Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials
Abstract
1. Introduction
1.1. Insomnia and Obstructive Sleep Apnea
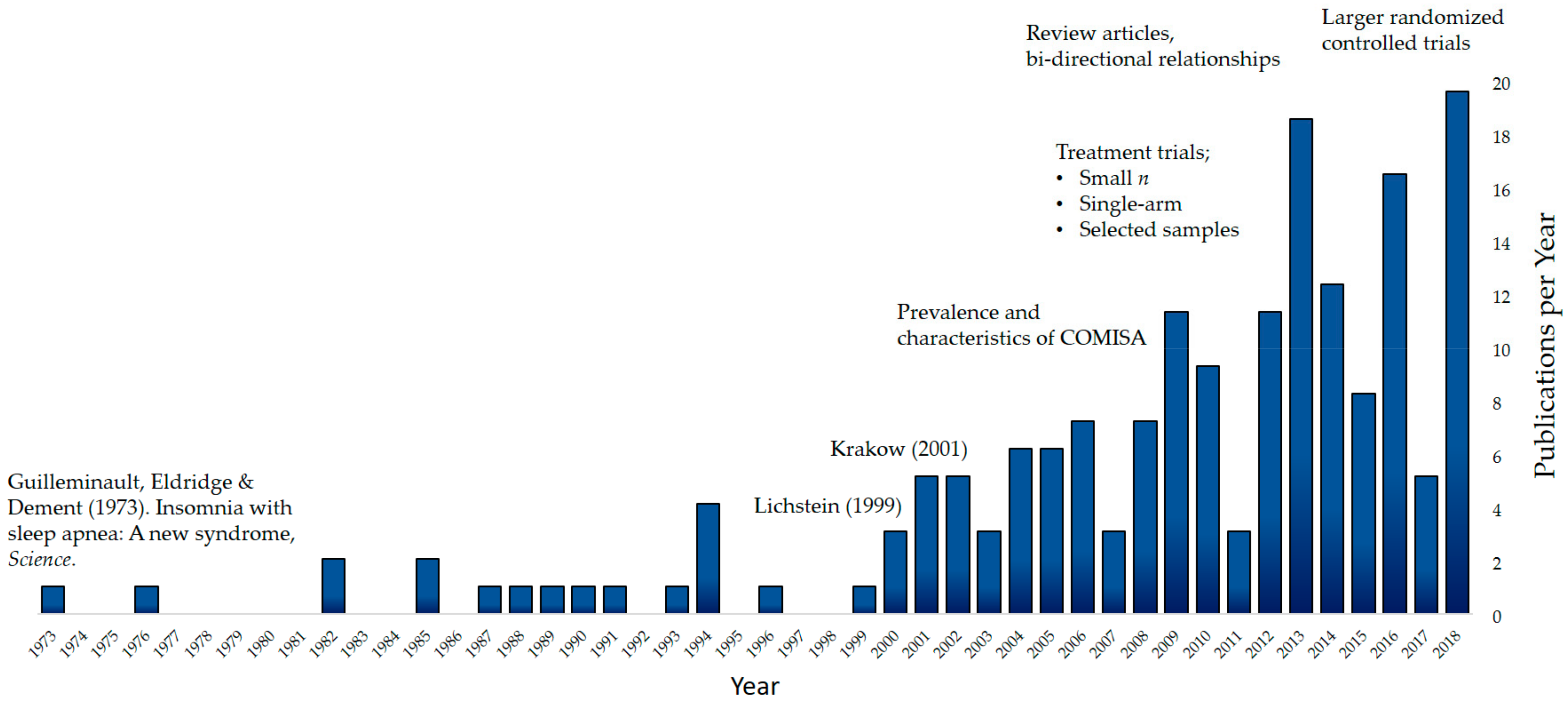
1.2. The Beginning of COMISA Research
1.3. COMISA Prevalence
1.4. Consequences of COMISA
1.5. Refining the Measurement of COMISA
2. Treatment of COMISA
2.1. Traditional Treatment Approaches
2.2. Combined Treatments for COMISA
2.3. Summary of Recent COMISA Randomized Controlled Trials
3. Bi-Directional Relationships in COMISA
4. Recommendations for Clinicians
- The majority of sleep clinics around the world currently specialize in the diagnosis and treatment of OSA whist neglecting the measurement and treatment of insomnia. However, 30%–50% of OSA patients report co-morbid insomnia symptoms, which reduce acceptance and use of CPAP therapy.
