Abstract
Background: Cognitive disorders are reported to be common in patients with primary Sjogren’s syndrome (pSS). In some cases, they are the first clinical manifestation, preceding the diagnosis of pSS by two years on average. Aim: A systematic review was conducted to explore cognitive impairment in pSS, with reference to diagnostic methods and their relationship with laboratory data and clinical manifestations. Materials and Methods: According to the PRISMA 2009 checklist, we carried out a comprehensive literature search in the three main bibliographic databases: MEDLINE, EMBASE, and PsycINFO (NICE HDAS interface). The following main search terms were used: primary Sjogren syndrome, neurological manifestations, fatigue, cognitive functions, psychiatric manifestations, mild cognitive impairment, dementia, and neurocognitive disorder. The search was made on 14 September, 2018. References from all selected studies were also examined. Inclusion criteria were: all studies and case-reports published in any language from 2002 that assessed the association of pSS (according to classification criteria proposed by the 2002 American/European collaborative group (AECG)) with all types of cognitive impairment (including dementia). Exclusion criteria were: reviews, abstracts, secondary Sjögren’s syndrome (SS), and all articles in which other classification criteria were used. Results: The initial search yielded 352 articles, of which 253 were excluded based on the title and abstract review. A total of 54 articles underwent a full-length review, and 32 articles were excluded. Data were extracted from 18 studies and three case-reports involving a total of 6196 participants. In most cases, cognitive dysfunction was a brain fog or a mild cognitive impairment (MCI). Occasionally, an autoimmune dementia was present. The relationship between pSS and degenerative dementias, such as Alzheimer’s disease (AD), was a controversial issue, even if some investigators hypothesized that pSS could be a risk factor. Several unmet needs were highlighted. First, some of the included studies had not reported the severity of pSS; hence, few correlations between disease severity and cognitive function were possible. Secondly, the evaluation of the pathogenetic role of comorbid diseases was often absent. The lack of information on the type of dementia represented a third critical point in the majority of the included studies. Conclusions: This systematic review confirmed that adequate studies on cognitive function in pSS are scarce, mostly performed on small-sized samples, and often conflicting. The routine assessment of cognitive function in patients with pSS seems advisable and it will help to elucidate some of the unmet needs highlighted by this review in future appropriately designed studies.
1. Introduction
1.1. Rationale
Sjögren’s syndrome (SS) is one of the most common connective tissue diseases. It typically presents with a lymphocytic infiltration of exocrine glands leading to sicca syndrome. SS can be present as primary SS (pSS), which is an entity by itself without an underlying autoimmune condition, or secondary SS (sSS) [1,2,3]. At least one-third of patients with pSS can present with extraglandular involvement, with neurological dysfunction being one of the most frequent conditions [4,5]. The rate of neurological symptoms in patients with pSS has been reported to range from 8.5% to 70%. Such a disparate range is a likely consequence of the use of different sets of diagnostic criteria for pSS and of different definitions of neurological syndromes. However, the diagnostic set is important, as there is a greater availability of evaluations of neurological function in patients hospitalized on neurology wards, compared with rheumatology wards [6,7]. The most frequent neurological complication of pSS is peripheral neuropathy, especially sensory polyneuropathy. Central nervous system (CNS) involvement is much less common and has been reported in less than 25% of patients. Simultaneous involvement of the peripheral and the central nervous systems is also possible [8]. In 25–60% of cases, neurological symptoms are the first clinical manifestation and can precede the diagnosis of pSS on an average of two years [9]. Cognitive disorders are reported to be common. Many patients reported mild or minimal subjective cognitive difficulties, often referred to as the so-called “brain-fog”. Brain fog represents a wide range of subjectively experienced cognitive difficulties, such as forgetfulness, memory lapses, mental confusion, reduced verbal fluency, and diminished ability to concentrate more severely in the presence of distractors or competing stimuli [10,11]. Sometimes, cognitive impairment has been thought to be a consequence of other manifestations of pSS, such as fatigue. Fatigue can be defined as an enduring, subjective sensation of generalized tiredness or exhaustion. Unlike weakness, fatigue can be alleviated by periods of rest. Most patients with rheumatic diseases complain of chronic fatigue [12]. Moreover, the relationship between pSS and the dementias, especially Alzheimer’s disease (AD), must be better assessed.
