Estimation of Brain Functional Connectivity in Patients with Mild Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Material
2.3. Procedure
2.4. MR Image Acquisition
2.5. Image Preprocessing
2.6. Regions of Interest
2.7. Statistical Analysis
2.8. Functional Connectivity
3. Results
3.1. Sociodemographic and Neuropsychological Characteristics
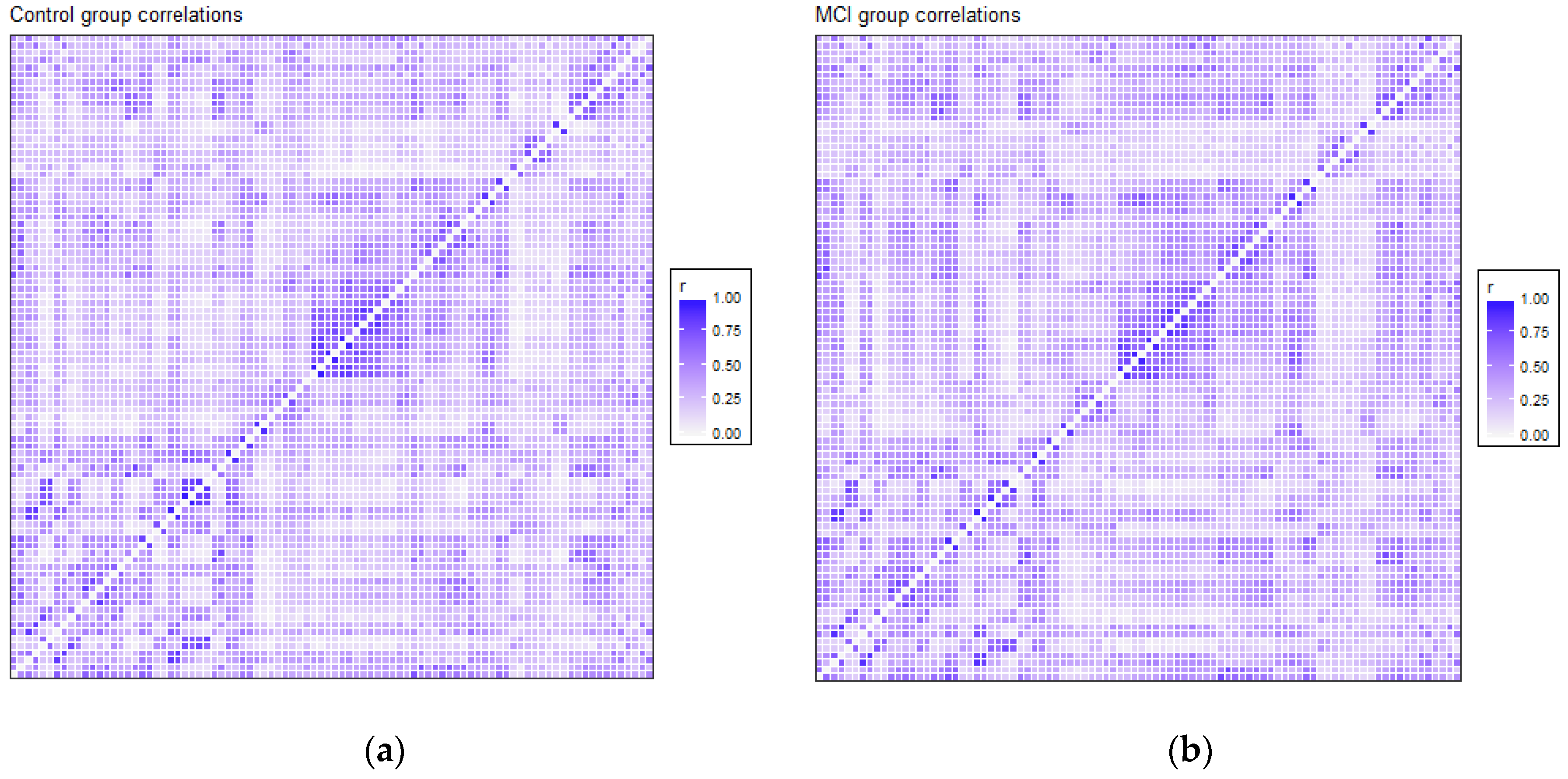
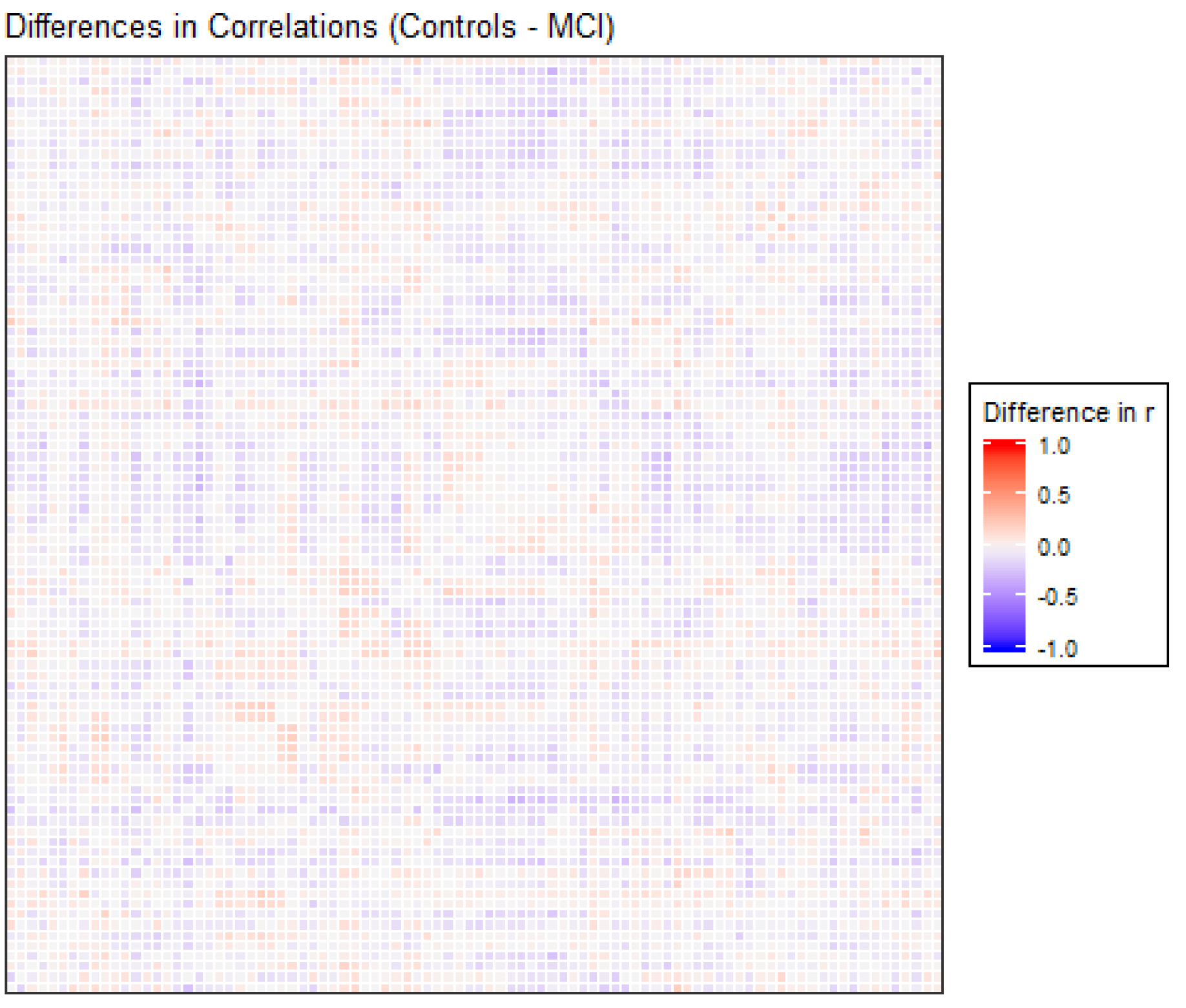
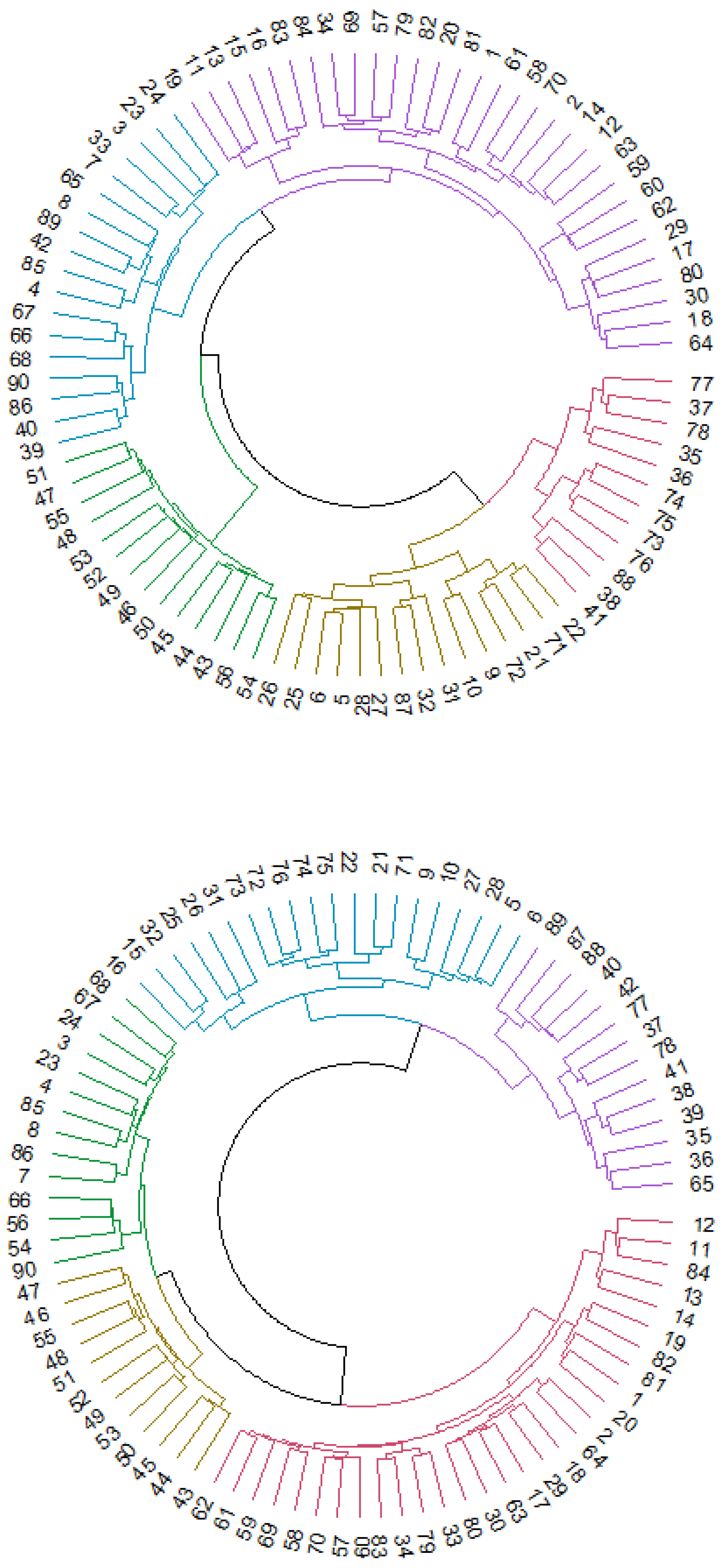
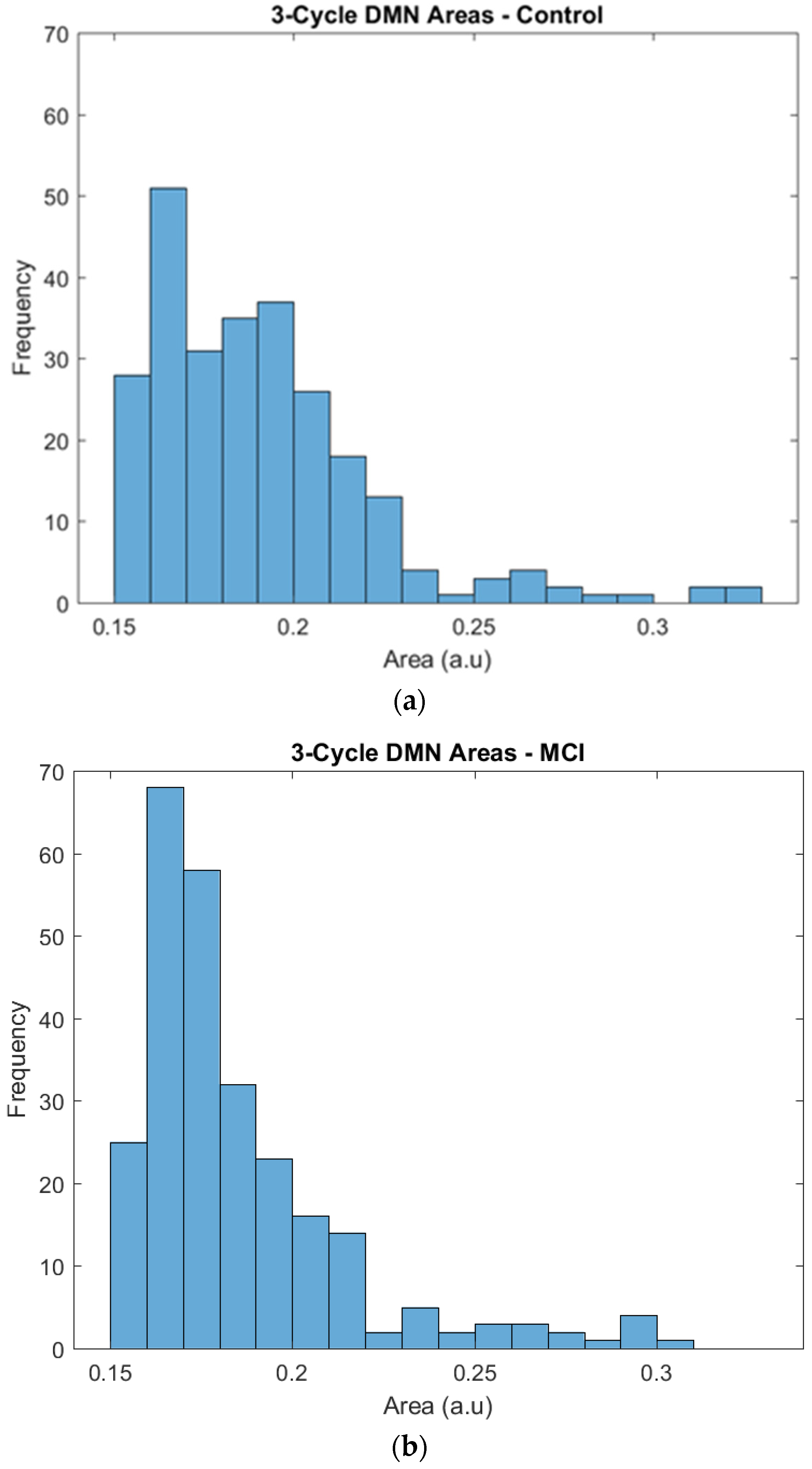
3.2. Functional Connectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ROI Number | Brain Region | ROI Number | Brain Region |
|---|---|---|---|
| 1 | Precentral_L | 46 | Cuneus_R |
| 2 | Precentral_R | 47 | Lingual_L |
| 3 | Frontal_Sup_L | 48 | Lingual_R |
| 4 | Frontal_Sup_R | 49 | Occipital_Sup_L |