- Co-morbid insomnia symptoms commonly reduce CPAP use and contribute to higher impairment of daytime functioning and quality of life. Therefore, the insomnia symptoms demand targeted diagnostic and treatment considerations, and should not be assumed to be a ‘secondary’ manifestation of the OSA.
- COMISA patients should be treated with both CBTi and CPAP therapy to improve insomnia symptoms, and increase CPAP acceptance and use.
- CBTi should be delivered by psychologists, or trained therapists, who can also provide motivational CPAP support.
5. Future Research Directions
- The diagnosis of co-morbid insomnia and OSA represents a complex task due to shared diagnostic symptoms. It is important to validate and refine insomnia measures in the presence of OSA.
- Investigate baseline symptoms and profiles which predict successful responses to different treatment combinations and sequences in COMISA.
- Investigate bi-directional relationships between COMISA, by examining 1) the effect of CBTi on manifestations and severity of OSA (e.g., AHI [109]), and 2) examine the effect of CPAP therapy on manifestations and severity of insomnia symptoms (e.g., sleep parameters, sleep misperceptions, ISI, etc.).
- Determine the most effective CBTi components and combinations to treat insomnia and improve CPAP adherence (for example, using isolated CBTi components such as bedtime restriction therapy to increase sleep efficiency before commencing CPAP therapy).
- Continue examining the efficacy of non-CPAP therapies in the presence of co-morbid insomnia symptoms, for patients who reject CPAP therapy.
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AHI | Apnea/hypopnea index |
| CBTi | Cognitive and behavioral therapy for insomnia |
| COMISA | Co-morbid insomnia and sleep apnea |
| CPAP | Continuous positive airway pressure |
| ISI | Insomnia severity index |
| OSA | Obstructive sleep apnea |
| PSG | Polysomnography |
| RCT | Randomized controlled trial |
References
- The American Academy of Sleep Medicine. International Classification of Sleep Disorders (ICSD-3), Diagnostic and Coding Manual, 3rd ed.; The American Academy of Sleep Medicine: Westchester, IL, USA, 2014. [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and Insomnia: State of the Science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef]
- Perlis, M.; Smith, M.T.; Pigeon, W.R. Etiology and pathophysiology of insomnia. In Principles and Practice in Sleep Medicine, 4th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2005; pp. 714–725. [Google Scholar]
- Spielman, A.J.; Caruso, L.S.; Glovinsky, P.B. A behavioral perspective on insomnia treatment. Psychiatry Clin. N. Am. 1987, 10, 541–553. [Google Scholar] [CrossRef]
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Reynolds, C.F. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009, 10, 952–960. [Google Scholar] [CrossRef]
- Qaseem, A.; Kansagara, D.; Forciea, M.A.; Cooke, M.; Denberg, T.D. Management of chronic insomnia disorder in adults: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2016, 165, 125–133. [Google Scholar] [CrossRef]
- Schutte-Rodin, S.; Broch, L.; Buysse, D.; Dorsey, C.; Sateia, M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J. Clin. Sleep Med. 2008, 4, 487–504. [Google Scholar]
- Ree, M.; Junge, M.; Cunnington, D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. 2017, 36, S43–S47. [Google Scholar] [CrossRef]
- Wilson, S.; Anderson, K.; Baldwin, D.; Dijk, D.-J.; Espie, A.; Espie, C.; Gringras, P.; Krystal, A.; Nutt, D.; Selsick, H. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J. Psychopharmacol. 2019, 33, 923–947. [Google Scholar] [CrossRef]
- Miller, C.B.; Valenti, L.; Harrison, C.M.; Bartlett, D.J.; Glozier, N.; Cross, N.E.; Grunstein, R.R.; Britt, H.C.; Marshall, N.S. Time trends in the family physician management of insomnia: The Australian experience (2000–2015). J. Clin. Sleep Med. 2017, 13, 785–790. [Google Scholar] [CrossRef]