1.2. Objectives
A systematic review was conducted to explore cognitive impairment in pSS, with emphasis on diagnostic methods and their relationship with laboratory data and with clinical manifestations. The relationship between pSS and dementias and the role of fatigue were the review’s main focus.
2. Materials and Methods
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. No review protocol exists.
2.1. Search Strategy
One author of this study (Isetta, M.) carried out a comprehensive literature search in the three main bibliographic databases: MEDLINE, EMBASE, and PsycINFO (NICE HDAS interface). The following main search terms were used: primary Sjogren syndrome, neurological manifestations, fatigue, cognitive functions, psychiatric manifestations, mild cognitive impairment, dementia, and neurocognitive disorder. The search was made on 14 September, 2018. References from all of the selected studies were also examined. In accordance with the PRISMA 2009 checklist [13], the full search terms used, together with the results of the searches in one of the three databases (MEDLINE), are detailed in the Supplemental material and methods section.
2.2. Inclusion Criteria
This review included all studies and case-reports published in any language from 2002 that assessed the association of pSS (according to classification criteria proposed by the 2002 American/European collaborative group (AECG)) with all types of cognitive impairment (including dementia). According to these criteria, the diagnosis of pSS can be made by fulfilling four out of six items. Two items include subjective xerophtalmia and xerostomia; two items include objective evidence of keratoconjunctivitis sicca and xerostomia; two items include a positive minor salivary gland biopsy with a focus score of ≥1/4 mm2 or the presence of anti-Ro/SSA or anti-La/SSB antibodies [14].
In 2012, new criteria were proposed by the Sjögren’s International Collaborative Clinical Alliances Cohort [15] and then approved in 2016 by the American College of Rheumatology (ACR) and by the European League against Rheumatism (EULAR). These classification criteria are based on the weighted sum of five items: anti-SSA/Ro antibody positivity and focal lymphocytic sialadenitis with a focus score of ≥1 foci/4 mm2, each scoring 3; an abnormal ocular staining score of ≥5 (or van Bijsterveld score of ≥4); a Schirmer’s test result of ≤5 mm/5 min; and an unstimulated salivary flow rate of ≤0.1 mL/min, each scoring 1. Individuals with signs or symptoms suggestive of SS, or both, who have a total score of ≥4 for the above items meet the criteria for primary SS [16]. Level of agreement between these criteria sets has been evaluated as excellent [17], but the real life application of the 2016 ACR/EULAR criteria is still infrequent [18].
2.3. Exclusion Criteria
Reviews, abstracts, and sSS were excluded from this review. All articles in which other classification criteria were used were also excluded.
2.4. Data Extraction
Two authors of this study (Martinez-Suarez, E. and Manzo, C.) independently reviewed the titles and abstracts of all identified citations. After reviewing the abstracts, data comparisons were conducted to ensure completeness and reliability. The inclusion criteria were independently applied to all identified studies. Differing decisions were resolved by consensus. Full-text versions of potentially relevant papers identified in the initial screening were retrieved. Data concerning study design, source of information, participant characteristics, SS, and assessment of cognitive function were independently extracted.
2.5. Assessment of Bias Risk
A subjective assessment of the methodological quality of observational studies was performed by all the authors using the Newcastle-Ottawa Scale, which is a quality assessment tool for non-randomized studies [19]. It uses a “star system” based on three major perspectives: the selection of the study groups (0–4 stars, or 0–5 stars for cross-sectional studies), the comparability of the groups by controlling for important and additional relevant factors (0–2 stars), and the ascertainment of the outcome of interest or exposure (0–3 stars). A total score of 3 or less was considered poor, 4–6 was considered moderate, and 7–10 was considered high quality. Studies scoring 3 or less were excluded from our review. Discrepant opinions between authors were resolved by consensus.
3. Results
3.1. Description of Included Studies
As reported in Figure 1, the initial search yielded 352 articles, of which 253 articles were excluded based on the title and abstract review. A total of 54 articles underwent a full-length review, and 32 articles were excluded (not a study in subject of interest = 5; studies having poor quality = 10; no outcome of interest = 18). Data were extracted from 18 studies and 3 case-reports, involving a total of 6196 subjects.
