Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Slice Preparation
2.3. Whole-Cell Patch-Clamp Recording
2.4. Data Analysis and Statistics
2.5. Drugs
3. Results
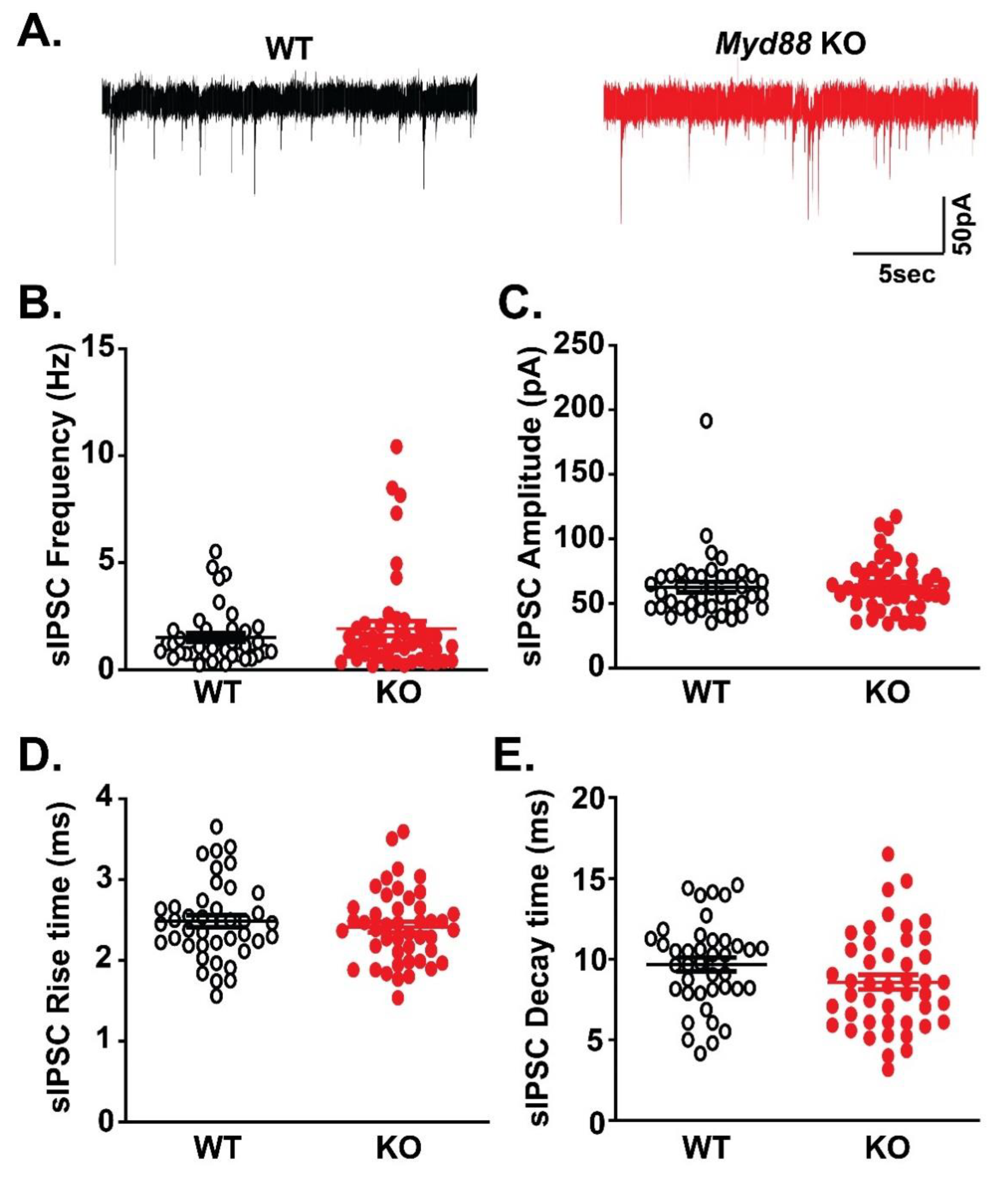
3.1. Basal GABAergic Transmission is Similar in the CeA of Myd88 KO and WT Mice
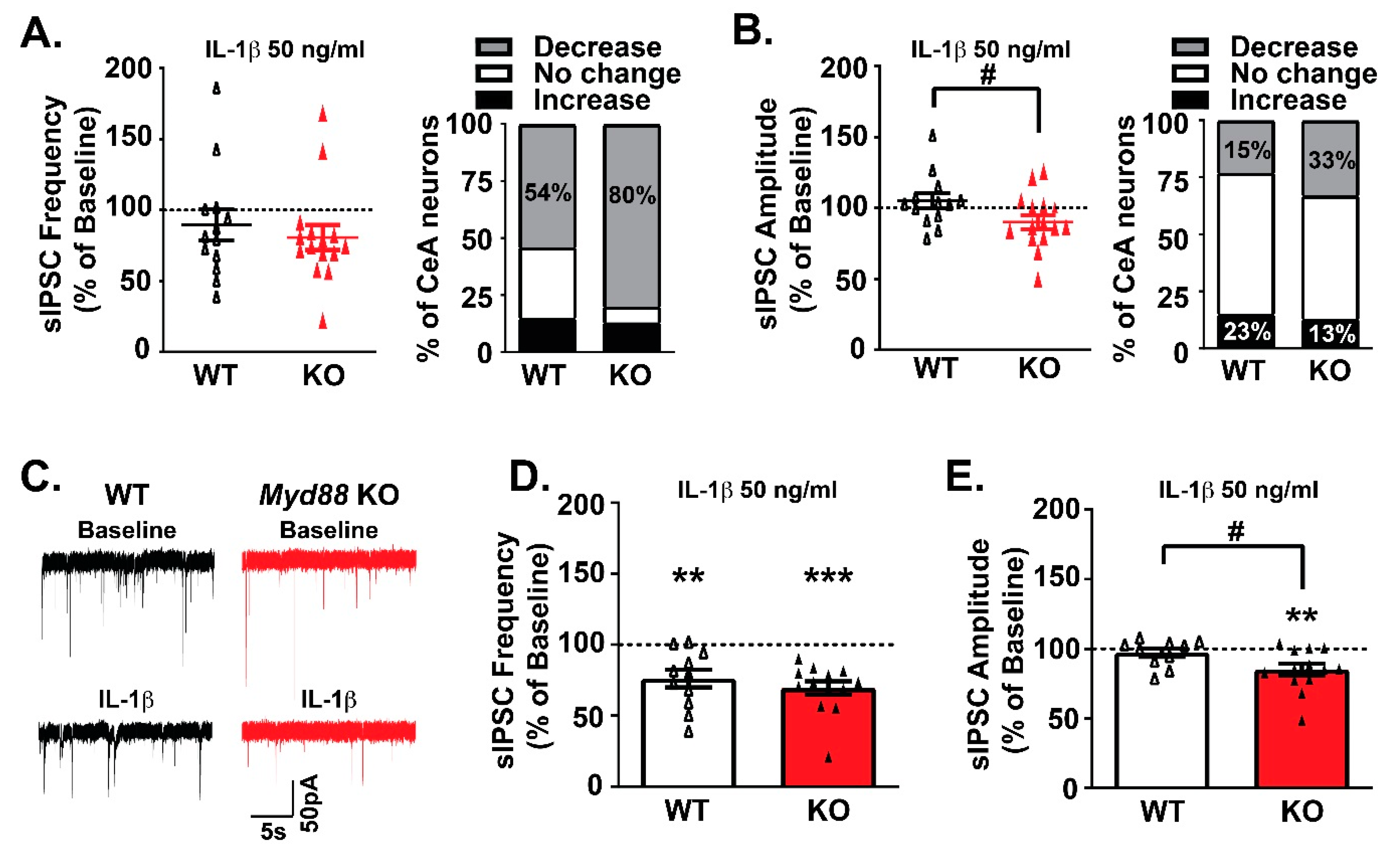
3.2. MyD88 Modulates IL-1β’s Actions at CeA GABA Synapses
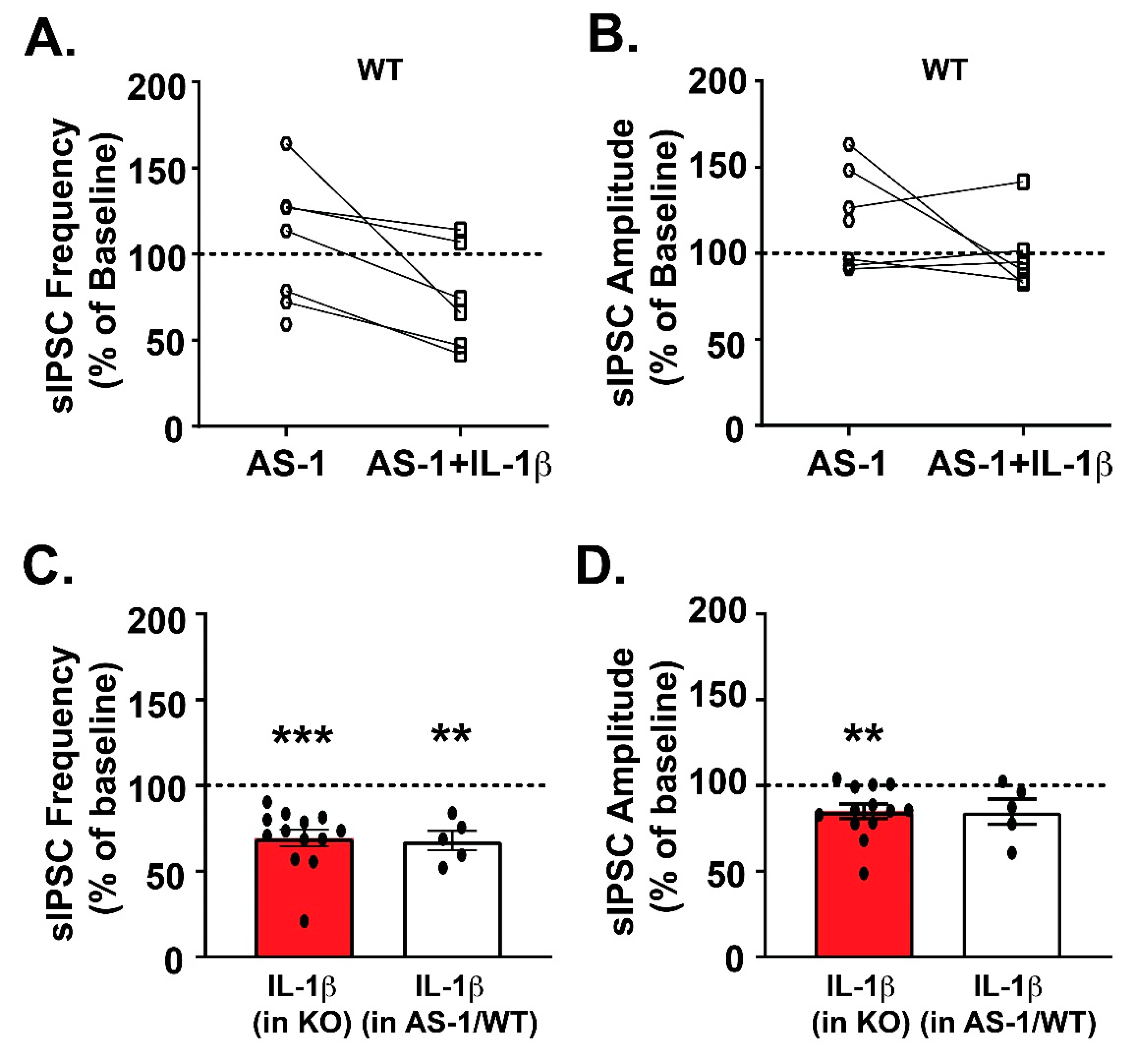
3.3. Pharmacological Inhibition of MyD88 Modulates IL-1β’s Action at CeA GABA Synapses Similar to Myd88 KO Mice
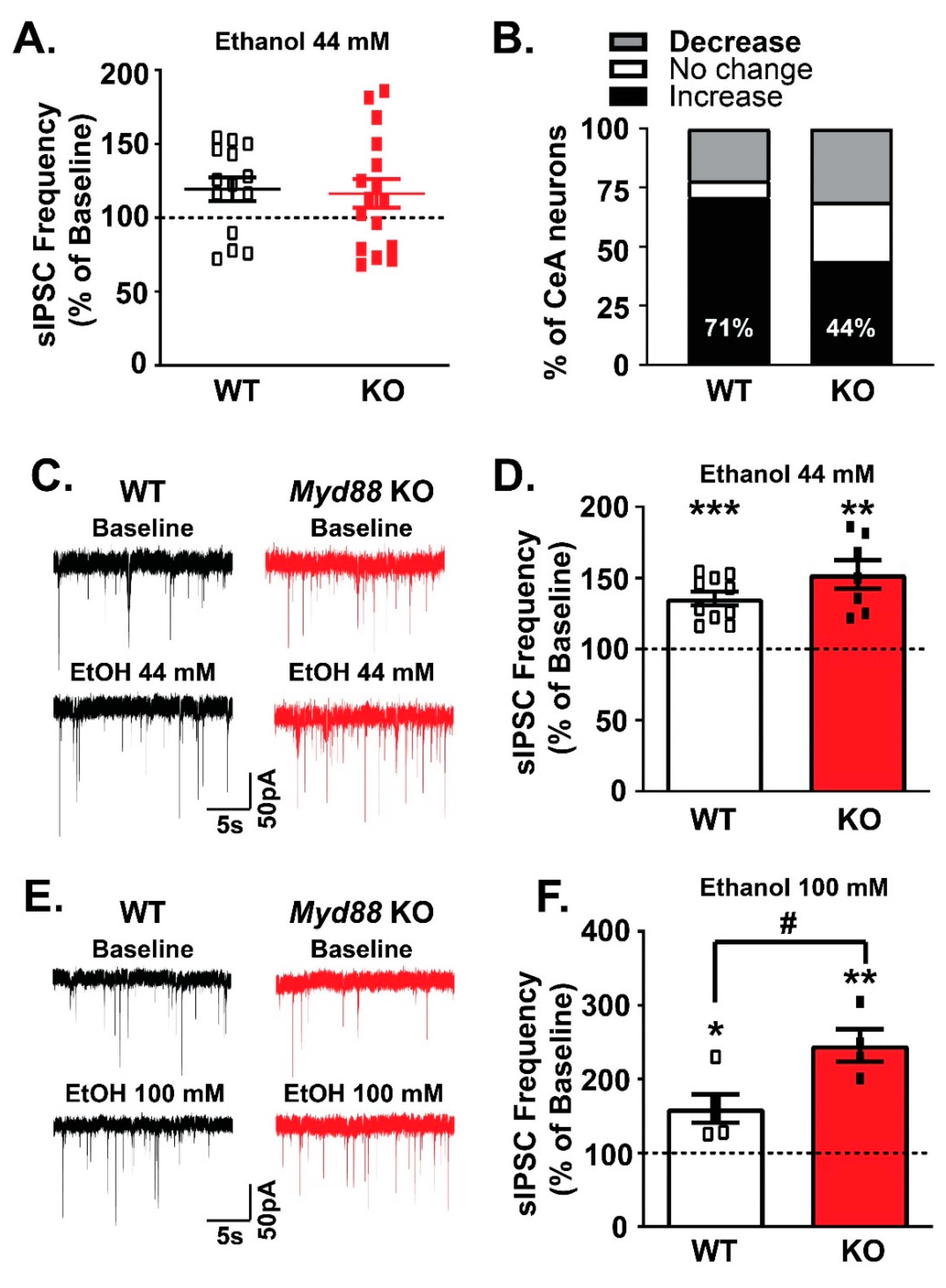
3.4. MyD88 Modulates Ethanol’s Facilitation of GABA Release in the CeA
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blanco, A.M.; Guerri, C. Ethanol intake enhances inflammatory mediators in brain: Role of glial cells and TLR4/IL-1RI receptors. Front. Biosci. 2007, 12, 2616–2630. [Google Scholar] [CrossRef][Green Version]
- McCarthy, G.M.; Warden, A.S.; Bridges, C.R.; Blednov, Y.A.; Harris, R.A. Chronic ethanol consumption: Role of TLR3/TRIF-dependent signaling. Addict. Biol. 2018, 23, 889–903. [Google Scholar] [CrossRef]
- Liu, J.; Lewohl, J.M.; Harris, R.A.; Iyer, V.R.; Dodd, P.R.; Randall, P.K.; Mayfield, R.D. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology 2006, 31, 1574–1582. [Google Scholar] [CrossRef]
- He, J.; Crews, F.T. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp. Neurol. 2008, 210, 349–358. [Google Scholar] [CrossRef]
- Mulligan, M.K.; Ponomarev, I.; Hitzemann, R.J.; Belknap, J.K.; Tabakoff, B.; Harris, R.A.; Crabbe, J.C.; Blednov, Y.A.; Grahame, N.J.; Phillips, T.J.; et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. Proc. Natl. Acad. Sci. USA 2006, 103, 6368–6373. [Google Scholar] [CrossRef]