- Kaufmann, C.N.; Spira, A.P.; Depp, C.A.; Mojtabai, R. Long-term use of benzodiazepines and nonbenzodiazepine hypnotics, 1999–2014. Psychiatr. Serv. 2017, 69, 235–238. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thor. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults: Adult obstructive sleep apnea task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef]
- Kapur, V.K.; Baldwin, C.M.; Resnick, H.E.; Gottlieb, D.J.; Nieto, F.J. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep 2005, 28, 472–478. [Google Scholar] [CrossRef]
- Sassani, A.; Findley, L.J.; Kryger, M.; Goldlust, E.; George, C.; Davidson, T.M. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep 2004, 27, 453–458. [Google Scholar] [CrossRef]
- Ellen, R.L.; Marshall, S.C.; Palayew, M.; Molnar, F.J.; Wilson, K.G.; Man-Son-Hing, M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J. Clin. Sleep Med. 2006, 2, 193–200. [Google Scholar]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Kushida, C.A.; Chediak, A.; Berry, R.B.; Brown, L.K.; Gozal, D.; Iber, C.; Parthasarathy, S.; Quan, S.F.; Rowley, J.A. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J. Clin. Sleep Med. 2008, 4, 157–171. [Google Scholar]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef]
- Weaver, T.E.; Grunstein, R.R. Adherence to continuous positive airway pressure therapy: The challenge to effective treatment. Proc. Am. Thor. Soc. 2008, 5, 173–178. [Google Scholar] [CrossRef]
- Guilleminault, C.; Eldridge, F.L.; Dement, W.C. Insomnia with sleep apnea: A new syndrome. Science 1973, 181, 856–858. [Google Scholar] [CrossRef]
- Guilleminault, C.; Eldridge, F.L.; Phillips, J.R.; Dement, W.C. Two occult causes of insomnia and their therapeutic problems. Arch. Gen. Psychiatry 1976, 33, 1241–1245. [Google Scholar] [CrossRef]
- Saaresranta, T.; Hedner, J.; Bonsignore, M.R.; Riha, R.L.; McNicholas, W.T.; Penzel, T.; Antalainen, U.; Kvamme, J.A.; Pretl, M.; Sliwinski, P. Clinical phenotypes and comorbidity in European sleep apnoea patients. PLoS ONE 2016, 11, e0163439. [Google Scholar] [CrossRef]
- Ye, L.; Pien, G.W.; Ratcliffe, S.J.; Björnsdottir, E.; Arnardottir, E.S.; Pack, A.I.; Benediktsdottir, B.; Gislason, T. The different clinical faces of obstructive sleep apnoea: A cluster analysis. Eur. Res. J. 2013, 44, 1600–1607. [Google Scholar] [CrossRef]
- Appleton, S.; Gill, T.; Taylor, A.; McEvoy, D.; Shi, Z.; Hill, C.; Reynolds, A.; Adams, R. Influence of gender on associations of obstructive sleep apnea symptoms with chronic conditions and quality of life. Int. J Environ. Res. Pub. Health 2018, 15, 930. [Google Scholar] [CrossRef]
- Lichstein, K.L. Secondary insomnia: A myth dismissed. Sleep Med. Rev. 2006, 10, 3–5. [Google Scholar] [CrossRef]
- Lichstein, K.; McCrae, C.; Wilson, N. Secondary insomnia: Diagnostic issues, cognitive-behavioral treatment, and future directions. Treating Sleep Disorders: Principles and Practice of Behavioral Sleep Medicine; Wiley: Hoboken, NJ, USA, 2003; pp. 286–304. [Google Scholar]
- Lichstein, K.L.; Nau, S.D.; McCrae, C.S.; Stone, K.C. Psychological and behavioral treatments for secondary insomnias. In Principles and Practice in Sleep Medicine, 4th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; Elsevier: Philadelphia, PA, USA, 2005. [Google Scholar]
- Stepanski, E.; Rybarczyk, B. Emerging research on the treatment and etiology of secondary or comorbid insomnia. Sleep Med. Rev. 2006, 10, 7–18. [Google Scholar] [CrossRef]
- National Institutes of Health. National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep 2005, 28, 1049–1057. [Google Scholar] [CrossRef]
- Morin, C.M.; Kowatch, R.A.; Barry, T.; Walton, E. Cognitive-behavior therapy for late-life insomnia. J. Consult. Clin. Psychol. 1993, 61, 137–146. [Google Scholar] [CrossRef]
- Stone, J.; Morin, C.M.; Hart, R.P.; Remsberg, S.; Mercer, J. Neuropsychological functioning in older insomniacs with or without obstructive sleep apnea. Psychol. Aging 1994, 9, 231. [Google Scholar] [CrossRef]