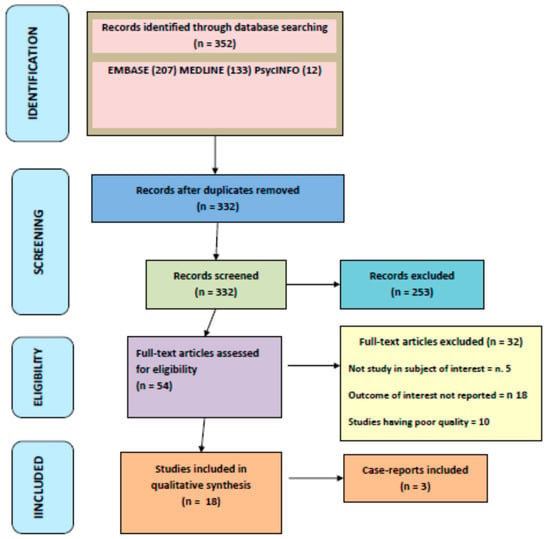
Figure 1.
PRISMA flow-chart of the systematic review process.
The characteristics of studies included in this review are outlined in Table 1.

Table 1.
Characteristics and main data of the studies included in this review.
3.2. pSS, Mild Cognitive Impairment (MCI), and Dementia
Six studies appraised the relationships between pSS, MCI, and dementia [22,25,26,27,32,33]. Yoshikawa et al. published a retrospective study and evaluated a cohort of 20 patients with pSS and dementia or cognitive dysfunction in general. Their age ranged from 56 to 92 years. They found that 12 patients were affected by dementia (four by Alzheimer’s type; six by vascular dementia; one by mixed dementia; one by normal pressure hydrocephalus), and eight by MCI (five of them had a vascular MCI). In all patients, brain magnetic resonance imaging (MRI) and single-photon emission computed tomography (SPECT) were performed. A correlation with depression was found, whereas no correlation with other extraglandular manifestations of pSS was observed [26].
In a case-control prospective study, Blanc et al. found that among 25 patients with pSS, 15 (60%) presented with cognitive dysfunction; five of these had dementia. Dementia was diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR). According to Petersen’s criteria, 10 of the patients had MCI (five non-amnestic single domain and five amnestic). Their age ranged from 30 to 74 years. The mean duration of cognitive complaints was 6.1 ± 5.6 years. Neuropsychological symptoms came first for six patients. Brain MRI was performed in all patients, and a trend towards a correlation was found between the severity of cognitive impairment and the degree of white matter lesions (WML) with a p-value = 0.03. No cases of MCI or dementia were found in the control group [32].
In a case-control study, Dziadkowiak et al. evaluated 30 consecutive patients with pSS (median age of 51 years), and found that cognitive impairment correlated with disease duration and with severity of inflammatory changes [33].
In 2015, Morreale et al. published their data regarding 87 patients with recent (<6 months) diagnosis of pSS and found that 31.1% had cognitive dysfunction compatible with dementia. Brain MRI was performed [25].
In 28 patients with pSS, Tezcan et al. found that 78.8% had cognitive impairment of the frontal- subcortical type and 11% had a severe cognitive impairment. No neuroimaging evaluation was performed. In two patients, there was no active extraglandular manifestation during neuropsychological assessment [22].
More recently, in a population study based on the Taiwan National Health Insurance Research Database (NHIRD), Lin et al. found that, among 4756 patients with pSS, 238 had dementia. However, the type of dementia was not specified. In NHIRD, parameters such as clinical severity or laboratory data are not recorded [27].
In these studies, brain MRI, when performed, mostly showed frontal and subcortical pathological findings. However, in several patients MRI scans were normal.
3.3. Fatigue and Cognitive Function in pSS
In pSS patients, fatigue prevalence is between 67% and 85 %, and it can be considered a good indicator of systemic activity [40,41]. Indeed, the assessment of fatigue is present in two of the most important pSS activity scales, namely the “Sjogren’s syndrome disease activity index (SSDAI)” [42,43] and the “Sjogren’s syndrome systemic clinical activity index (SCAI)” [44]. A strong correlation was observed between the presence of subjective cognitive symptoms and fatigue severity, and dysfunction in attention, executive functions, working memory, and verbal memory among objective cognitive tests [45]. Among the studies included in this review, eight also had assessed fatigue [20,22,28,29,30,33,34,36]. The case-control study published in 2016 by Kocer et al. was of great interest; 32 patients with pSS were evaluated through comprehensive neuropsychological tests and neuroimaging. In the other six studies included in this review, neuroimaging was not performed. Fatigue was evaluated by using the Fatigue Severity Scale (FSS). FSS is a nine-item self-administered instrument. The scores for each item range from one to seven, with the lower score indicating less fatigue. A FSS score ≥4 reliably differentiates subjects with fatigue from controls. In this study, no statistically significant differences were observed in terms of all neuropsychological tests, depression, fatigue severity, health state, and daily-life activities between PSS and control cases without depression. These findings indicate the presence of a pattern of cognitive impairment without the negative effects of depression in patients with pSS. Nevertheless, the authors found a correlation between fatigue and cognitive dysfunction, with a p-value <0.05 when they were compared with the healthy control group. In particular, a significant negative correlation between the Clock Drawing and the SF-36 was observed. However, they highlighted that fatigue may not affect neuropsychological-tests performance in well-selected patients [29]. In the other included studies, a certain terminological confusion was found. In particular, confusion among fatigue, weakness, and stiffness was particularly frequent.
