Applying a Sensing-Enabled System for Ensuring Safe Anterior Cingulate Deep Brain Stimulation for Pain
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Stimulation and Local Field Potential Recordings
2.3. Data Analysis
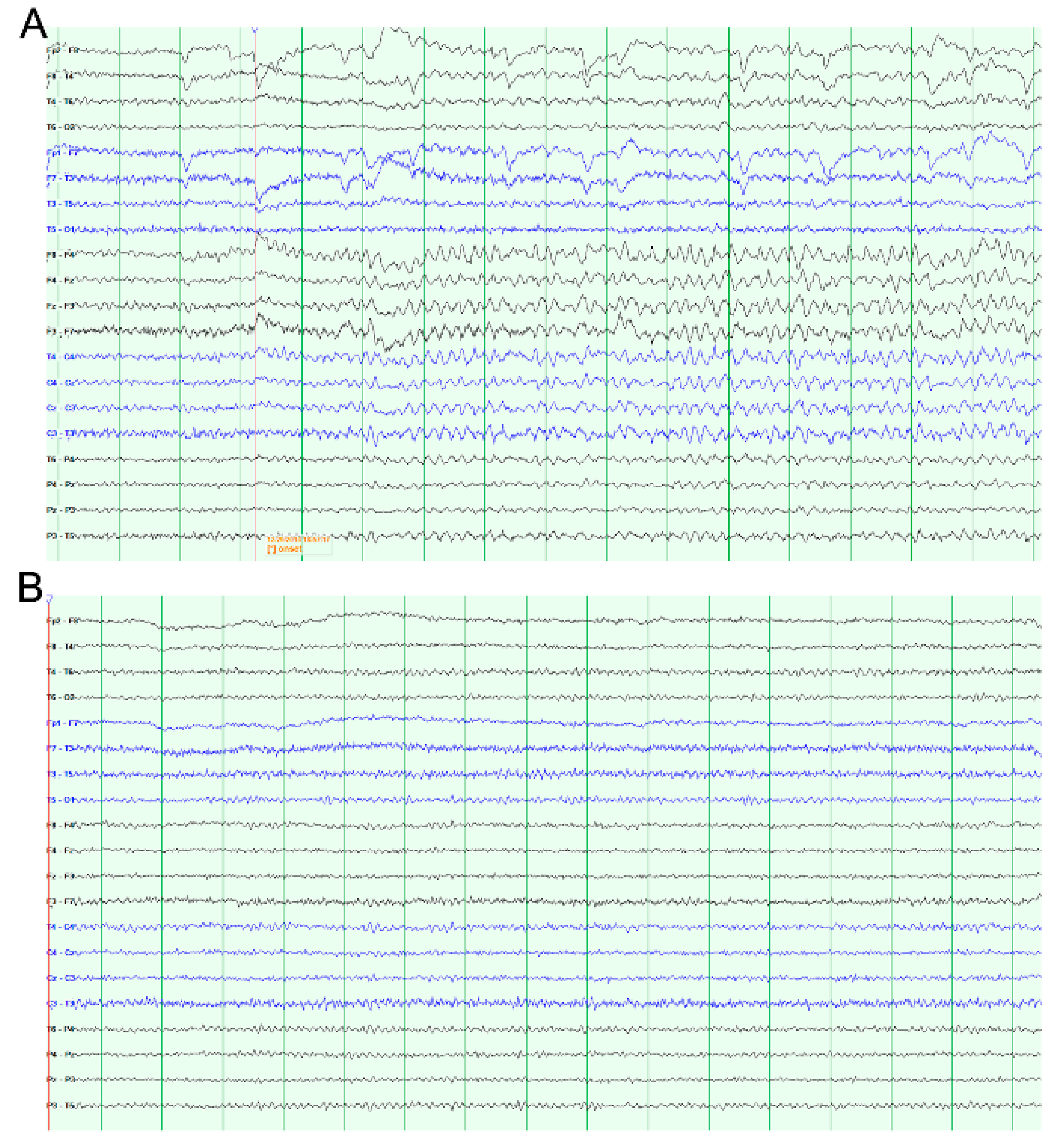
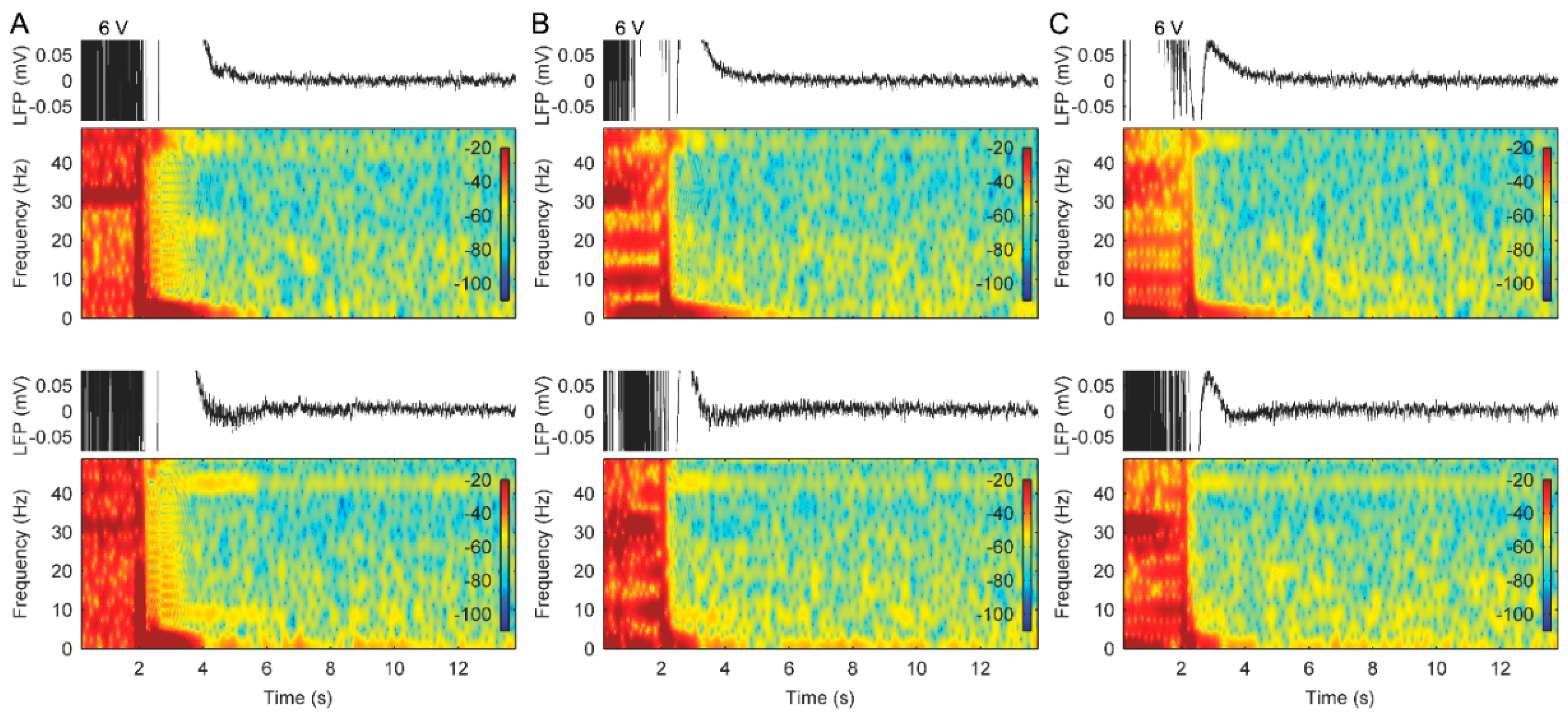
3. Results
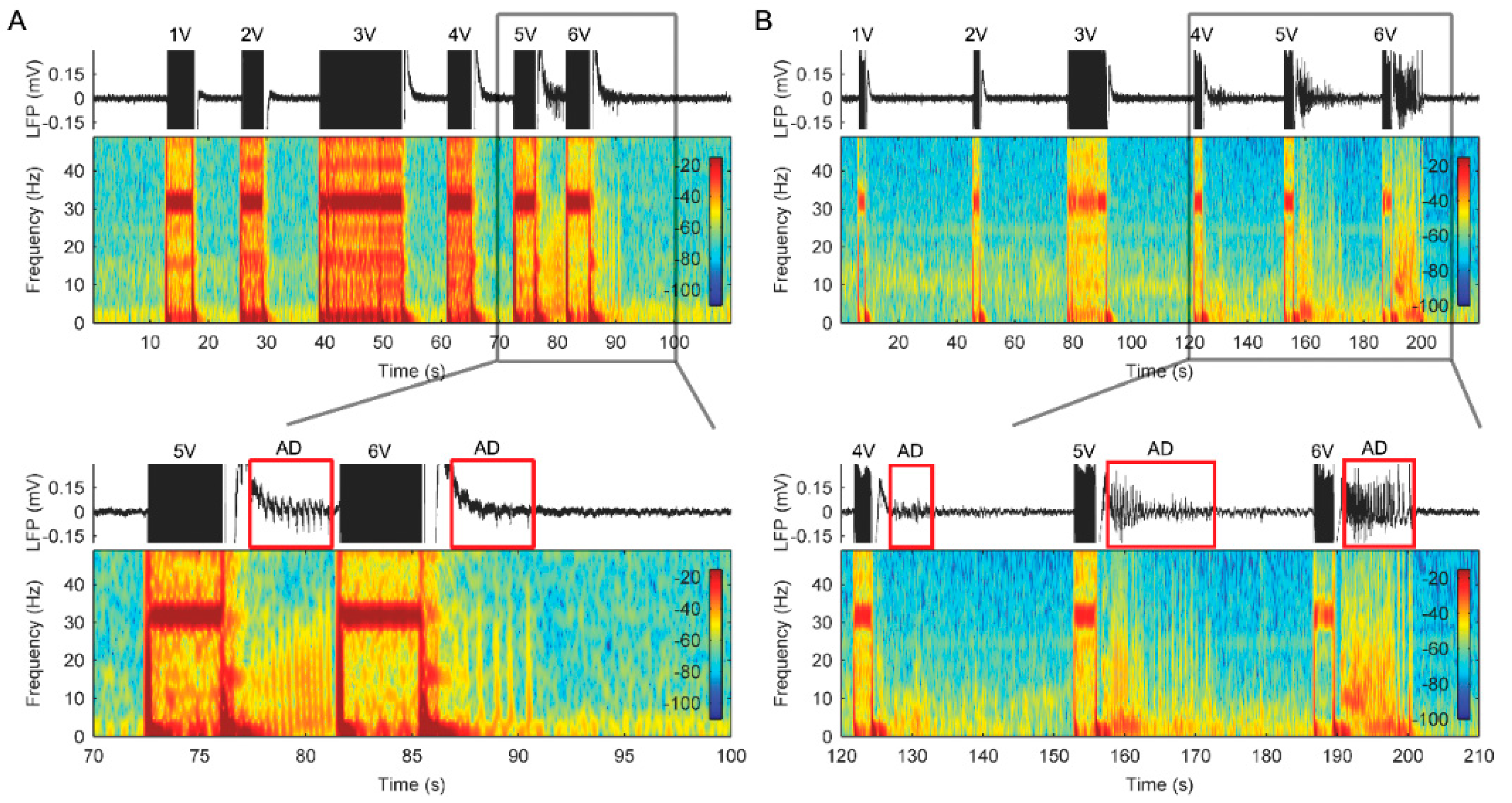
3.1. AD Activity in ACC LFPs Is Induced Following Stimulation
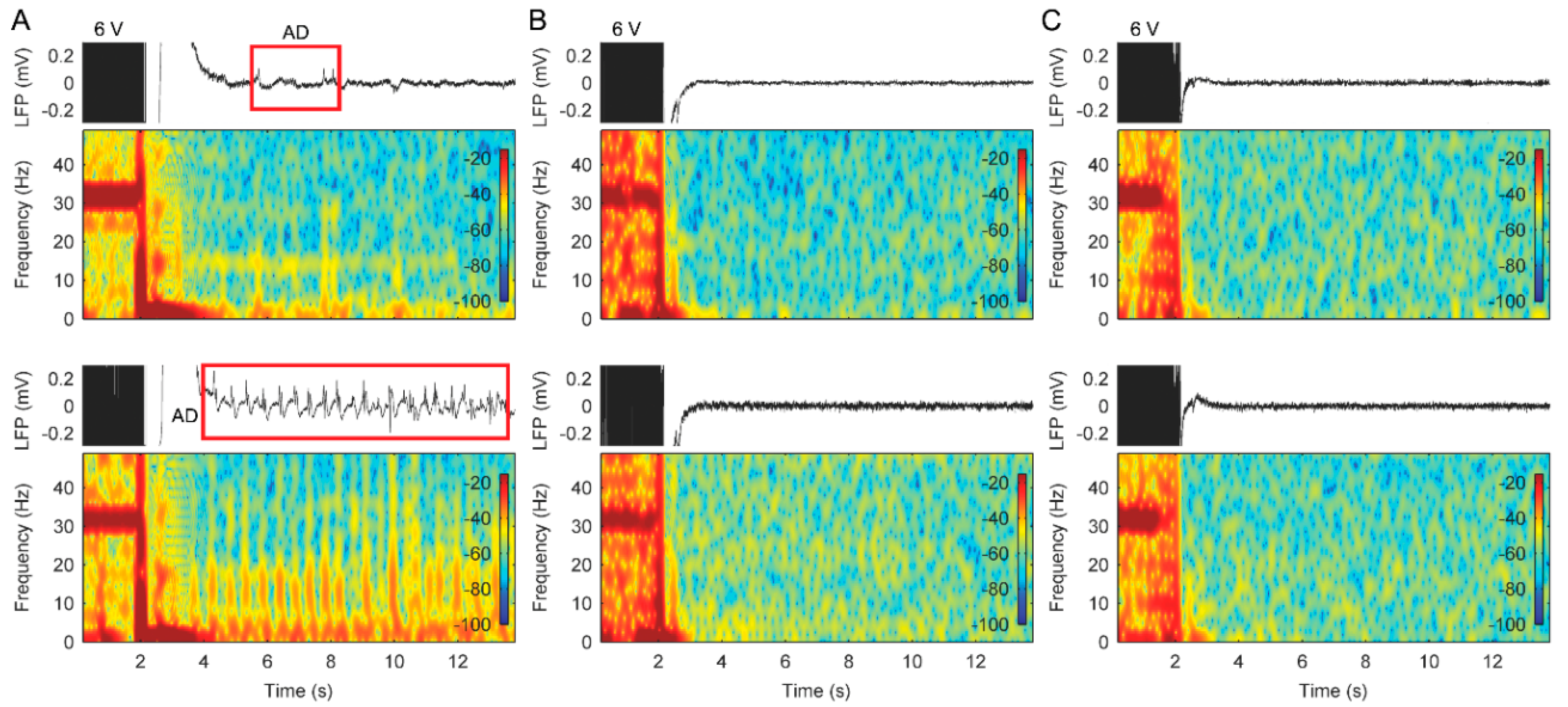
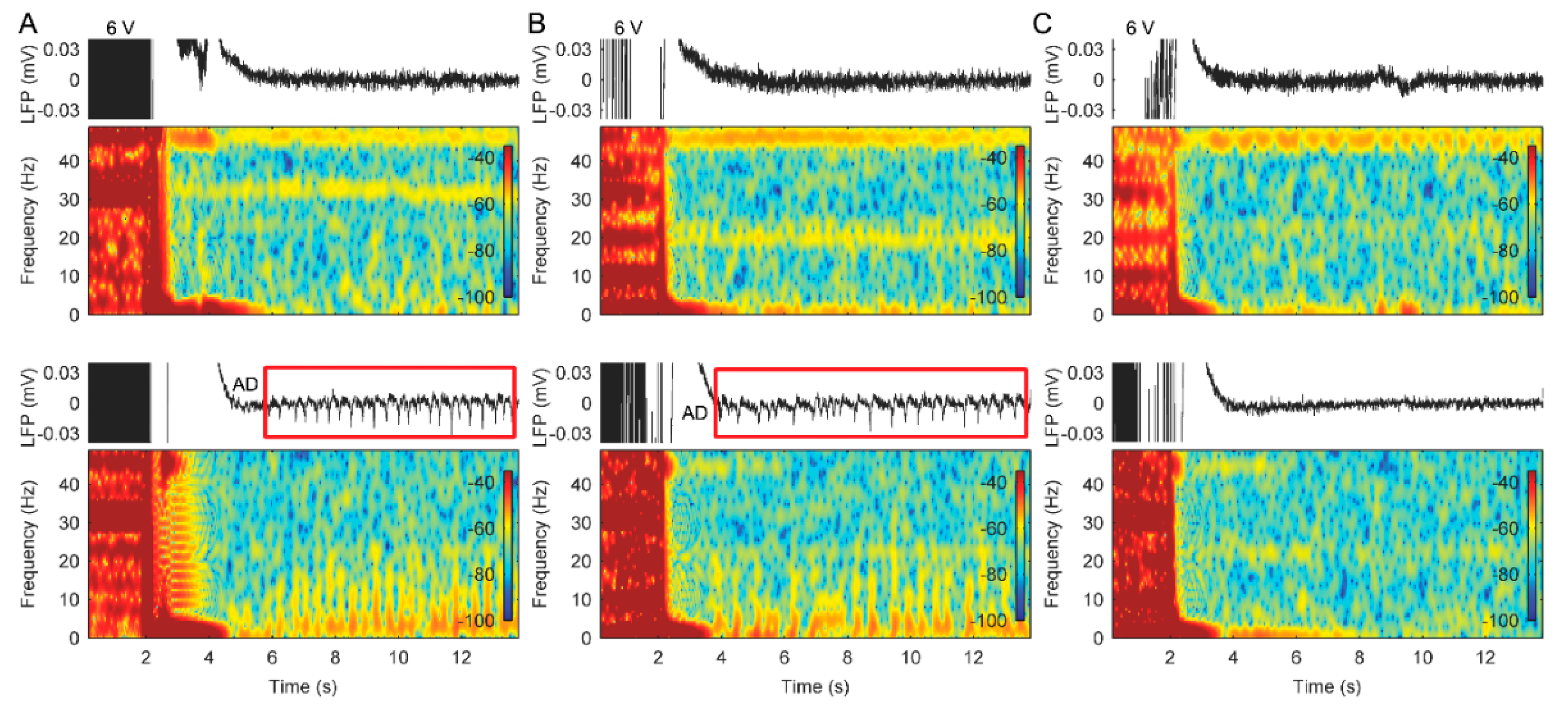
3.2. Stimulation with Slowly Ramped on/off during Cycling Successfully Eliminates ADs
3.3. The Use of Cycled Stimulation with Slow Ramps Provides Sustained Pain Relief without Seizures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.I.; Blomstedt, P.; Zrinzo, L. Deep brain stimulation between 1947 and 1987: The untold story. Neurosurg. Focus 2010, 29, E1. [Google Scholar] [CrossRef] [PubMed]