- Coleman, R.M.; Roffwarg, H.P.; Kennedy, S.J.; Guilleminault, C.; Cinque, J.; Cohn, M.A.; Karacan, I.; Kupfer, D.J.; Lemmi, H.; Miles, L.E.; et al. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. J. Am. Med. Assoc. 1982, 147, 997–1003. [Google Scholar]
- Lichstein, K.L.; Riedel, B.W.; Lester, K.W.; Aguillard, R.N. Occult sleep apnea in a recruited sample of older adults with insomnia. J. Consult. Clin. Psychol. 1999, 67, 405–410. [Google Scholar] [CrossRef]
- Krakow, B.; Melendrez, D.; Ferreira, E.; Clark, J.; Warner, T.D.; Sisley, B.; Sklar, D. Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 2001, 120, 1923–1929. [Google Scholar] [CrossRef]
- Luyster, F.S.; Buysse, D.J.; Strollo, P.J. Comorbid insomnia and obstructive sleep apnea: Challenges for clinical practice and research. J. Clin. Sleep Med. 2010, 6, 196–204. [Google Scholar]
- Sweetman, A.; Lack, L.C.; Catcheside, P.G.; Antic, N.A.; Chai-Coetzer, C.L.; Smith, S.S.; Douglas, J.A.; McEvoy, R.D. Developing a successful treatment for co-morbid insomnia and sleep apnoea. Sleep Med. Rev. 2017, 33, 28–38. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Vaillant, M.L.; Paris, A.; Pigeanne, T.; Leclair-Visonneau, L.; Bizieux-Thaminy, A.; Alizon, C.; Humeau, MP.; Nguyen, XL.; Rouault, B.; et al. Relationship between OSA clinical phenotypes and CPAP treatment outcomes. Chest 2016, 149, 288–290. [Google Scholar] [CrossRef]
- Pien, G.W.; Ye, L.; Keenan, B.T.; Maislin, G.; Björnsdóttir, E.; Arnardottir, E.S.; Benediktsdottir, B.; Gislason, T.; Pack, A.I. Changing Faces of OSA: Treatment Effects by Cluster Designation in the Icelandic Sleep Apnea Cohort. Sleep 2018, 41, zsx201. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, R.; Lei, F.; Zhou, J.; Zhang, J.; Wing, Y.-K.; Sanford, L.D.; Tang, X. Worldwide and regional prevalence rates of co-occurrence of insomnia and insomnia symptoms with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 45, 1–17. [Google Scholar] [CrossRef]
- Wallace, D.M.; Vargas, S.S.; Schwartz, S.J.; Aloia, M.S.; Shafazand, S. Determinants of continuous positive airway pressure adherence in a sleep clinic cohort of South Florida Hispanic veterans. Sleep Breath. 2013, 17, 351–363. [Google Scholar] [CrossRef]
- Mysliwiec, V.; Matsangas, P.; Baxter, T.; McGraw, L.; Bothwell, N.E.; Roth, B.J. Comorbid insomnia and obstructive sleep apnea in military personnel: Correlation with polysomnographic variables. Mil. Med. 2014, 179, 294–300. [Google Scholar] [CrossRef]
- Lang, C.J.; Appleton, S.L.; Vakulin, A.; McEvoy, R.D.; Wittert, G.A.; Martin, S.A.; Catcheside, P.G.; Antic, N.A.; Lack, L.; Adams, R. Co-morbid OSA and insomnia increases depression prevalence and severity in men. Respirology 2017, 22, 1407–1415. [Google Scholar]
- Tasbakan, M.; Gunduz, C.; Pirildar, S.; Basoglu, O.K. Quality of life in obstructive sleep apnea is related to female gender and comorbid insomnia. Sleep Breath. 2018, 22, 1013–1020. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, K.T.; Moon, H.J.; Korostyshevskiy, V.R.; Motamedi, G.K.; Yang, K.K. Comorbid Insomnia with Obstructive Sleep Apnea: Clinical Characteristics and Risk Factors. J. Clin. Sleep Med. 2018, 14, 409–417. [Google Scholar] [CrossRef]
- Wallace, D.M.; Sawyer, A.; Shafazand, S. Comorbid insomnia symptoms predict lower 6-month adherence to CPAP in US veterans with obstructive sleep apnea. Sleep Breath. 2018, 22, 5–15. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Adamo, D.; Kufel, T. Comorbid insomnia and sleep apnea in Veterans with post-traumatic stress disorder. Sleep Breath. 2018, 22, 23–31. [Google Scholar] [CrossRef]