3.4. Laboratory Data and Clinical Manifestations
In the majority of the studies included in this review, correlations between extraglandular manifestations, laboratory data, and cognitive dysfunction were not assessed or not found. Delalande et al.’s study was particularly interesting, because they found that in 47% of patients sicca symptoms were absent [4]. The potential pathogenetic role of antibodies against the NR-2 subtype of N-methyl-D-aspartate (NMDA) receptors was particularly evaluated in the study performed by Lauvsnes et al. The authors found that cognitive dysfunction and depression occurred more frequently in pSS patients with anti-NR2 antibodies than in pSS patients without them. A correlation between anti-NR2 antibodies, cognitive dysfunction, and hippocampus volume was also highlighted in a subgroup of their pSS patients [35].
4. Discussion
This systematic review highlighted that cognitive impairment can occur in patients with pSS. Many individuals reported mild cognitive difficulties, often referred to as the so-called “brain fog”. We know that brain fog is a characteristic of fibromyalgia; some investigators have referred to it as fibro-fog [46,47]. A brain fog has been reported in 50 – 80% of fibromyalgic patients [46,47,48]. Is the brain fog of fibromyalgic patients identical to that of patients with pSS? Is there a “Sjo-fog”? In this review, it is postulated and argued that brain fog in pSS is not different from that of fibromyalgia. As in fibromyalgia, its physiological basis in pSS is not clearly understood, but it has been suggested to be multifactorial with pain, depression, sleep, and some drugs being the most important determinants [46,47,48,49]. In clinical practice, the possibility that brain fog can be related to a concomitant or overlapping fibromyalgia must be carefully considered [49,50].
A possible immune-mediated endothelitis has been hypothesized in brain fog in patients with pSS, but this review did not identify studies confirming this hypothesis. This unmet need should be addressed in ad hoc future studies.
Dementia is less commonly described in the literature, with subcortical dementia as the most frequent clinical finding [20,21,22,23,24]. Furthermore, pSS is common in the elderly [51] and older age can be a common factor with AD. In a nation-wide retrospective population study, which was not included in this review because it did not meet the inclusion criteria, a higher prevalence of AD was found in pSS patients older than 70 years than in non-pSS patients, suggesting that pSS should be considered as a risk factor for AD [52]. In another prospective study, recruiting patients with dementia in a memory clinic, patients with pSS accounted for 7.5% of those with cognitive impairment [23].
This systematic review suggests that four important points must be taken into account in clinical practice:
1) Dementia can occur in patients with pSS. With increasing ageing, subcortical dementia is usually due to vascular brain pathology or synucleinopathies (i.e., Parkinson’s disease and Lewy body disease), but it can also be associated with pSS in a small proportion of cases (5%) [8], although the actual causal mechanisms or relationship remains to be elucidated from current available knowledge.
2) An autoimmune-induced dementia is possible in pSS. Autoimmune dementia is, by definition, reversible. It may be misdiagnosed as a primary, degenerative dementia, leading to serious therapeutic errors.
3) Normal findings in brain MRI are frequent in patients with pSS.
4) Cognitive dysfunction can be the first clinical manifestation of pSS.
Hence, it is very important that clinicians look for sicca syndrome or for laboratory data suggestive of pSS when direct observation and neurologic tests detect problems with information processing, attention, memory, or executive function, even if brain MRI is normal or in the presence of a few WML. SPECT assessment should be considered, as it revealed a hypoperfusion in parietal, temporal, and frontal lobes in more than 50% of patients having normal MRI brain when cognitive dysfunction was present. This SPECT pattern was present only in 17% of pSS patients without cognitive dysfunction [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,53].