| 5 | Frontal_Sup_Orb_L | 50 | Occipital_Sup_R |
| 6 | Frontal_Sup_Orb_R | 51 | Occipital_Mid_L |
| 7 | Frontal_Mid_L | 52 | Occipital_Mid_R |
| 8 | Frontal_Mid_R | 53 | Occipital_Inf_L |
| 9 | Frontal_Mid_Orb_L | 54 | Occipital_Inf_R |
| 10 | Frontal_Mid_Orb_R | 55 | Fusiform_L |
| 11 | Frontal_Inf_Oper_L | 56 | Fusiform_R |
| 12 | Frontal_Inf_Oper_R | 57 | Postcentral_L |
| 13 | Frontal_Inf_Tri_L | 58 | Postcentral_R |
| 14 | Frontal_Inf_Tri_R | 59 | Parietal_Sup_L |
| 15 | Frontal_Inf_Orb_L | 60 | Parietal_Sup_R |
| 16 | Frontal_Inf_Orb_R | 61 | Parietal_Inf_L |
| 17 | Rolandic_Oper_L | 62 | Parietal_Inf_R |
| 18 | Rolandic_Oper_R | 63 | SupraMarginal_L |
| 19 | Supp_Motor_Area_L | 64 | SupraMarginal_R |
| 20 | Supp_Motor_Area_R | 65 | Angular_L |
| 21 | Olfactory_L | 66 | Angular_R |