- Kimpel, M.W.; Strother, W.N.; McClintick, J.N.; Carr, L.G.; Liang, T.; Edenberg, H.J.; McBride, W.J. Functional gene expression differences between inbred alcohol-preferring and -non-preferring rats in five brain regions. Alcohol 2007, 41, 95–132. [Google Scholar] [CrossRef]
- Flatscher-Bader, T.; Zuvela, N.; Landis, N.; Wilce, P.A. Smoking and alcoholism target genes associated with plasticity and glutamate transmission in the human ventral tegmental area. Hum. Mol. Genet. 2008, 17, 38–51. [Google Scholar] [CrossRef]
- Liu, J.; Lewohl, J.M.; Harris, R.A.; Dodd, P.R.; Mayfield, R.D. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol. Clin. Exp. Res. 2007, 31, 1460–1466. [Google Scholar] [CrossRef]
- Mayfield, J.; Arends, M.A.; Harris, R.A.; Blednov, Y.A. Genes and Alcohol Consumption: Studies with Mutant Mice. Int. Rev. Neurobiol. 2016, 126, 293–355. [Google Scholar] [CrossRef]
- Robinson, G.; Most, D.; Ferguson, L.B.; Mayfield, J.; Harris, R.A.; Blednov, Y.A. Neuroimmune pathways in alcohol consumption: Evidence from behavioral and genetic studies in rodents and humans. Int. Rev. Neurobiol. 2014, 118, 13–39. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Benavidez, J.M.; Geil, C.; Perra, S.; Morikawa, H.; Harris, R.A. Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav. Immun. 2011, 25, S92–S105. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Benavidez, J.M.; Black, M.; Mayfield, J.; Harris, R.A. Role of interleukin-1 receptor signaling in the behavioral effects of ethanol and benzodiazepines. Neuropharmacology 2015, 95, 309–320. [Google Scholar] [CrossRef]