The first observation of a correlation between CNS involvement and laboratory data is in the series of eight patients described by Alexander et al. in 1982, in which a direct pathogenetic role of the antiRo (SSA) antibodies was suggested [54]. However, cognitive dysfunction may occur even in the seronegative forms of pSS [20,54], and this must be taken into account in clinical practice.
NR2 receptors are present in particularly high-density in the hippocampus. Binding of anti-NR2 antibodies to these receptors causes neuronal death through an increase of intracellular levels of calcium. Anti-NR2 antibodies may play a pathogenetic role in cognitive dysfunction in pSS, and correlation between their levels and the severity of cognitive dysfunction have been suggested [32]. However, this hypothesis should be confirmed in multicenter, large studies.
Finally, the roles of cryoglobulinemia and antiphospholipid syndrome were more than anecdotal in this review [18].
Fatigue is a frequent and destabilizing manifestation in patients with pSS and it can influence their cognitive performances. This review identified only one good-quality article that evaluated this relationship, confirming a bidirectional interference between fatigue and cognitive functions [29]. The interference of pain was not always taken into account. For example, testing patients during pSS relapses should favor it. From the review, it is not possible to tease out the influence of fatigue and pain on cognitive functions in patients with pSS.
Our review identified areas that demand further insight. First, some of the included studies did not report the severity of pSS [17,21,23,25,28,29], and few correlations between disease severity and cognitive function were possible. Second, the evaluation of the pathogenetic role of comorbid diseases was often absent [17,21,22,23,24,25]. The lack of information on the type of dementia represented a third critical point in the majority of the included studies [7,20,22,24,25,26,27,28,30,31,32,33,34].
5. Conclusions
Cognitive dysfunction can be present in patients with pSS, and, in some cases, it is the first clinical manifestation.
In most cases, a brain fog or MCI are present. Occasionally, pSS can cause an autoimmune dementia that would be reversible by definition. However, the relationship between pSS and degenerative dementias, such as AD, is not clear, even if some investigators have hypothesized that pSS could be a risk factor. In elderly onset SS, older age can be a common risk factor.
When cognitive impairment is diagnosed in a middle-aged patient, a search for sicca syndrome or for laboratory data suggestive of pSS should be considered. However, the possibility that sicca symptoms can be absent in pSS patients with cognitive dysfunction, as well as that cognitive dysfunction can be present in seronegative forms of pSS, must be taken into account.
The routine assessment of cognitive function in patients with pSS seems advisable, and will inform the future studies designed to explore some of the knowledge gaps highlighted by this review.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3425/9/4/85/s1. Box 1: Full search terms used together with the results of the searches in MEDLINE.
Author Contributions
Conceptualization, C.M. and J.S.-M.; methodology, C.M., J.S.-M., and M.I.; software, M.I.; validation, C.M., E.M.-S., M.K., and M.I.; formal analysis, C.M., E.M.-S., and M.K.; investigation, M.I., E.M.-S., C.M.; data curation, E.M.-S., C.M., and M.K.; writing and original draft preparation, C.M.; writing – review and editing, J.S.-M., M.I., M.K., E.M.-S., and C.M.; supervision, J.S.-M. and C.M.; project administration, M.I.
Funding
This research received no external funding.
Conflicts of Interest
The Authors declare no conflict of interest.