| 22 | Olfactory_R | 67 | Precuneus_L |
| 23 | Frontal_Sup_Medial_L | 68 | Precuneus_R |
| 24 | Frontal_Sup_Medial_R | 69 | Paracentral_Lobule_L |
| 25 | Frontal_Med_Orb_L | 70 | Paracentral_Lobule_R |
| 26 | Frontal_Med_Orb_R | 71 | Caudate_L |
| 27 | Rectus_L | 72 | Caudate_R |
| 28 | Rectus_R | 73 | Putamen_L |
| 29 | Insula_L | 74 | Putamen_R |
| 30 | Insula_R | 75 | Pallidum_L |
| 31 | Cingulum_Ant_L | 76 | Pallidum_R |
| 32 | Cingulum_Ant_R | 77 | Thalamus_L |
| 33 | Cingulum_Mid_L | 78 | Thalamus_R |
| 34 | Cingulum_Mid_R | 79 | Heschl_L |
| 35 | Cingulum_Post_L | 80 | Heschl_R |
| 36 | Cingulum_Post_R | 81 | Temporal_Sup_L |
| 37 | Hippocampus_L | 82 | Temporal_Sup_R |
| 38 | Hippocampus_R | 83 | Temporal_Pole_Sup_L |
| 39 | ParaHippocampal_L | 84 | Temporal_Pole_Sup_R |
| 40 | ParaHippocampal_R | 85 | Temporal_Mid_L |