- Philip, R.; Catcheside, P.; Stevens, D.; Lovato, N.; McEvoy, D.; Vakulin, A. Comorbid insomnia and sleep apnoea is associated with greater neurocognitive impairment compared with OSA alone. J. Sleep Res. 2017, 40, e260. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.C.; Lambert, S.; Gradisar, M.; Harris, J. Does co-morbid obstructive sleep apnea impair the effectiveness of cognitive and behavioral therapy for insomnia? Sleep Med. 2017, 39, 38–46. [Google Scholar] [CrossRef]
- Buysse, D.J.; Ancoli-Israel, S.; Edinger, J.D.; Lichstein, K.L.; Morin, C.M. Recommendations for a standard research assessment of insomnia. Sleep 2006, 29, 1155–1173. [Google Scholar] [CrossRef]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef]
- Partinen, M.; Gislason, T. Basic Nordic Sleep Questionnaire (BNSQ): A quantitated measure of subjective sleep complaints. J. Sleep Res. 1995, 4, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Hoch, C.C.; Yeager, A.L.; Kupfer, D.J. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar] [PubMed]
- Sweetman, A.; Lack, L.C.; McEvoy, D. Refining the Measurement of Insomnia in Patients with Obstructive Sleep Apnea: Commentary on Wallace and Wohlgemuth. Predictors of Insomnia Severity Index Profiles in US veterans with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, in press. [Google Scholar]
- Duarte, R.; Magalhães-da-Silveira, J.F.; Oliveira-e-Sá, T.S.; Rabahi, M.F.; Mello, F.C.; Gozal, D. Predicting Obstructive Sleep Apnea in Patients with Insomnia: A Comparative Study with Four Screening Instruments. Lung 2019, 197, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000, 118, 372–379. [Google Scholar] [CrossRef]
- Espie, C.A.; Kyle, S.D.; Hames, P.; Gardani, M.; Fleming, L.; Cape, J. The Sleep Condition Indicator: A clinical screening tool to evaluate insomnia disorder. BMJ Open 2014, 4, e004183. [Google Scholar] [CrossRef]
- Wallace, D.M.; Wohlgemuth, W.K. Predictors of Insomnia Severity Index Profiles in US Veterans with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2019, in press. [Google Scholar]
- Glidewell, R.N.; Renn, B.N.; Roby, E.; Orr, W.C. Predictors and patterns of insomnia symptoms in OSA before and after PAP therapy. Sleep Med. 2014, 15, 899–905. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Catcheside, P.; Antic, N.; Smith, S.; Chai-Coetzer, C.L.; Douglas, P.; O’Grady, A.; Dunn, N.; Robinson, J.; et al. Cognitive and behavioral therapy for insomnia increases the use of continuous positive airway pressure therapy in obstructive sleep apnea participants with co-morbid insomnia: A randomized clinical trial. Sleep 2019. [Google Scholar] [CrossRef]
- Philip, P.; Bioulac, S.; Altena, E.; Morin, C.M.; Ghorayeb, I.; Coste, O.; Monteyrol, P.J.; Micoulaud-Franchi, JA. Specific insomnia symptoms and self-efficacy explain CPAP compliance in a sample of OSAS patients. PLoS ONE 2018, 13, e0195343. [Google Scholar] [CrossRef]
- Ji, X.B.C.; Ellis, J.G.; Hale, L.; Grandner, M.A. Disassembling insomnia symptoms and their associations with depressive symptoms in a community sample: The differential role of sleep symptoms, daytime symptoms, and perception symptoms of insomnia. Sleep Health 2019, 5, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.L.; Bakoyannis, G.; Rand, K.L.; Ensrud, K.E.; Guthrie, K.A.; Joffe, H.; McCurry, S.M.; Newton, K.M.; Carpenter, J.S. Confirmatory factor analysis of the Insomnia Severity Index (ISI) and invariance across race: A pooled analysis of MsFLASH data. Menopause 2019, 26, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Barthlen, G.M.; Lange, D.J. Unexpectedly severe sleep and respiratory pathology in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 2000, 7, 299–302. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Chung, S. A case of obstructive sleep apnea syndrome presenting as paradoxical insomnia. Psychiatry Investig. 2010, 7, 75–78. [Google Scholar] [CrossRef]
- Smith, S.S.; Dunn, N.; Douglas, J.; Jorgensen, G. Sleep onset insomnia is associated with reduced adherence to CPAP therapy. Sleep Biol. Rhythm. 2009, 7, 74. [Google Scholar]