References
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Mariette, X.; Criswell, L.A. Primary Sjögren’s Syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- McCoy, S.S.; Baer, A.N. Neurological Complications of Sjögren’s Syndrome: Diagnosis and Management. Curr. Treat. Options Rheumatol. 2017, 3, 275–288. [Google Scholar] [CrossRef]
- Perzyńska-Mazan, J.; Maślińska, M.; Gasik, R. Neurological manifestations of primary Sjögren’s syndrome. Reumatologia 2018, 56, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Gono, T.; Kawaguchi, Y.; Katsumata, Y.; Takagi, K.; Tochimoto, A.; Baba, S.; Okamoto, Y.; Ota, Y.; Yamanaka, H. Clinical manifestations of neurological involvement in primary Sjögren’s syndrome. Clin. Rheumatol. 2011, 30, 485–490. [Google Scholar] [CrossRef]
- Ye, W.; Chen, S.; Huang, X.; Qin, W.; Zhang, T.; Zhu, X.; Lin, C.; Wang, X. Clinical features and risk factors of neurological involvement in Sjögren’s syndrome. BMC Neurosci. 2018, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.; Teixeira, F.; Martins Silva, A.; Vasconcelos, C.; Farinha, F.; Santos, E. Frequent involvement of central nervous system in primary Sjögren syndrome. Rheumatol. Int. 2015, 35, 289–294. [Google Scholar] [CrossRef]
- Posso-Osorio, I.; Naranjo-Escobar, J.; Loaiza, D.M.; Polo, M.; Echeverri, A.; Tobon, G.J. Neurological involvement in primary Sjogren’s syndrome. Curr. Rheumatol. Rev. 2018. [Google Scholar] [CrossRef]
- Manzo, C.; Serra-Mestres, J. Considerations on fibrofog and fibromyalgic discognition in older patients. Giornale Italiano di Reumatologia Clinica 2016, 3, 59–75. [Google Scholar]
- Serra-Mestres, J. Problemas de memoria no debidos a demencia: Conceptos y mecanismos. Inf. Psiquiatr. 2016, 224, 13–20. [Google Scholar]
- Helme, C.; Hegarty, R.S.M.; Stebbings, S.; Treharne, G.J. “I actually just really need to stop work sometimes”: Exploring fatigue-related barriers to employment among people with rheumatic diseases. Musculoskelet. Care 2018. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef]
- Shiboski, S.C.; Shiboski, C.H.; Criswell, L.A.; Baer, A.N.; Challacombe, S.; Lanfranchi, H.; Schiødt, M.; Umehara, H.; Vivino, F.; Zhao, Y.; et al. American College of Rheumatology Classification Criteria for Sjögren’s Syndrome: A Data-Driven, Expert Consensus Approach in the SICCA Cohort. Arthritis Care Res. 2012, 64, 475–487. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef]
- Le Goff, M.; Cornec, D.; Jousse-Joulin, S.; Costa, S.; Guellec, D.; Marhadour, T.; Le Berre, R.; Genestet, S.; Cochener, B.; Boisrame-Gastrin, S.; et al. Comparison of 2002 AECG and 2016 ACR/EULAR classification criteria and added value of salivary gland ultrasonography in a patient cohort with suspected primary Sjögren’s syndrome. Arthritis Res. Ther. 2017, 19, 269. [Google Scholar] [CrossRef]
- Franceschini, F.; Cavazzana, I.; Andreoli, L.; Tincani, A. The 2016 classification criteria for primary Sjogren’s syndrome: What’s new? BMC Med. 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of non-randomized studies in metaanalyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Segal, B.M.; Pogatchnik, B.; Holker, E.; Liu, H.; Sloan, J.; Rhodus, N.; Moser, K.L. Primary Sjogren’s syndrome: Cognitive symptoms, mood, and cognitive performance. Acta Neurol. Scand. 2012, 125, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Le Guern, V.; Belin, C.; Henegar, C.; Moroni, C.; Maillet, D.; Lacau, C.; Dumas, J.L.; Vigneron, N.; Guillevin, L. Cognitive function and 99mTc-ECD brain SPECT are significantly correlated in patients with primary Sjogren syndrome: A case-control study. Ann. Rheum. Dis. 2010, 69, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, M.E.; Kocer, E.B.; Haznedaroglu, S.; Sonmez, C.; Nercan, R.; Yucel, A.A.; Irkec, C.; Bitik, B.; Goker, B. Primary Sjögren’s syndrome is associated with significant cognitive dysfunction. Int. J. Rheum. Dis. 2016, 19, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Delalande, S.; de Seze, J.; Fauchais, A.