- Foltz, E.L.; White, L.E., Jr. Pain “relief” by frontal cingulumotomy. J. Neurosurg. 1962, 19, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Hassenbusch, S.J.; Pillay, P.K.; Barnett, G.H. Radiofrequency cingulotomy for intractable cancer pain using stereotaxis guided by magnetic resonance imaging. Neurosurgery 1990, 27, 220. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, A.; Harsh, V.; Pereira, E.A.C.; Aziz, T.Z. Cingulotomy for medically refractory cancer pain. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef]
- Cohen, R.A.; Kaplan, R.F.; Moser, D.J.; Jenkins, M.A.; Wilkinson, H. Impairments of attention after cingulotomy. Neurology 1999, 53, 819. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Kosslyn, S.M.; Cosgrove, G.; Cassem, E.H.; Price, B.H.; Nierenberg, A.A.; Rauch, S.L. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychoogy 2001, 39, 219–230. [Google Scholar] [CrossRef]
- Boccard, S.G.; Fitzgerald, J.J.; Pereira, E.A.; Moir, L.; Van Hartevelt, T.J.; Kringelbach, M.L.; Green, A.L.; Aziz, T.Z. Targeting the affective component of chronic pain: A case series of deep brain stimulation of the anterior cingulate cortex. Neurosurgery 2014, 74, 628–635. [Google Scholar] [CrossRef]
- Pereira, E.A.; Green, A.L.; Aziz, T.Z. Deep brain stimulation for pain. Handbook Clin. Neurol. 2013, 116, 277–294. [Google Scholar]
- Maslen, H.; Cheeran, B.; Pugh, J.; Pycroft, L.; Boccard, S.; Prangnell, S.; Green, A.L.; FitzGerald, J.; Savulescu, J.; Aziz, T. Unexpected Complications of Novel Deep Brain Stimulation Treatments: Ethical Issues and Clinical Recommendations. Neuromodulation 2018, 21, 135–143. [Google Scholar] [CrossRef]
- Blume, W.T.; Jones, D.C.; Pathak, P. Properties of after-discharges from cortical electrical stimulation in focal epilepsies. Clin. Neurophysiol. 