- Montesinos, J.; Gil, A.; Guerri, C. Nalmefene Prevents Alcohol-Induced Neuroinflammation and Alcohol Drinking Preference in Adolescent Female Mice: Role of TLR4. Alcohol. Clin. Exp. Res. 2017, 41, 1257–1270. [Google Scholar] [CrossRef]
- Marshall, S.A.; McKnight, K.H.; Blose, A.K.; Lysle, D.T.; Thiele, T.E. Modulation of Binge-like Ethanol Consumption by IL-10 Signaling in the Basolateral Amygdala. J. Neuroimmune Pharmacol. 2017, 12, 249–259. [Google Scholar] [CrossRef]
- Marshall, S.A.; Casachahua, J.D.; Rinker, J.A.; Blose, A.K.; Lysle, D.T.; Thiele, T.E. IL-1 receptor signaling in the basolateral amygdala modulates binge-like ethanol consumption in male C57BL/6J mice. Brain Behav. Immun. 2016, 51, 258–267. [Google Scholar] [CrossRef]
- Harris, R.A.; Bajo, M.; Bell, R.L.; Blednov, Y.A.; Varodayan, F.P.; Truitt, J.M.; de Guglielmo, G.; Lasek, A.W.; Logrip, M.L.; Vendruscolo, L.F.; et al. Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J. Neurosci. 2017, 37, 1139–1155. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Black, M.; Chernis, J.; Da Costa, A.; Mayfield, J.; Harris, R.A. Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol. Clin. Exp. Res. 2017, 41, 516–530. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Black, M.; Benavidez, J.M.; Da Costa, A.; Mayfield, J.; Harris, R.A. Sedative and Motor Incoordination Effects of Ethanol in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol. Clin. Exp. Res. 2017, 41, 531–540. [Google Scholar] [CrossRef]
