Stromal Vascular Fraction Cell Therapy for a Stroke Patient—Cure without Side Effects
Abstract
1. Introduction
2. Methods
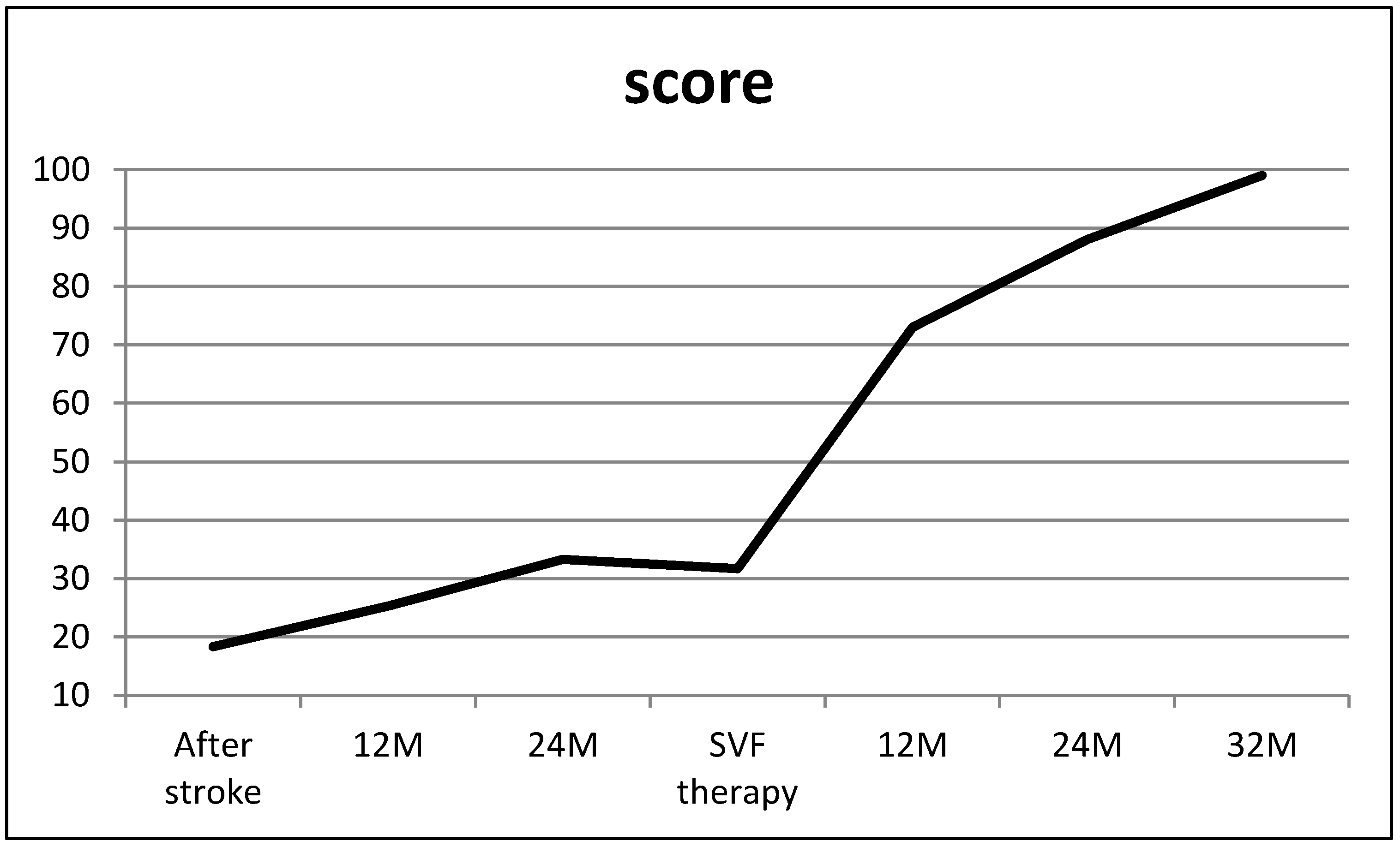
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhasin, A.; Kumaran, S.S.; Bhatia, R.; Mohanty, S.; Srivastava, M.V.P. Safety and Feasibility of Autologous Mesenchymal Stem Cell Transplantation in Chronic Stroke in Indian patients. A four-year follow up. J. Stem. Cells Regen. Med. 2017, 13, 14–19. [Google Scholar] [PubMed]
- Strioga, M.; Viswanathan, S.; Darinskas, A.; Slaby, O.; Michalek, J. Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 2012, 21, 2724–2752. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.; Moster, R.; Lukac, L.; Proefrock, K.; Petrasovic, M.; Rybar, J.; Chaloupka, A.; Darinskas, A.; Michalek, J.; Kristek, J.; et al. Stromal vascular fraction cells of adipose and connective tissue in patients with osteoarthritis: A case control prospective multi-centric non-randomized study. Glob. Surg. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Williams, L.S.; Weinberger, M.; Harris, L.E.; Clark, D.O.; Biller, J. Development of a stroke-specific quality of life scale. Stroke 1999, 30, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Caruana, G.; Bonomini, S.; Libondi, G. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast Reconstr. Surg. 2014, 133, 1406–1409. [Google Scholar] [PubMed]
- Michalek, J.; Vrablikova, A.; Darinskas, A.; Lukac, L.; Prucha, J.; Skopalik, J.; Travnik, J.; Cibulka, M.; Dudasova, Z. Stromal vascular fraction cell therapy for osteoarthritis in elderly: Multicenter case-control study. J. Clin. Orthop. Trauma 2019, 10, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 2018 13, e01958917. [Google Scholar] [CrossRef]
- Qayyum, A.A.; Mathiasen, A.B.; Mygind, N.D.; Kühl, J.T.; Jørgensen, E.; Helqvist, S.; Elberg, J.J.; Kofoed, K.F.; Vejlstrup, N.G.; Fischer-Nielsen, A.; et al. Adipose-Derived Stromal Cells for Treatment of Patients with Chronic Ischemic Heart Disease (MyStromalCell Trial): A Randomized Placebo-Controlled Study. Stem Cells Int. 2017, 2017, 5237063. