Endogenous Neurostimulation and Physiotherapy in Cluster Headache: A Clinical Case
Abstract
1. Introduction
2. Methods
2.1. Patient
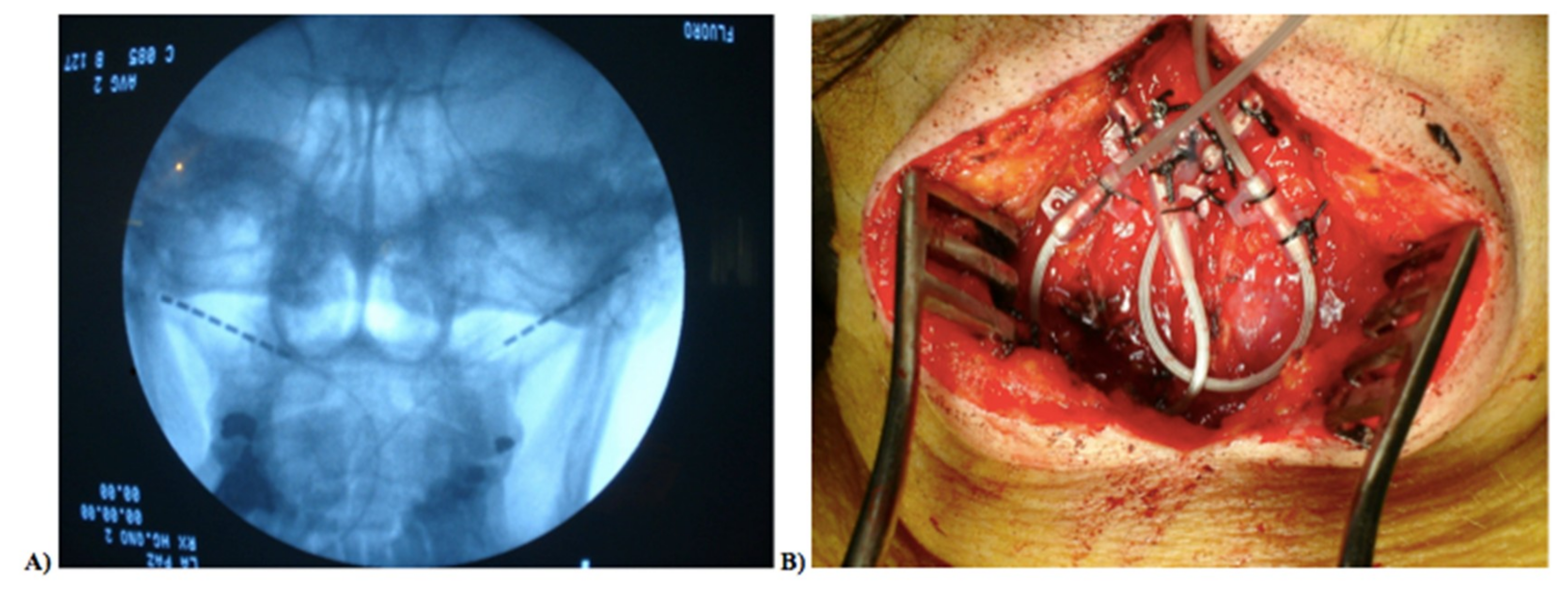
2.2. Neurostimulator Implantation Procedure
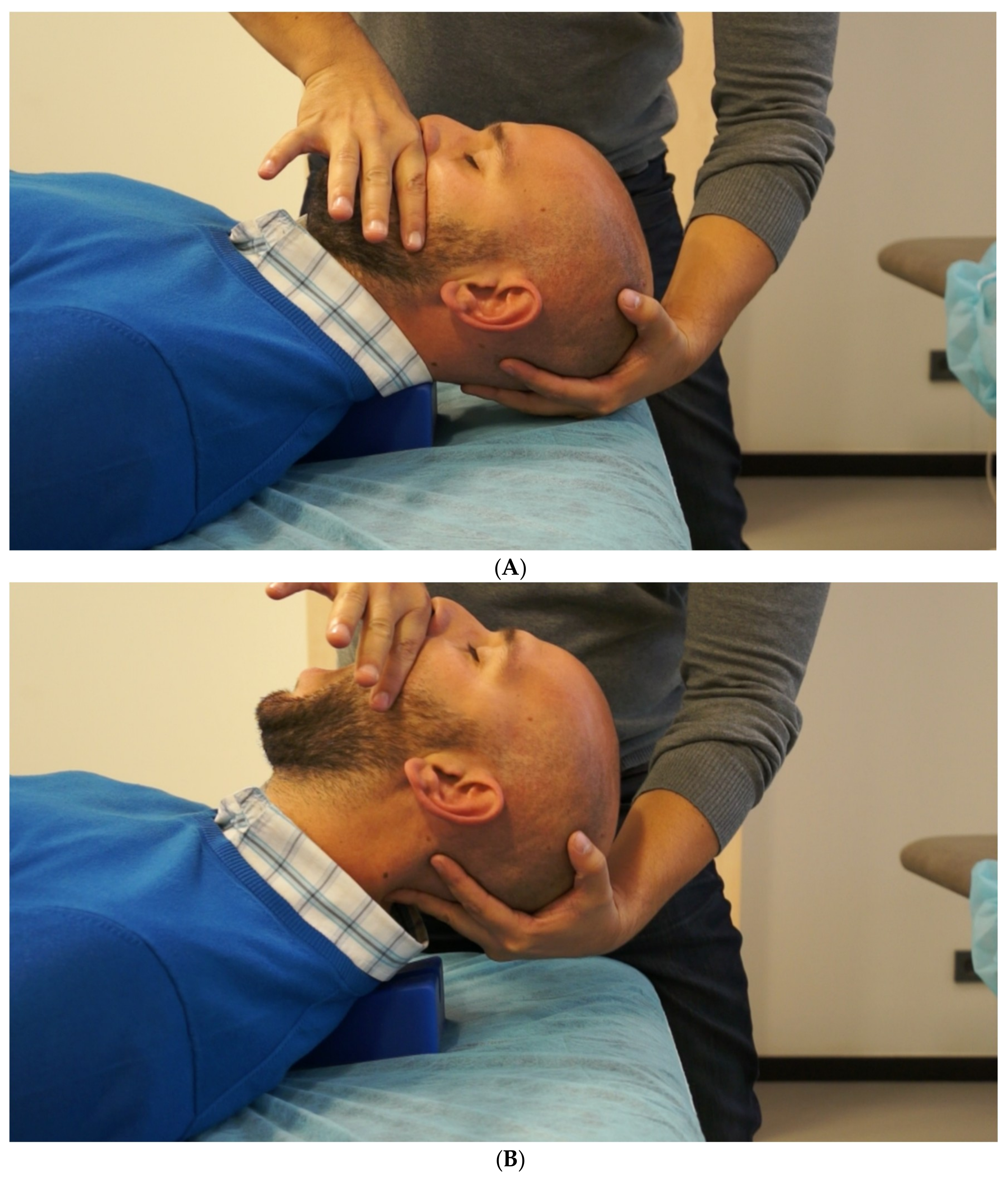
2.3. Evaluation
2.4. Postsurgical Physiotherapy Approach
3. Results and Discussion
Author Contributions
Funding
Conflicts of Interest
Clinical Implications
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Fischera, M.; Marziniak, M.; Gralow, I.; Evers, S. The incidence and prevalence of cluster headache: A meta-analysis of population-based studies. Cephalalgia 2008, 28, 614–618. [Google Scholar] [CrossRef]
- Meyers, S.L. Cluster headache and trigeminal autonomic cephalgias. Dis. Mon. 2015, 61, 236–239. [Google Scholar] [CrossRef]
- May, A.; Bahra, A.; Büchel, C.; Frackowiak, R.S.; Goadsby, P.J. PET and MRA findings in cluster headache and MRA in experimental pain. Neurology 2000, 55, 1328–1335. [Google Scholar] [CrossRef]
- Naegel, S.; Holle, D.; Desmarattes, N.; Theysohn, N.; Diener, H.-C.; Katsarava, Z.; Obermann, M. Cortical plasticity in episodic and chronic cluster headache. Neuroimage Clin. 2014, 6, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, T.; Goadsby, P.J. Stimulation of the greater occipital nerve induces increased central excitability of dural afferent input. Brain 2002, 125, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Afridi, S.K.; Shields, K.G.; Bhola, R.; Goadsby, P.J. Greater occipital nerve injection in primary headache syndromes--prolonged effects from a single injection. Pain 2006, 122, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, A.; Vandenheede, M.; Rossi, P.; Aloj, F.; Sauli, E.; Pierelli, F.; Schoenen, J. Suboccipital injection with a mixture of rapid- and long-acting steroids in cluster headache: A double-blind placebo-controlled study. Pain 2005, 118, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.P.; Stiles, M.A.; Siow, H.C.; Rozen, T.D.; Young, W.B.; Silberstein, S.D. Greater occipital nerve blockade for cluster headache. Cephalalgia 2002, 22, 520–522. [Google Scholar] [CrossRef]
- Leone, M.; Proietti Cecchini, A. Long-term use of daily sumatriptan injections in severe drug-resistant chronic cluster headache. Neurology 2015, 86, 194–195. [Google Scholar] [CrossRef]