- Corrigan, F.; Wu, Y.; Tuke, J.; Coller, J.K.; Rice, K.C.; Diener, K.R.; Hayball, J.D.; Watkins, L.R.; Somogyi, A.A.; Hutchinson, M.R. Alcohol-induced sedation and synergistic interactions between alcohol and morphine: A key mechanistic role for Toll-like receptors and MyD88-dependent signaling. Brain Behav. Immun. 2015, 45, 245–252. [Google Scholar] [CrossRef]
- Wu, Y.; Lousberg, E.L.; Moldenhauer, L.M.; Hayball, J.D.; Coller, J.K.; Rice, K.C.; Watkins, L.R.; Somogyi, A.A.; Hutchinson, M.R. Inhibiting the TLR4-MyD88 signalling cascade by genetic or pharmacological strategies reduces acute alcohol-induced sedation and motor impairment in mice. Br. J. Pharmacol. 2012, 165, 1319–1329. [Google Scholar] [CrossRef]
- Crews, F.T.; Lawrimore, C.J.; Walter, T.J.; Coleman, L.G., Jr. The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017, 122, 56–73. [Google Scholar] [CrossRef]
- Mayfield, J.; Ferguson, L.; Harris, R.A. Neuroimmune signaling: A key component of alcohol abuse. Curr. Opin. Neurobiol. 2013, 23, 513–520. [Google Scholar] [CrossRef]
- Montesinos, J.; Alfonso-Loeches, S.; Guerri, C. Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol. Clin. Exp. Res. 2016, 40, 2260–2270. [Google Scholar] [CrossRef]
- Freire, D.; Reyes, R.E.; Baghram, A.; Davies, D.L.; Asatryan, L. P2 × 7 Receptor Antagonist A804598 Inhibits Inflammation in Brain and Liver in C57BL/6J Mice Exposed to Chronic Ethanol and High Fat Diet. J. Neuroimmune Pharmacol. 2019, 14, 263–277. [Google Scholar] [CrossRef]
- Petrakis, I.L.; Ralevski, E.; Gueorguieva, R.; Sloan, M.E.; Devine, L.; Yoon, G.; Arias, A.J.; Sofuoglu, M. Targeting neuroinflammation with minocycline in heavy drinkers. Psychopharmacology 2019, 236, 3013–3021. [Google Scholar] [CrossRef]