-L.; Hachulla, E.; Stojkovic, T.; Ferriby, D.; Dubucquoi, S.; Pruvo, J.-P.; Vermersch, P.; Hatron, P.-Y. Neurologic manifestations in primary Sjögren syndrome: A study of 82 patients. Medicine 2004, 83, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.N.; Hora, J.S.; Salgado, M.C. A short neuropsychological evaluation of patients with primary Sjögren’s syndrome. Arq. Neuropsiquiatr. 2014, 72, 38–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morreale, M.; Francia, A.; Marchione, P.; Manuppella, F.; Giacomini, P. Intracranial hemodynamic changes in primary Sjögren syndrome: A transcranial Doppler case-control study. Neurol. Sci. 2015, 36, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Hatate, J.; Toratani, N.; Sugiura, S.; Shimizu, Y.; Takahash, T.; Ito, T.; Fukunaga, R. Prevalence of Sjögren’s syndrome with dementia in a memory clinic. J. Neurol. Sci. 2012, 322, 217–221. [Google Scholar] [CrossRef]
- Lin, T.-M.; Chen, W.-S.; Sheu, J.-J.; Chen, Y.-H.; Chen, J.-H.; Chang, C.-C. Autoimmune rheumatic diseases increase dementia risk in middle-aged patients: A nationwide cohort study. PLoS ONE 2018, 13, e0186475. [Google Scholar] [CrossRef]
- Omma, A.; Tecer, D.; Kucuksahin, O.; Sandikci, S.C.; Yildiz, F.; Erten, S. Do the European League Against Rheumatism (EULAR) Sjögren’s syndrome outcome measures correlate with impaired quality of life, fatigue, anxiety and depression in primary Sjögren’s syndrome? Arch. Med. Sci. 2018, 14, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Koçer, B.; Tezcan, M.E.; Batur, H.Z.; Haznedaroğlu, S.; Göker, B.; İrkeç, C.; Çetinkaya, R. Cognition, depression, fatigue, and quality of life in primary Sjögren’s syndrome: Correlations. Brain Behav. 2016, 6, e00586. [Google Scholar] [CrossRef]
- Wouters, E.J.M.; van Leeuwen, N.V.; Bossema, E.R.; Kruize, A.A.; Bootsma, H.; Bijlsma, J.W.J.; Geenen, R. Physical activity and physical activity cognitions are potential factors maintaining fatigue in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2012, 71, 668–673. [Google Scholar] [CrossRef]
- Hartkamp, A.; Geenen, R.; Bijl, M.; Kruize, A.A.; Godaert, G.L.R.; Derksen, R.H.W.M. Serum cytokine levels related to multiple dimensions of fatigue in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 2004, 63, 1335–1337. [Google Scholar] [CrossRef]
- Blanc, F.; Longato, N.; Jung, B.; Kleitz, C.; Di Bitonto, L.; Cretin, B.; Collongues, N.; Sordet, C.; Fleury, M.; Poindron, V.; et al. Cognitive Dysfunction and Dementia in Primary Sjögren’s Syndrome. ISRN Neurol. 2013, 19, 501327. [Google Scholar] [CrossRef] [PubMed]
- Dziadkowiak, E.; Sebastian, A.; Wiland, P.; Waliszewska-Prosol, M.; Zagrajek, M.; Ejma, M. Endogenous event-related potentials in patients with primary Sjögren’s syndrome without central nervous system involvement. Scand. J. Rheumatol. 2015, 44, 487–494. [Google Scholar] [CrossRef]
- Goodchild, C.E.; Treharne, G.J.; Booth, D.A.; Bowman, S.J. Daytime patterning of fatigue and its associations with the previous night’s discomfort and poor sleep among women with primary Sjögren’s syndrome or rheumatoid arthritis. Musculoskelet. Care 2010, 8, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Lauvsnes, M.B.; Maroni, S.S.; Appenzeller, S.; Beyer, M.K.; Greve, O.J.; Kvaloy, J.T.; Harboe, E.; Goransson, L.G.; Tjensvoll, A.B.; Omdal, R. Memory dysfunction in primary Sjögren’s syndrome is associated with anti-NR2 antibodies. Arthritis Rheum. 2013, 65, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Hackett, K.L.; Deary, V.; Deane, K.H.; Newton, J.L.; Ng, W.-F.; Rapley, T. Experience of sleep disruption in primary Sjögren’s syndrome: A focus group study. Br. J. Occup. Ther. 2018, 81, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rosado, S.N.; Silveira, V.; Reis, A.I.; Gordinho, A.; Noronha, C. Catatonia and psychosis as manifestations of primary Sjogren’s syndrome. Eur. J. Case Rep. Intern. Med. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Kimura, A.; Kato, S.; Koumura, A.; Sakurai, T.; Tanaka, Y.; Hozumi, I.; Sunden, Y.; Orba, Y.; Sawa, H.; et al. Progressive multifocal leukoencephalopathy and CD4+ T-lymphocytopenia in a patient with Sjögren syndrome. J. Neurol. Sci. 2008, 268, 195–198. [Google Scholar] [CrossRef]