- Ekbom, K.; Monstad, I.; Prusinski, A.; Cole, J.A.; Pilgrim, A.J.; Noronha, D. Subcutaneous sumatriptan in the acute treatment of cluster headache: A dose comparison study. The Sumatriptan Cluster Headache Study Group. Acta Neurol. Scand. 1993, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Law, S.; Derry, S.; Moore, R.A. Triptans for acute cluster headache. Cochrane Database Syst. Rev. 2013, 7, CD008042. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Leone, M.; Afra, J.; Linde, M.; Sándor, P.S.; Evers, S.; Goadsby, P.J. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur. J. Neurol. 2006, 13, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.S.; Barloese, M.C.; Jensen, R.H. Oxygen treatment of cluster headache: A review. Cephalalgia 2014, 34, 1079–1087. [Google Scholar] [CrossRef]
- Bratbak, D.F.; Nordgård, S.; Stovner, L.J.; Linde, M.; Folvik, M.; Bugten, V.; Tronvik, E. Pilot study of sphenopalatine injection of onabotulinumtoxinA for the treatment of intractable chronic cluster headache. Cephalalgia 2015, 36, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Starling, A.J.; Pringsheim, T.M.; Becker, W.J.; Schwedt, T.J. Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines. Headache J. Head Face Pain 2016, 56, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Gregor, N.; Schlesiger, C.; Akova-Ozturk, E.; Kraemer, C.; Husstedt, I.-W.; Evers, S. Treatment of Cluster Headache Attacks With Less Than 6 mg Subcutaneous Sumatriptan. Headache J. Head Face Pain 2005, 45, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Antonaci, F.; Ramusino, M.C.; Nappi, G. The Neuropharmacology of Cluster Headache and other Trigeminal Autonomic Cephalalgias. Curr. Neuropharmacol. 2015, 13, 304–323. [Google Scholar] [CrossRef]
- Savoldi, F.; Bono, G.; Manzoni, G.C.; Micieli, G.; Lanfranchi, M.; Nappi, G. Lithium salts in cluster headache treatment. Cephalalgia 1983, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Bussone, G.; Leone, M.; Peccarisi, C.; Micieli, G.; Granella, F.; Magri, M.; Manzoni, G.C.; Nappi, G. Double blind comparison of lithium and verapamil in cluster headache prophylaxis. Headache 1990, 30, 411–417. [Google Scholar] [CrossRef]
- Cohen, A.S.; Matharu, M.S.; Goadsby, P.J. Electrocardiographic abnormalities in patients with cluster headache on verapamil therapy. Neurology 2007, 69, 668–675. [Google Scholar] [CrossRef]
- Leone, M.; Franzini, A.; Proietti Cecchini, A.; Mea, E.; Broggi, G.; Bussone, G. Costs of hypothalamic stimulation in chronic drug-resistant cluster headache: Preliminary data. Neurol. Sci. 2009, 30, 43–47. [Google Scholar] [CrossRef]
- Nesbitt, A.D.; Marin, J.C.A.; Tompkins, E.; Ruttledge, M.H.; Goadsby, P.J. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology 2015, 84, 1249–1253. [Google Scholar] [CrossRef]