- Franklin, K.M.; Hauser, S.R.; Lasek, A.W.; McClintick, J.; Ding, Z.M.; McBride, W.J.; Bell, R.L. Reduction of alcohol drinking of alcohol-preferring (P) and high-alcohol drinking (HAD1) rats by targeting phosphodiesterase-4 (PDE4). Psychopharmacology 2015, 232, 2251–2262. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Cohen, P. The TLR and IL-1 signalling network at a glance. J. Cell Sci. 2014, 127, 2383–2390. [Google Scholar] [CrossRef]
- Jain, A.; Kaczanowska, S.; Davila, E. IL-1 Receptor-Associated Kinase Signaling and Its Role in Inflammation, Cancer Progression, and Therapy Resistance. Front. Immunol. 2014, 5, 553. [Google Scholar] [CrossRef]
- Balka, K.R.; De Nardo, D. Understanding early TLR signaling through the Myddosome. J. Leukoc. Biol. 2019, 105, 339–351. [Google Scholar] [CrossRef]
- Motshwene, P.G.; Moncrieffe, M.C.; Grossmann, J.G.; Kao, C.; Ayaluru, M.; Sandercock, A.M.; Robinson, C.V.; Latz, E.; Gay, N.J. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 2009, 284, 25404–25411. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Bajo, M.; Madamba, S.G.; Roberto, M.; Blednov, Y.A.; Sagi, V.N.; Roberts, E.; Rice, K.C.; Harris, R.A.; Siggins, G.R. Innate immune factors modulate ethanol interaction with GABAergic transmission in mouse central amygdala. Brain Behav. Immun. 2014, 40, 191–202. [Google Scholar] [CrossRef]
- Bajo, M.; Varodayan, F.P.; Madamba, S.G.; Robert, A.J.; Casal, L.M.; Oleata, C.S.; Siggins, G.R.; Roberto, M. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front. Pharmacol. 2015, 6, 49. [Google Scholar] [CrossRef]