2004, 115, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, S.; Hopfengärtner, R.; Rössler, K.; Müller, T.; Olmes, D.G.; Lang, J.; Köhn, J.; Onugoren, M.D.; Heyne, J.; Schwab, S.; et al. Afterdischarges elicited by cortical electric stimulation in humans: When do they occur and what do they mean? Epilepsy Behav. 2018, 87, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.G.; Bancaud, J.; Talairach, J.; Bonis, A.; Szikla, G. Comparative Value of Spontaneous and Chemically and Electrically Induced Seizures in Establishing the Lateralization of Temporal Lobe Seizures. Epilepsia 1979, 20, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Lesser, R.P.; Kim, S.H.; Beyderman, L.; Miglioretti, D.L.; Webber, W.R.S.; Bare, M.; Cysyk, B.; Krauss, G.; Gordon, B. Brief bursts of pulse stimulation terminate afterdischarges caused by cortical stimulation. Neurology 1999, 53, 2073. [Google Scholar] [CrossRef]
- Lesser, R.P.; Lüders, H.; Klem, G.; Dinner, D.S.; Morris, H.H.; Hahn, J. Cortical Afterdischarge and Functional Response Thresholds: Results of Extraoperative Testing. Epilepsia 1984, 25, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Lesser, R.P.; Lee, H.W.; Webber, W.R.S.; Prince, B.; Crone, N.E.; Miglioretti, D.L. Short-term variations in response distribution to cortical stimulation. Brain 2008, 131, 1528–1539. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Webber, W.; Crone, N.; Miglioretti, D.L.; Lesser, R.P. When is electrical cortical stimulation more likely to produce afterdischarges? Clin. Neurophysiol. 2010, 121, 14–20. [Google Scholar] [CrossRef]
- Stypulkowski, P.; Stanslaski, S.; Denison, T.; Giftakis, J. Chronic Evaluation of a Clinical System for Deep Brain Stimulation and Recording of Neural Network Activity. Ster. Funct. Neurosurg. 2013, 91, 220–232. [Google Scholar] [CrossRef]
- Stypulkowski, P.H.; Stanslaski, S.R.; Jensen, R.M.; Denison, T.J.; Giftakis, J.E. Brain Stimulation for Epilepsy—Local and Remote Modulation of Network Excitability. Brain Stimul. 2014, 7, 350–358. [Google Scholar] [CrossRef]
- Rouse, A.G.; Stanslaski, S.R.; Cong, P.; Jensen, R.M.; Afshar, P.; Ullestad, D.; Gupta, R.; Molnar, G.F.; Moran, D.W.; Denison, T.J.; et al. A Chronic Generalized Bi-directional Brain-Machine Interface. J. Neural Eng. 2011, 8, 036018. [Google Scholar] [CrossRef]
- Stanslaski, S.; Afshar, P.; Cong, P.; Giftakis, J.; Stypulkowski, P.; Carlson, D.; Linde, D.; Ullestad, D.; Avestruz, A.-T.; Denison, T. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device With Concurrent Sensing and Stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Denison, T.J.; Afshar, P.; Stanslaski, S.R. Simultaneous Physiological Sensing and Stimulation with Saturation Detection. Google Patents 2016. Available online: https://patents.patsnap.com/v/US10080898-simultaneous-physiological-sensing-and-stimulation-with-saturation-detection.html (accessed on 25 September 2018).