- Goadsby, P.J.; de Coo, I.F.; Silver, N.; Tyagi, A.; Ahmed, F.; Gaul, C.; Jensen, R.H.; Diener, H.-C.; Solbach, K.; Straube, A.; et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: A randomized, double-blind, sham-controlled ACT2 study. Cephalalgia 2017, 38, 959–969. [Google Scholar] [CrossRef]
- Cosentino, G.; Brighina, F.; Brancato, S.; Valentino, F.; Indovino, S.; Fierro, B. Transcranial magnetic stimulation reveals cortical hyperexcitability in episodic cluster headache. J. Pain 2015, 16, 53–59. [Google Scholar] [CrossRef]
- Hodaj, H.; Alibeu, J.-P.; Payen, J.-F.; Lefaucheur, J.-P. Treatment of Chronic Facial Pain Including Cluster Headache by Repetitive Transcranial Magnetic Stimulation of the Motor Cortex with Maintenance Sessions: A Naturalistic Study. Brain Stimul. 2015, 8, 801–807. [Google Scholar] [CrossRef]
- Schwedt, T.J.; Vargas, B. Neurostimulation for Treatment of Migraine and Cluster Headache. Pain Med. 2015, 16, 1827–1834. [Google Scholar] [CrossRef]
- Jürgens, T.P.; Barloese, M.; May, A.; Láinez, J.M.; Schoenen, J.; Gaul, C.; Goodman, A.M.; Caparso, A.; Jensen, R.H. Long-term effectiveness of sphenopalatine ganglion stimulation for cluster headache. Cephalalgia 2017, 37, 423–434. [Google Scholar] [CrossRef]
- Magis, D.; Schoenen, J. Advances and challenges in neurostimulation for headaches. Lancet. Neurol. 2012, 11, 708–719. [Google Scholar] [CrossRef]
- Chaibi, A.; Russell, M.B. Manual therapies for cervicogenic headache: A systematic review. J. Headache Pain 2012, 13, 351–359. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. CARE Group the CARE guidelines: Consensus-based clinical case report guideline development. J. Clin. Epidemiol. 2014, 67, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-V.; Lin, C.-P.; Hung, C.-Y.; Özçakar, L.; Wang, T.-G.; Chen, W.-S. Sonographic Nerve Tracking in the Cervical Region: A Pictorial Essay and Video Demonstration. Am. J. Phys. Med. Rehabil. 2016, 95, 862–870. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Ortega-Santiago, R.; Cuadrado, M.L.; López-de-Silanes, C.; Pareja, J.A. Bilateral Widespread Mechanical Pain Hypersensitivity as Sign of Central Sensitization in Patients with Cluster Headache. Headache J. Head Face Pain 2011, 51, 384–391. [Google Scholar] [CrossRef]
- Kinser, A.M.; Sands, W.A.; Stone, M.H. Reliability and validity of a pressure algometer. J. Strength Cond. Res. 2009, 23, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Bulut, T.; Akgun, U.; Ozcan, C.; Unver, B.; Sener, M. Inter- and intra-tester reliability of sensibility testing in digital nerve repair. J. Hand Surg. Eur. 2015, 41, 621–623. [Google Scholar] [CrossRef]