| 41 | Amygdala_L | 86 | Temporal_Mid_R |
| 42 | Amygdala_R | 87 | Temporal_Pole_Mid_L |
| 43 | Calcarine_L | 88 | Temporal_Pole_Mid_R |
| 44 | Calcarine_R | 89 | Temporal_Inf_L |
| 45 | Cuneus_L | 90 | Temporal_Inf_R |
| Positive Differences | Negative Differences | ||||
|---|---|---|---|---|---|
| First ROI | Second ROI | Difference | First ROI | Second ROI | Difference |
| 8 | 10 | 0.3051 | 88 | 89 | −0.332 |
| 41 | 84 | 0.2899 | 19 | 49 | −0.3008 |
| 28 | 43 | 0.2894 | 19 | 59 | −0.3006 |
| 37 | 84 | 0.2873 | 53 | 89 | −0.2997 |
| 28 | 47 | 0.2728 | 19 | 50 | −0.2994 |
| 28 | 45 | 0.2723 | 18 | 88 | −0.2979 |
| 10 | 53 | 0.2701 | 13 | 60 | −0.2783 |
| 78 | 83 | 0.2671 | 4 | 53 | −0.2751 |
| 78 | 84 | 0.2568 | 56 | 64 | −0.2666 |
| 31 | 41 | 0.253 | 53 | 85 | −0.2664 |
| 10 | 55 | 0.252 | 8 | 88 | −0.266 |