- Fontaine, D.; Hamani, C.; Lozano, A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: Critical review of the literature. J. Neurosurg. 2009, 110, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.M.; Treister, R.; Raij, T.; Pascual-Leone, A.; Park, L.; Nurmikko, T.; Lenz, F.; Lefaucheur, J.-P.; Lang, M.; Hallett, M.; et al. Transcranial magnetic stimulation of the brain: Guidelines for pain treatment research. Pain 2015, 156, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Cruccu, G.; Aziz, T.Z.; Garcia-Larrea, L.; Hansson, P.; Jensen, T.S.; Lefaucheur, J.-P.; Simpson, B.A.; Taylor, R.S. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur. J. Neurol. 2007, 14, 952–970. [Google Scholar] [CrossRef] [PubMed]
- Kalamangalam, G.P.; Tandon, N.; Slater, J.D. Dynamic mechanisms underlying afterdischarge: A human subdural recording study. Clin. Neurophysiol. 2014, 125, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R. The intrinsic electrophysiological properties of mammalian neurons: Insights into central nervous system function. Science 1988, 242, 1654–1664. [Google Scholar] [CrossRef]
- Morales, J.C.; Roncagliolo, M.; Fuenzalida, M.; Wellmann, M.; Nualart, F.J.; Bonansco, C.; Álvarez-Ferradas, C. A new rapid kindling variant for induction of cortical epileptogenesis in freely moving rats. Front. Cell. Neurosci. 2014, 8, 200. [Google Scholar] [CrossRef]
- De Ridder, D.; De Mulder, G.; Verstraeten, E.; Van Der Kelen, K.; Sunaert, S.; Smits, M.; Kovacs, S.; Verlooy, J.; Van De Heyning, P.; Moller, A.R. Primary and Secondary Auditory Cortex Stimulation for Intractable Tinnitus. ORL 2006, 68, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Afshar, P.; Khambhati, A.; Stanslaski, S.; Carlson, D.; Jensen, R.; Linde, D.; Dani, S.; Lazarewicz, M.; Cong, P.; Giftakis, J.; et al. A translational platform for prototyping closed-loop neuromodulation systems. Front. Neural. Circuits 2012, 6, 117. [Google Scholar] [CrossRef] [PubMed]
- Miocinovic, S.; Somayajula, S.; Chitnis, S.; Vitek, J.L. History, Applications, and Mechanisms of Deep Brain Stimulation. JAMA Neurol. 2013, 70, 163. [Google Scholar] [CrossRef]
- Dougherty, D.D.; Rezai, A.R.; Carpenter, L.L.; Howland, R.H.; Bhati, M.T.; O’Reardon, J.P.; Eskandar, E.N.; Baltuch, G.H.; Machado, A.D.; Kondziolka, D.; et al. A Randomized Sham-Controlled Trial of Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum for Chronic Treatment-Resistant Depression. Boil. Psychiatry 2015, 78, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Lozano, A.M.; Fosdick, L.; Chakravarty, M.M.; Leoutsakos, J.-M.; Munro, C.; Oh, E.; Drake, K.E.; Lyman, C.H.; Rosenberg, P.B.; Anderson, W.S.; et al. A Phase II Study of Fornix Deep Brain Stimulation in Mild Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 54, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Holtzheimer, P.E.; Husain, M.M.; Lisanby, S.H.; Taylor, S.F.; A Whitworth, L.; McClintock, S.; Slavin, K.V.; Berman, J.; McKhann, G.M.; Patil, P.G.; et al. Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 2017, 4, 839–849. [Google Scholar] [CrossRef]