- Olson, L.E.; Millar, A.L.; Dunker, J.; Hicks, J.; Glanz, D. Reliability of a clinical test for deep cervical flexor endurance. J. Manip. Physiol. Ther. 2006, 29, 134–138. [Google Scholar] [CrossRef]
- Sauro, K.M.; Rose, M.S.; Becker, W.J.; Christie, S.N.; Giammarco, R.; Mackie, G.F.; Eloff, A.G.; Gawel, M.J. HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache 2010, 50, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Olmedilla Zafra, A.; Ortega Toro, E.; Cano, L.A. Validation of the Pain Catastrophizing Scale in Spanish athletes. Cuadernos Psicologia Deporte 2013, 13, 83–93. [Google Scholar] [CrossRef]
- Andrade Ortega, J.A.; Delgado Martínez, A.D.; Almécija Ruiz, R. Validation of the Spanish version of the Neck Disability Index. Spine 2010, 35, E114–E118. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Chamorro, L.; Luque, A.; Dal-Ré, R.; Badia, X.; Baró, E. Validation of the Spanish versions of the Montgomery-Asberg depression and Hamilton anxiety rating scales. Med. Clin. 2002, 118, 493–499. [Google Scholar] [CrossRef]
- Holdgate, A.; Asha, S.; Craig, J.; Thompson, J. Comparison of a verbal numeric rating scale with the visual analogue scale for the measurement of acute pain. Emerg. Med. 2003, 15, 441–446. [Google Scholar] [CrossRef]
- La Touche, R.; París-Alemany, A.; Mannheimer, J.S.; Angulo-Díaz-Parreño, S.; Bishop, M.D.; Lopéz-Valverde-Centeno, A.; von Piekartz, H.; Fernández-Carnero, J. Does mobilization of the upper cervical spine affect pain sensitivity and autonomic nervous system function in patients with cervico-craniofacial pain?: A randomized-controlled trial. Clin. J. Pain 2013, 29, 205–215. [Google Scholar] [CrossRef]
- Harris, K.D.; Heer, D.M.; Roy, T.C.; Santos, D.M.; Whitman, J.M.; Wainner, R.S. Reliability of a measurement of neck flexor muscle endurance. Phys Ther. 2005, 85, 1349–1355. [Google Scholar]
- Rodrigo, M.D.; Quero, J.; Cía, P.; Escartín, R.; Acín, P.; Bono, C.; Polo, C. Estimulación eléctrica invasiva de C2-C3 en el tratamiento del dolor cefálico y facial: Neuralgia occipital. Migraña transformada. Cefalea en racimos. Algias faciales. Rev. De La Soc. Española Del Dolor 2008, 15, 382–391. [Google Scholar]
- Pedersen, J.L.; Barloese, M.; Jensen, R.H. Neurostimulation in cluster headache: A review of current progress. Cephalalgia 2013, 33, 1179–1193. [Google Scholar] [CrossRef]