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.D.; Lopes-Pacheco, M.; Paz, A.H.R.; Cruz, F.F.; Melo, E.B.; de Oliveira, M.V.; Xisto, D.G.; Capelozzi, V.L.; Morales, M.M.; Pelosi, P.; et al. Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Lung Tissue Differentially Mitigate Lung and Distal Organ Damage in Experimental Acute Respiratory Distress Syndrome. Crit. Care Med. 2018, 46, e132–e140. [Google Scholar] [CrossRef] [PubMed]
- Philandrianos, C.; Serrero, M.; Grimaud, F.; Magalon, J.; Visée, C.; Velier, M.; Francois, P.; Orsoni, P.; Magalon, G.; Grimaud, J.C.; et al. First clinical case report of local microinjection of autologous fat and adipose-derived stromal vascular fraction for perianal fistula in Crohn’s disease. Stem Cell Res. Ther. 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Hsieh, W.L.; Kao, Ch.L.; Chan, R.C. Principles of rehabilitation for common chronic neurologic diseases in the elderly. J. Clin. Geront. Geriatr. 2012, 3, 5–13. [Google Scholar] [CrossRef]
- Ouyang, Q.; Li, F.; Xie, Y.; Han, J.; Zhang, Z.; Feng, Z.; Su, D.; Zou, X.; Cai, Y.; Zou, Y.; et al. Meta-analysis of the safety and efficacy of stem cell therapies for ischemic stroke in preclinical and clinical studies. Stem Cells Dev. 2019. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalek, J.; Vrablikova, A.; Heinrich, K.G.; Dudasova, Z. Stromal Vascular Fraction Cell Therapy for a Stroke Patient—Cure without Side Effects. Brain Sci. 2019, 9, 55. https://doi.org/10.3390/brainsci9030055
Michalek J, Vrablikova A, Heinrich KG, Dudasova Z. Stromal Vascular Fraction Cell Therapy for a Stroke Patient—Cure without Side Effects. Brain Sciences. 2019; 9(3):55. https://doi.org/10.3390/brainsci9030055
Chicago/Turabian StyleMichalek, Jaroslav, Alena Vrablikova, Karl Georg Heinrich, and Zuzana Dudasova. 2019. "Stromal Vascular Fraction Cell Therapy for a Stroke Patient—Cure without Side Effects" Brain Sciences 9, no. 3: 55. https://doi.org/10.3390/brainsci9030055
APA StyleMichalek, J., Vrablikova, A., Heinrich, K. G., & Dudasova, Z. (2019). Stromal Vascular Fraction Cell Therapy for a Stroke Patient—Cure without Side Effects. Brain Sciences, 9(3), 55. https://doi.org/10.3390/brainsci9030055