- Quinn, E.J.; Blumenfeld, Z.; Velisar, A.; Koop, M.M.; Shreve, L.A.; Trager, M.H.; Hill, B.C.; Kilbane, C.; Henderson, J.M.; Bronte-Stewart, H. Beta oscillations in freely moving Parkinson’s subjects are attenuated during deep brain stimulation. Mov. Disord. 2015, 30, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Velisar, A.; Syrkin-Nikolau, J.; Blumenfeld, Z.; Trager, M.H.; Afzal, M.F.; Prabhakar, V.; Bronte-Stewart, H. Dual threshold neural closed loop deep brain stimulation in Parkinson disease patients. Brain Stimul. 2019, 12, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, J.J.; Klassen, B.T.; Worrell, G.A.; Lee, K.H.; Shin, C.; Zhao, C.Z.; Brown, D.A.; Goerss, S.J.; Kall, B.A.; Stead, M. Anterior nuclear deep brain stimulation guided by concordant hippocampal recording. Neurosurg. Focus 2015, 38, E9. [Google Scholar] [CrossRef] [PubMed]
- Shute, J.B.; Okun, M.S.; Opri, E.; Molina, R.; Rossi, P.J.; Martinez-Ramirez, D.; Foote, K.D.; Gunduz, A. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. NeuroImage Clin. 2016, 12, 165–172. [Google Scholar] [CrossRef]
- Swann, N.C.; De Hemptinne, C.; Miocinovic, S.; Qasim, S.; Ostrem, J.L.; Galifianakis, N.B.; Luciano, M.S.; Wang, S.S.; Ziman, N.; Taylor, R.; et al. Chronic multisite brain recordings from a totally implantable bidirectional neural interface: Experience in 5 patients with Parkinson’s disease. J. Neurosurg. 2018, 128, 605–616. [Google Scholar] [CrossRef]


| Patient | Age at Surgery/Sex | Etiology | Onset of Seizure after Surgery | Seizure Symptoms | DBS Settings at Onset of Seizures | Anti-Epileptic Drugs | DBS Settings with Seizure Free | Follow-Up |
|---|---|---|---|---|---|---|---|---|
| 1 | 46/F | Whole spine pain secondary to multiple spinal interventions | 20 months | 1) Focal non-motor onset with impaired awareness 2) Nocturnal generalized tonic-clonic seizures (maximum frequency reported: 1 event per month, lasting up to 45 min) | 5 V 130 Hz 450 μs | Levetiracetam Clobazam Sodium valproate | 6 V 130 Hz 450 μs 8-s ramp 3 min ON/11 min OFF | Seizure free for 17 months |
| 2 | 51/M | Whole body pain secondary to excision of ependymoma of cervical spinal cord | 60 months | Focal non-motor onset with impaired awareness (maximum frequency reported: 1 event per h) | 8.5 V 130 Hz 450 μs | no | 6 V 130 Hz 450 μs 8-s ramp1 min ON/1 min OFF | Seizure free for 6 months |
| 3 | 49/M | Right hemi body pain secondary to posterior fossa decompression for Arnold–Chiari malformation | 12 months | Focal non-motor onset with impaired awareness (maximum frequency reported: 50 events per day) | 8.5 V 130 Hz 450 μs | Levetiracetam Oxcarbazepine | 6 V 130 Hz 450 μs 8-s ramp1 min ON/1 min OFF | Seizure free for 1 month (then system removed due to infection) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Cheeran, B.; Green, A.L.; Denison, T.J.; Aziz, T.Z. Applying a Sensing-Enabled System for Ensuring Safe Anterior Cingulate Deep Brain Stimulation for Pain. Brain Sci. 2019, 9, 150. https://doi.org/10.3390/brainsci9070150
Huang Y, Cheeran B, Green AL, Denison TJ, Aziz TZ. Applying a Sensing-Enabled System for Ensuring Safe Anterior Cingulate Deep Brain Stimulation for Pain. Brain Sciences. 2019; 9(7):150. https://doi.org/10.3390/brainsci9070150
Chicago/Turabian StyleHuang, Yongzhi, Binith Cheeran, Alexander L. Green, Timothy J. Denison, and Tipu Z. Aziz. 2019. "Applying a Sensing-Enabled System for Ensuring Safe Anterior Cingulate Deep Brain Stimulation for Pain" Brain Sciences 9, no. 7: 150. https://doi.org/10.3390/brainsci9070150
APA StyleHuang, Y., Cheeran, B., Green, A. L., Denison, T. J., & Aziz, T. Z. (2019). Applying a Sensing-Enabled System for Ensuring Safe Anterior Cingulate Deep Brain Stimulation for Pain. Brain Sciences, 9(7), 150. https://doi.org/10.3390/brainsci9070150