- Bajo, M.; Herman, M.A.; Varodayan, F.P.; Oleata, C.S.; Madamba, S.G.; Harris, R.A.; Blednov, Y.A.; Roberto, M. Role of the IL-1 receptor antagonist in ethanol-induced regulation of GABAergic transmission in the central amygdala. Brain Behav. Immun. 2015, 45, 189–197. [Google Scholar] [CrossRef][Green Version]
- Roberto, M.; Madamba, S.G.; Stouffer, D.G.; Parsons, L.H.; Siggins, G.R. Increased GABA release in the central amygdala of ethanol-dependent rats. J. Neurosci. 2004, 24, 10159–10166. [Google Scholar] [CrossRef]
- Roberto, M.; Madamba, S.G.; Moore, S.D.; Tallent, M.K.; Siggins, G.R. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc. Natl. Acad. Sci. USA 2003, 100, 2053–2058. [Google Scholar] [CrossRef]
- Bajo, M.; Cruz, M.T.; Siggins, G.R.; Messing, R.; Roberto, M. Protein kinase C epsilon mediation of CRF- and ethanol-induced GABA release in central amygdala. Proc. Natl. Acad. Sci. USA 2008, 105, 8410–8415. [Google Scholar] [CrossRef]
- Nie, Z.; Schweitzer, P.; Roberts, A.J.; Madamba, S.G.; Moore, S.D.; Siggins, G.R. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 2004, 303, 1512–1514. [Google Scholar] [CrossRef]
- Dodt, H.U.; Zieglgansberger, W. Visualizing unstained neurons in living brain slices by infrared DIC-videomicroscopy. Brain Res. 1990, 537, 333–336. [Google Scholar] [CrossRef]
- Patel, R.R.; Khom, S.; Steinman, M.Q.; Varodayan, F.P.; Kiosses, W.B.; Hedges, D.M.; Vlkolinsky, R.; Nadav, T.; Polis, I.; Bajo, M.; et al. IL-1beta expression is increased and regulates GABA transmission following chronic ethanol in mouse central amygdala. Brain Behav. Immun. 2019, 75, 208–219. [Google Scholar] [CrossRef]