- Suraiya, S.; Lavie, P. Sleep onset insomnia in sleep apnea patients: Influence on acceptance of nCPAP treatment. Sleep Med. 2006, 7, S85. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Smith, M.T.; Birnbaum, S.; Collop, N.A. Sleep maintenance insomnia complaints predict poor CPAP adherence: A clinical case series. Sleep Med. 2010, 11, 772–776. [Google Scholar] [CrossRef]
- Nguyên, X.; Chaskalovic, J.; Rakotonanahary, D.; Fleury, B. Insomnia symptoms and CPAP compliance in OSAS patients: A descriptive study using data mining methods. Sleep Med. 2010, 11, 777–784. [Google Scholar] [CrossRef]
- Mysliwiec, V.; Capaldi, V.; Gill, J.; Baxter, T.; O’Reilly, B.M.; Matsangas, P.; Roth, B.J. Adherence to positive airway pressure therapy in U.S. military personnel with sleep apnea improves sleepiness, sleep quality, and depressive symptoms. Mil. Med. 2015, 180, 475–482. [Google Scholar] [CrossRef]
- Guilleminault, C.; Palombini, L.; Poyares, D.; Chowdhuri, S. Chronic insomnia, premenopausal women, and sleep disordered breathing part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J. Psychosom. Res. 2002, 53, 617–623. [Google Scholar] [CrossRef]
- Melendrez, D.C.; Krakow, B.J.; Johnston, L.; Sisley, B.; Warner, T.D. A prospective study on the treatment of complex insomnia—Insomnia plus sleep disordered breathing—In a small series of crime victims with PTSD. Sleep 2001, 24, A120. [Google Scholar]
- Guilleminault, C.; Davis, K.; Huynh, N.T. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep 2008, 31, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.H.; Martin, J.L.; Josephson, K.; Fiorentino, L.; Dzierzewski, J.M.; Jouldjian, S.; Rodriguez-Tapia, J.C.; Mitchell, M.N.; Alessi, C. Efficacy of cognitive behavioral therapy for insomnia in older adults with occult sleep-disordered breathing. Psychosom. Med. 2016, 78, 629. [Google Scholar] [CrossRef] [PubMed]
- Alessi, C.; Martin, J.; Fung, C.; Dzierzewski, J.; Fiorentino, L.; Stepnowsky, C.; Song, Y.; Rodriguez, R.C.; Zeidler, M.; Mitchell, M. 0407 Randomized Controlled Trial of an Integrated Behavioral Treatment in Veterans with Obstructive Sleep Apnea and Coexisting Insomnia. Sleep 2018, 41 (Suppl. 1), A155. [Google Scholar] [CrossRef]
- Machado, M.A.C.; de Carvalho, L.B.C.; Juliano, M.L.; Taga, M.; do Prado, L.B.F.; do Prado, G.F. Clinical co-morbidities in obstructive sleep apnea syndrome treated with mandibular repositioning appliance. Respir. Med. 2005, 100, 988–995. [Google Scholar]
- Krakow, B.; Melendrez, D.; Sisley, B.; Warner, T.D.; Krakow, J. Nasal dilator strip therapy for chronic sleep maintenance insomnia: A case series. Sleep Breath. 2004, 8, 133–140. [Google Scholar] [CrossRef]
- Krakow, B.; Melendrez, D.; Sisley, B.; Warner, T.D.; Krakow, J.; Leahigh, L.; Lee, S. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: A randomized controlled trial. Sleep Breath. 2006, 10, 16–28. [Google Scholar] [CrossRef]
- Wickwire, E.M.; Schumacher, J.A.; Richert, A.C.; Baran, A.S.; Roffwarg, H.P. Combined insomnia and poor CPAP compliance: A case study and discussion. Clin. Case Stud. 2008, 7, 267–286. [Google Scholar] [CrossRef]
- Bjorvatn, B.; Berge, T.; Lehmann, S.; Pallesen, S.; Saxvig, IW. No Effect of a Self-help Book for Insomnia in Patients with Obstructive Sleep Apnea and Comorbid Chronic Insomnia—A Randomized Controlled Trial. Front. Psychol. 2018, 9, 2413. [Google Scholar] [CrossRef]
- Bjorvatn, B.; Fiske, E.; Pallesen, S. A self-help book is better than sleep hygiene advice for insomnia: A randomized controlled comparative study. Scand. J. Psychol. 2011, 52, 580–585. [Google Scholar] [CrossRef]
- Pallesen, S.; Bjorvatn, B.; Nordhus, I.H.; Sivertsen, B.; Hjornevik, M.; Morin, C.M. A new scale for measuring insomnia: The Bergen Insomnia Scale. Percept. Mot. Ski. 2008, 107, 691–706. [Google Scholar] [CrossRef]
- Alessi, C. Novel Treatment of Comorbid Insomnia and Sleep Apnea in Older Veterans. 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT02027558 (accessed on 1 October 2019).