- Hirohata, M.; Yasukawa, Y.; Ishida, C.; Komai, K.; Yamada, M. Reversible cortical lesions in primary Sjögren’s syndrome presenting with meningoencephalitis as an initial manifestation. J. Neurol. Sci. 2005, 232, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Arends, S.; Meiners, P.M.; Moerman, R.V.; Kroese, F.G.; Brouwer, E.; Spijkervet, F.K.; Vissink, A.; Bootsma, H. Physical fatigue characterises patient experience of primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2017, 35, 255–261. [Google Scholar] [PubMed]
- Bodewes, I.L.A.; van der Spek, P.J.; Leon, L.G.; Wijkhuijs, A.J.M.; van Helden-Meeuwsen, C.G.; Tas, L.; Schreurs, M.W.J.; van Daele, P.L.A.; Katsikis, P.D.; Versnel, M.A. Fatigue in Sjögren’s Syndrome: A Search for Biomarkers and Treatment Targets. Front. Immunol. 2019, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Seror, R.; Ravaud, P.; Bowman, S.J.; Baron, G.; Tzioufas, A.; Theander, E.; Gottenberg, J.-E.; Bootsma, H.; Mariette, X.; Vitali, C. EULAR Sjogren’s syndrome disease activity index: Development of a consensus systemic disease activity index for primary Sjogren’s syndrome. Ann. Rheum. Dis. 2010, 69, 1103–1109. [Google Scholar] [CrossRef]
- Seror, R.; Bowman, S.J.; Brito-Zeron, P.; Theander, E.; Bootsma, H.; Tzioufas, A.; Gottemberg, J.E.; Roman-Casals, M.; Dorner, T.; Ravaud, P.; et al. EULAR Sjögren’s syndrome disease activity index (ESSDAI): A user guide. RMD Open 2015, 1, e000022. [Google Scholar] [CrossRef]
- Bowman, S.J.; Sutcliffe, N.; Isenberg, D.A.; Goldblatt, F.; Adler, M.; Price, E.; Canavan, A.; Hamburger, J.; Richards, A.; Regan, S.R.M.; et al. Sjögren’s Systemic Clinical Activity Index (SCAI)–A systemic disease activity measure for use in clinical trials in primary Sjögren’s syndrome. Rheumatology 2007, 46, 1845–1851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coates, T.; Slavotinek, J.P.; Rischmueller, M.; Schultz, D.; Anderson, C.; Dellamelva, M. Cerebral white matter lesions in primary Sjögren’s syndrome: A controlled study. J. Rheumatol. 1999, 26, 1301–1305. [Google Scholar]
- Ambrose, K.R.; Gracely, R.H.; Glass, J.M. Fibromyalgia dyscognition: Concepts and issue. Reumatismo 2012, 64, 206–215. [Google Scholar] [CrossRef]
- Torta, R.G.V.; Tesio, V.; Ieraci, V.; Castelli, L.; Zizzi, F.B. Fibro-fog. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 96), S6–S8. [Google Scholar]
- Glass, J.M.; Park, D.C. Cognitive dysfunction in fibromyalgia. Curr. Rheumatol. Rep. 2001, 3, 123–127. [Google Scholar] [CrossRef]
- Choi, B.Y.; Oh, H.J.; Lee, Y.J.; Song, Y.W. Prevalence and clinical impact of fibromyalgia in patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2016, 34 (Suppl. 96), S9–S13. [Google Scholar]
- Kang, J.H.; Lin, H.C. Comorbidities in patients with primary Sjogren’s syndrome: A registry-based case-control study. J. Rheumatol. 2010, 37, 1188–1194. [Google Scholar] [CrossRef]
- Manzo, C.; Maslinska, M. Primary Sjogren’s syndrome in the elderly: Does age of onset make a difference? EMJ Rheumatol. 2018, 5, 75–82. [Google Scholar]
- Liliang, P.-C.; Liang, C.-L.; Lu, K.; Yang, S.-N.; Hsieh, M.-T.; Tai, Y.-C.; Wang, K.-W. Population-based study suggests an increased risk of Alzheimer’sdisease in Sjögren’s syndrome. Clin. Rheumatol. 2018, 37, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.; Provost, T.; Stevens, M.; Alexander, G.E. Neurologic complications of primary Sjögren’s syndrome. Medicine 1982, 61, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Segal, B.M.; Pogatchnik, B.; Henn, L.; Rudser, K.; Sivils, K.M. Pain severity and neuropathic pain symptoms in primary Sjögren’s syndrome: A comparison study of seropositive and seronegative Sjögren’s syndrome patients. Arthritis Care Res. 2013, 65, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).