- Davis, C.N.; Mann, E.; Behrens, M.M.; Gaidarova, S.; Rebek, M.; Rebek, J., Jr.; Bartfai, T. MyD88-dependent and -independent signaling by IL-1 in neurons probed by bifunctional Toll/IL-1 receptor domain/BB-loop mimetics. Proc. Natl. Acad. Sci. USA 2006, 103, 2953–2958. [Google Scholar] [CrossRef]
- Otis, T.S.; De Koninck, Y.; Mody, I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc. Natl. Acad. Sci. USA 1994, 91, 7698–7702. [Google Scholar] [CrossRef]
- Bartfai, T.; Behrens, M.M.; Gaidarova, S.; Pemberton, J.; Shivanyuk, A.; Rebek, J., Jr. A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc. Natl. Acad. Sci. USA 2003, 100, 7971–7976. [Google Scholar] [CrossRef]
- Hu, Y.; Li, T.J.; Wang, Y.; Guo, L.; Shan, X.; Li, J.; Chen, Q.; Li, Y. IL-1R I/MyD88-TIR mimic AS-1 inhibits the activation of MyD88-dependent signaling pathway induced by IL-1β in vitro. J. Nanjing Med. Univ. 2007, 21, 354–358. [Google Scholar] [CrossRef]
- Cruz, M.T.; Bajo, M.; Maragnoli, M.E.; Tabakoff, B.; Siggins, G.R.; Roberto, M. Type 7 Adenylyl Cyclase is Involved in the Ethanol and CRF Sensitivity of GABAergic Synapses in Mouse Central Amygdala. Front. Neurosci. 2011, 4, 207. [Google Scholar] [CrossRef]
- Herman, M.A.; Contet, C.; Justice, N.J.; Vale, W.; Roberto, M. Novel Subunit-Specific Tonic GABA Currents and Differential Effects of Ethanol in the Central Amygdala of CRF Receptor-1 Reporter Mice. J. Neurosci. 2013, 33, 3284–3298. [Google Scholar] [CrossRef]
- Mody, I.; Pearce, R.A. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004, 27, 569–575. [Google Scholar] [CrossRef]
- Jimenez, V.A.; Herman, M.A.; Cuzon Carlson, V.C.; Walter, N.A.; Grant, K.A.; Roberto, M. Synaptic adaptations in the central amygdala and hypothalamic paraventricular nucleus associated with protracted ethanol abstinence in male rhesus monkeys. Neuropsychopharmacology 2019, 44, 982–993. [Google Scholar] [CrossRef]