- Ong, J.C.; Crawford, M.R.; Wyatt, J.K.; Fogg, L.F.; Turner, A.D.; Dawson, S.C.; Edinger, J.D.; Kushida, C.A.; Abbott, S.M.; Malkani, R.G. 0379 A Randomized Controlled Trial Of CBT-I and CPAP For Comorbid Insomnia and OSA: Initial Findings from the MATRICS Study. Sleep 2019, 42 (Suppl. 1), A154. [Google Scholar] [CrossRef]
- Crawford, M.R.; Turner, A.D.; Wyatt, J.K.; Fogg, L.F.; Ong, J.C. Evaluating the treatment of obstructive sleep apnea comorbid with insomnia disorder using an incomplete factorial design. Contemp. Clin. Trials 2016, 47, 146–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Edinger, J.; Manber, R. Stepped-Care Management of Insomnia Co-Occurring with Sleep Apnea; National Institute of Health: Denver, CO, USA, 2016. [Google Scholar]
- Van Ryswyk, E.; Anderson, C.S.; Antic, N.A.; Barbe, F.; Bittencourt, L.; Freed, R.; Heeley, E.; Liu, Z.; Loffler, K.; Lorenzio-Filho, G.; et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnea and cardiovascular disease. Sleep 2019, 36, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.C.; Crawford, M.R.; Kong, A.; Park, M.; Cvengros, J.A.; Crisostomo, M.I.; Alexander, E.I.; Whatt, J.K. Management of obstructive sleep apnea and comorbid insomnia: A mixed-methods evaluation. Behav. Sleep Med. 2016, 15, 180–197. [Google Scholar]
- Olsen, S.L.; Smith, S.S.; Oei, T.; Douglas, J. Motivational interviewing (MINT) improves continuous positive airway pressure (CPAP) acceptance and adherence: A randomised controlled trial. J. Consult. Clin. Psychol. 2012, 80, 151–163. [Google Scholar] [CrossRef]
- Wozniak, D.; Lasserson, T.J.; Smith, I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2014, 1. [Google Scholar] [CrossRef]
- Benetó, A.; Gomez-Siurana, E.; Rubio-Sanchez, P. Comorbidity between sleep apnea and insomnia. Sleep Med. Rev. 2009, 13, 287–293. [Google Scholar] [CrossRef]
- Lack, L.; Sweetman, A. Diagnosis and treatment of insomnia comorbid with obstructive sleep apnea. Sleep Med. Clin. 2016, 11, 379–388. [Google Scholar] [CrossRef]
- Eckert, D.J.; Parikh, S.; White, D.P.; Jordan, A.S.; Merchia, P.; Malhotra, A. Sleep deprivation impairs genioglossus muscle responsiveness. Am. J. Respir. Crit. Care Med. 2011, 183, A6163. [Google Scholar]
- Parikh, S.; White, D.; Jordan, A.; Merchia, P.; Malhotra, A.; Eckert, D. 36 Hours of Sleep Deprivation Reduces Genioglossus Muscle Activity during Hypercapnia and Inspiratory Resistive Loads during Wakefulness. Sleep 2011, 34, A153. [Google Scholar]
- Leiter, J.C.; Knuth, S.L.; Bartlett, D. The effect of sleep deprivation on activity of the genioglossus muscle. Am. Rev. Res. Dies. 1985, 132, 1242–1245. [Google Scholar]
- Persson, H.E.; Svanborg, E. Sleep deprivation worsens obstructive sleep apnea: Comparison between diurnal and nocturnal polysomnography. Chest 1996, 109, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Marks, G.; Grunstein, R. Does sleep deprivation worsen mild obstructive sleep apnea? Sleep 2003, 26, 1038–1041. [Google Scholar] [CrossRef] [PubMed]
- Guilleminault, C.; Rosekind, M. The arousal threshold: Sleep deprivation, sleep fragmentation, and obstructive sleep apnea syndrome. Bull. Eur. Physiopathol. Respir. 1981, 17, 341–349. [Google Scholar]
- Guilleminault, C.; Silvestri, R.; Mondini, S.; Coburn, S. Aging and sleep apnea: Action of benzodiazepine, acetazolamide, alcohol, and sleep deprivation in a healthy elderly group. J. Gerontol. 1984, 39, 655–661. [Google Scholar] [CrossRef]
- Stoohs, R.A.; Dement, W.C. Snoring and sleep-related breathing abnormality during partial sleep deprivation. N. Engl. J. Med. 1993, 328, 1279. [Google Scholar] [CrossRef]
- Younes, M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am. J. Crit. Care Med. 2004, 169, 623–633. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia. Sleep Med. Rev. 1997, 1, 97–108. [Google Scholar] [CrossRef]
- Ratnavadivel, R.; Chau, N.; Stadler, D.; Yeo, A.; McEvoy, D.R.; Catcheside, P.G. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J. Clin. Sleep Med. 2009, 5, 519–524. [Google Scholar]
- Janssen, H.C.; Venekamp, L.N.; Peeters, G.A.; Pijpers, A.; Pevernagie, D.A. Management of insomnia in sleep disordered breathing. Eur. Res. Rev. 2019, 28, 190080. [Google Scholar] [CrossRef]
- Sweetman, A.; Lack, L.; Catcheside, P.; Antic, N.; Smith, S.; Chai-Coetzer, C.L.; Douglas, P.; O’Grady, A.; Dunn, N.; Robinson, J.; et al. The Effect of Cognitive and Behavioural Therapy for Insomnia on Changes in Sleep Architecture and AHI in Patients with Co-Occurring Insomnia and Sleep Apnea; World Sleep: Vancouver, BC, Canada, 2019; Available online: https://www.researchgate.net/publication/334506579_The_effect_of_cognitive_and_behavioural_therapy_for_insomnia_on_changes_in_sleep_architecture_and_AHI_in_patients_with_co-occurring_insomnia_and_sleep_apnea (accessed on 1 October 2019).