- Piovesan, E.J.; Kowacs, P.A.; Tatsui, C.E.; Lange, M.C.; Ribas, L.C.; Werneck, L.C. Referred pain after painful stimulation of the greater occipital nerve in humans: Evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 2001, 21, 107–109. [Google Scholar] [CrossRef]
- Busch, V.; Gaul, C. Exercise in migraine therapy is there any evidence for efficacy? A critical review. Headache 2008, 48, 890–899. [Google Scholar] [CrossRef]
- Hall, T.; Chan, H.T.; Christensen, L.; Odenthal, B.; Wells, C.; Robinson, K. Efficacy of a C1-C2 self-sustained natural apophyseal glide (SNAG) in the management of cervicogenic headache. J. Orthop. Sports Phys. Ther. 2007, 37, 100–107. [Google Scholar] [CrossRef]
- Skyba, D.A.; Radhakrishnan, R.; Rohlwing, J.J.; Wright, A.; Sluka, K.A. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain 2003, 106, 159–168. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Müller, M.; Blum, B.; Straube, A. Correlation of headache frequency and psychosocial impairment in migraine: A cross-sectional study. Headache 2014, 54, 861–871. [Google Scholar] [CrossRef]
- Chaibi, A.; Russell, M.B. Manual therapies for primary chronic headaches: A systematic review of randomized controlled trials. J. Headache Pain 2014, 15, 67. [Google Scholar] [CrossRef]
- Santos, F.M.; Silva, J.T.; Giardini, A.C.; Rocha, P.A.; Achermann, A.P.P.; Alves, A.S.; Britto, L.R.G.; Chacur, M. Neural mobilization reverses behavioral and cellular changes that characterize neuropathic pain in rats. Mol. Pain 2012, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Wilbrink, L.A.; Louter, M.A.; Teernstra, O.P.M.; van Zwet, E.W.; Huygen, F.J.P.M.; Haan, J.; Ferrari, M.D.; Terwindt, G.M. Allodynia in cluster headache. Pain 2017, 158, 1113–1117. [Google Scholar] [CrossRef]
- Ferreira, P.H.; Ferreira, M.L.; Maher, C.G.; Herbert, R.D.; Refshauge, K. Specific stabilisation exercise for spinal and pelvic pain: A systematic review. Aust. J. Physiother. 2006, 52, 79–88. [Google Scholar] [CrossRef]
- Gil-Martínez, A.; Kindelan-Calvo, P.; Agudo-Carmona, D.; Muñoz-Plata, R.; López-de-Uralde-Villanueva, I.; La Touche, R. Therapeutic exercise as treatment for migraine and tension-type headaches: A systematic review of randomised clinical trials. Rev. Neurol. 2013, 57, 433–443. [Google Scholar]
| Pre | Post | 3 months | 4 months | % of change | |
|---|---|---|---|---|---|
| PPT | % Pre-4 months | ||||
| V1 right | 1.12 | 0.75 | 1.71 | 1.49 | 33.03% |
| V2 right | 1.62 | 1.22 | 2.45 | 2.77 | 70.98% |
| V3 right | 1.39 | 0.94 | 2.5 | 1.67 | 20.14% |
| Temporalis M1 right | 2.58 | 2.13 | 4.17 | 2.73 | 5.81% |