- Gilpin, N.W.; Herman, M.A.; Roberto, M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol. Psychiatry 2015, 77, 859–869. [Google Scholar] [CrossRef]
- Blednov, Y.A.; Ponomarev, I.; Geil, C.; Bergeson, S.; Koob, G.F.; Harris, R.A. Neuroimmune regulation of alcohol consumption: Behavioral validation of genes obtained from genomic studies. Addict. Biol. 2012, 17, 108–120. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajo, M.; Patel, R.R.; Hedges, D.M.; Varodayan, F.P.; Vlkolinsky, R.; Davis, T.D.; Burkart, M.D.; Blednov, Y.A.; Roberto, M. Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala. Brain Sci. 2019, 9, 361. https://doi.org/10.3390/brainsci9120361
Bajo M, Patel RR, Hedges DM, Varodayan FP, Vlkolinsky R, Davis TD, Burkart MD, Blednov YA, Roberto M. Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala. Brain Sciences. 2019; 9(12):361. https://doi.org/10.3390/brainsci9120361
Chicago/Turabian StyleBajo, Michal, Reesha R. Patel, David M. Hedges, Florence P. Varodayan, Roman Vlkolinsky, Tony D. Davis, Michael D. Burkart, Yuri A. Blednov, and Marisa Roberto. 2019. "Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala" Brain Sciences 9, no. 12: 361. https://doi.org/10.3390/brainsci9120361
APA StyleBajo, M., Patel, R. R., Hedges, D. M., Varodayan, F. P., Vlkolinsky, R., Davis, T. D., Burkart, M. D., Blednov, Y. A., & Roberto, M. (2019). Role of MyD88 in IL-1β and Ethanol Modulation of GABAergic Transmission in the Central Amygdala. Brain Sciences, 9(12), 361. https://doi.org/10.3390/brainsci9120361