- Sériès, F. Can improving sleep influence sleep-disordered breathing? Drugs 2009, 69 (Suppl. 2), 77–91. [Google Scholar] [CrossRef]
- Mercer, J.D.; Bootzin, R.R.; Lack, L. Insomniacs’ perception of wake instead of sleep. Sleep 2002, 25, 559–566. [Google Scholar] [CrossRef]
- Björnsdóttir, E.; Janson, C.; Sigurdsson, J.F.; Gehrman, P.; Perlis, M.; Juliusson, S.; Arnardottir, E.S.; Kuna, S.T.; Pack, A.I.; Gislason, T. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep 2013, 36, 1901–1909. [Google Scholar] [CrossRef]
| Study | n | Diagnostic Criteria | CBTi Intervention | Control | CPAP Follow-up | Insomnia Outcome | CPAP Use |
|---|---|---|---|---|---|---|---|
| Alessi et al., 2018 [79,87] | 125 | ICSD-3, AHI ≥ 15 | Five session CBTi and behavioral CPAP adherence program, delivered concurrently with CPAP | Sleep Education Program | Objective CPAP data at 6 months. | CBTi group showed greater improvement during treatment. | CBTi group showed 78, and 48 min greater CPAP use, at the 3 and 6 month follow-ups, respectively. |
| Bjorvatn et al., 2018 [84] | 134 | DSM-5, ICSD-3, AHI ≥ 5 | Previously validated self-help CBTi book, delivered concurrently with CPAP | Sleep hygiene advice | Objective CPAP data at 3 months. | No difference in improvement of ISI or Bergen Insomnia Scale between groups. | No significant difference between groups. Mean difference = 1 minute. |
| Ong et al., 2019 [88,89] | 121 | ICSD-2, AHI ≥ 5 | Four session CBTi program, delivered before vs. concurrently with CPAP | No treatment, monitoring | Objective CPAP data at 3 months | CBTi groups reported greater ISI improvement during treatment. | No significant difference between CBTi and CPAP-only groups. CBTi before CPAP (M use = 148 min, SD = 137) CBTi with CPAP (M use = 152 min, SD = 155) CPAP only (M use = 181 min, SD = 155). |
| Sweetman et al., 2019 [64] | 145 | ICSD-3, AHI ≥ 15 | Four session CBTi program delivered before CPAP | No treatment | Objective CPAP data at 6 months. | CBTi group reported greater improvement of the ISI, sleep diary parameters, and dysfunctional beliefs about sleep during treatment. | CBTi group showed 61 min greater CPAP use over the first 6 months. CBTi before CPAP (M use = 265, SD = 166) CPAP only (M use = 204, SD = 153). |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sweetman, A.; Lack, L.; Bastien, C. Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials. Brain Sci. 2019, 9, 371. https://doi.org/10.3390/brainsci9120371
Sweetman A, Lack L, Bastien C. Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials. Brain Sciences. 2019; 9(12):371. https://doi.org/10.3390/brainsci9120371
Chicago/Turabian StyleSweetman, Alexander, Leon Lack, and Célyne Bastien. 2019. "Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials" Brain Sciences 9, no. 12: 371. https://doi.org/10.3390/brainsci9120371
APA StyleSweetman, A., Lack, L., & Bastien, C. (2019). Co-Morbid Insomnia and Sleep Apnea (COMISA): Prevalence, Consequences, Methodological Considerations, and Recent Randomized Controlled Trials. Brain Sciences, 9(12), 371. https://doi.org/10.3390/brainsci9120371