| Temporalis M2 right | 3.28 | 3.19 | 5.7 | 5.16 | 57.32% |
| V1 left | 0.72 | 0.62 | 1.52 | 1.29 | 79.17% |
| V2 left | 1.27 | 1.08 | 2.86 | 3.1 | 144.09% |
| V3 left | 1.37 | 0.88 | 3.03 | 2.41 | 75.91% |
| Temporalis M1 left | 1.69 | 2.09 | 3.28 | 3.56 | 110.65% |
| Temporalis M2 left | 2.85 | 3.19 | 5.35 | 4.64 | 62.81% |
| Mastoid P right | 3.54 | 3.34 | 4.54 | 4.64 | 31.07% |
| Mastoid P left | 2.81 | 2.14 | 3.52 | 4.2 | 49.47% |
| Greater occipital N right | 4.75 | 3.8 | 4.68 | 4.04 | −14.95% |
| Greater occipital N left | 4.34 | 3.26 | 4.64 | 3.42 | −21.20% |
| Tibialis M right | 4.98 | 6.31 | 17.6 | 10.96 | 120.08% |
| Tibialis M left | 5.84 | 5.17 | 17.08 | 12.68 | 117.12% |
| Craniocervical flexion test | |||||
| time (s) | 3.06 | 8.22 | 12.47 | 24.06 | - |
| Fatigue | 21.5 | 22 | 43 | 60.5 | - |
| Physiological characteristic | |||||
| differences Pre-4 months | |||||
| HIT-6 | 63 | 61 | 50 | 54 | −9 |
| NDI | 18 | 12 | 13 | 13 | −5 |
| PCS | 17 | 10 | 10 | 10 | −7 |
| PCS rumination | 8 | 7 | 7 | 7 | −1 |
| PCS magnification | 0 | 0 | 0 | 0 | 0 |
| PCS helplessness | 9 | 3 | 3 | 3 | −6 |
| HDRS | 20 | 18 | 14 | 14 | −6 |
| Months | Frequency | Intensity | Duration | Abortive Treatment |
|---|---|---|---|---|
| May | 2 | 9 | 25 | SS-OT |
| June | 3 | 9.33 | 23.33 | SS |
| July | 7 | 9.71 | 31.43 | SS-OT |
| August | 2 | 9.50 | 35 | SS |
| September | 0 | - | - | - |
| October | 0 | - | - | - |
| November | 4 | 8.50 | 33.75 | SS-OT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Fernández, G.; de-la-Puente-Ranea, L.; Gandía-González, M.; Gil-Martínez, A. Endogenous Neurostimulation and Physiotherapy in Cluster Headache: A Clinical Case. Brain Sci. 2019, 9, 60. https://doi.org/10.3390/brainsci9030060
Navarro-Fernández G, de-la-Puente-Ranea L, Gandía-González M, Gil-Martínez A. Endogenous Neurostimulation and Physiotherapy in Cluster Headache: A Clinical Case. Brain Sciences. 2019; 9(3):60. https://doi.org/10.3390/brainsci9030060
Chicago/Turabian StyleNavarro-Fernández, Gonzalo, Lucía de-la-Puente-Ranea, Marisa Gandía-González, and Alfonso Gil-Martínez. 2019. "Endogenous Neurostimulation and Physiotherapy in Cluster Headache: A Clinical Case" Brain Sciences 9, no. 3: 60. https://doi.org/10.3390/brainsci9030060
APA StyleNavarro-Fernández, G., de-la-Puente-Ranea, L., Gandía-González, M., & Gil-Martínez, A. (2019). Endogenous Neurostimulation and Physiotherapy in Cluster Headache: A Clinical Case. Brain Sciences, 9(3), 60. https://doi.org/10.3390/brainsci9030060






