CrossRef]
- Nie, D.-Y.; Ma, Q.-H.; Law, J.W.; Chia, C.-P.; Dhingra, N.K.; Shimoda, Y.; Yang, W.-L.; Gong, N.; Chen, Q.-W.; Xu, G.; et al. Oligodendrocytes regulate formation of nodes of Ranvier via the recognition molecule OMgp. Neuron Glia Biol. 2006, 2, 151–164. [Google Scholar] [CrossRef][Green Version]
- West, R.K.; Maynard, M.E.; Leasure, J.L. Binge ethanol effects on prefrontal cortex neurons, spatial working memory and task-induced neuronal activation in male and female rats. Physiol. Behav. 2018, 188, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Zamora-Martinez, E.R.; Edwards, S.; Mandyam, C.D. Structural reorganization of pyramidal neurons in the medial prefrontal cortex of alcohol dependent rats is associated with altered glial plasticity. Brain Struct. Funct. 2015, 220, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Cerghet, M.; Skoff, R.P.; Bessert, D.; Zhang, Z.; Mullins, C.; Ghandour, M.S. Proliferation and Death of Oligodendrocytes and Myelin Proteins Are Differentially Regulated in Male and Female Rodents. J. Neurosci. 2006, 26, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Moore, S.; Crawford, D.K.; Hannsun, G.; Sasidhar, M.V.; Tan, K.; Molaie, D.; Tiwari-Woodruff, S.K.; Tiwari-Woodruff, S.K. Attenuation of corpus callosum axon myelination and remyelination in the absence of circulating sex hormones. Brain Pathol. 2013, 23, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Ffrench-Constant, C.; Raff, M.C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature 1986, 319, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Dawson, M. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef]
- Sim, F.J.; Lang, J.K.; Waldau, B.; Roy, N.S.; Schwartz, T.E.; Pilcher, W.H.; Chandross, K.J.; Natesan, S.; Merrill, J.E.; Goldmanm, S.A. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann. Neurol. 2006, 59, 763–779. [Google Scholar] [CrossRef]
- Jung-Testas, I.; Renoir, M.; Bugnard, H.; Greene, G.L.; Baulieu, E.-E. Demonstration of steroid hormone receptors and steroid action in primary cultures of rat glial cells. J. Steroid Biochem. Mol. Biol. 1992, 41, 621–631. [Google Scholar] [CrossRef]
- Bava, S.; Tapert, S.F. Adolescent Brain Development and the Risk for Alcohol and Other Drug Problems. Neuropsychol. Rev. 2010, 20, 398–413. [Google Scholar] [CrossRef]
- Baker, S.T.; Yűcel, M.; Fornito, A.; Allen, N.B.; Lubman, D.I. A systematic review of diffusion weighted MRI studies of white matter microstructure in adolescent substance users. Neurosci. Biobehav. Rev. 2013, 37, 1713–1723. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Tapert, S.F.; Sullivan, E.V.; Jacobus, J.; Meloy, M.J.; Rohlfing, T.; Pfefferbaum, A. Brain Development in Heavy Drinking Adolescents. Am. J. Psychiatry 2015, 172, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Pla, A.; Miñarro, J.; Guerri, C. Neuroimmune activation and myelin changes in adolescent rats exposed to high-dose alcohol and associated cognitive dysfunction: A review with reference to human adolescent drinking. Alcohol Alcohol. 2014, 49, 187–192. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, E.R.; Silva-Gotay, A.; Vargas Riad, W.; Bengston, L.; N. Richardson, H. Sex Differences in the Effect of Alcohol Drinking on Myelinated Axons in the Anterior Cingulate Cortex of Adolescent Rats. Brain Sci. 2019, 9, 167. https://doi.org/10.3390/brainsci9070167
Tavares ER, Silva-Gotay A, Vargas Riad W, Bengston L, N. Richardson H. Sex Differences in the Effect of Alcohol Drinking on Myelinated Axons in the Anterior Cingulate Cortex of Adolescent Rats. Brain Sciences. 2019; 9(7):167. https://doi.org/10.3390/brainsci9070167
Chicago/Turabian StyleTavares, Elizabeth R., Andrea Silva-Gotay, Wanette Vargas Riad, Lynn Bengston, and Heather N. Richardson. 2019. "Sex Differences in the Effect of Alcohol Drinking on Myelinated Axons in the Anterior Cingulate Cortex of Adolescent Rats" Brain Sciences 9, no. 7: 167. https://doi.org/10.3390/brainsci9070167
APA StyleTavares, E. R., Silva-Gotay, A., Vargas Riad, W., Bengston, L., & N. Richardson, H. (2019). Sex Differences in the Effect of Alcohol Drinking on Myelinated Axons in the Anterior Cingulate Cortex of Adolescent Rats. Brain Sciences, 9(7), 167. https://doi.org/10.3390/brainsci9070167