| 28 | 48 | 0.2514 | 14 | 88 | −0.2598 |
| 10 | 89 | 0.2511 | 52 | 64 | −0.2591 |
| 32 | 41 | 0.2494 | 19 | 52 | −0.258 |
| 25 | 28 | 0.2417 | 18 | 40 | −0.2577 |
| 16 | 70 | 0.2399 | 13 | 18 | −0.2576 |
| 10 | 25 | 0.2386 | 18 | 26 | −0.255 |
| 77 | 83 | 0.2362 | 1 | 18 | −0.2536 |
| 76 | 79 | 0.2346 | 42 | 88 | −0.2525 |
| 40 | 84 | 0.2341 | 14 | 26 | −0.2507 |
| 28 | 41 | 0.2339 | 22 | 42 | −0.2478 |
| 28 | 46 | 0.2327 | 18 | 32 | −0.2471 |
| 1 | 65 | 0.2324 | 5 | 18 | −0.2442 |
| 34 | 61 | 0.2299 | 60 | 88 | −0.2436 |
| 33 | 41 | 0.2297 | 62 | 80 | −0.2429 |
| 28 | 44 | 0.2275 | 46 | 85 | −0.2417 |
| 4 | 10 | 0.2272 | 35 | 46 | −0.2384 |
| 39 | 84 | 0.2269 | 19 | 60 | −0.2376 |
| 33 | 39 | 0.2218 | 8 | 53 | −0.2373 |
| 57 | 65 | 0.2211 | 18 | 59 | −0.2368 |
| 34 | 41 | 0.2199 | 18 | 25 | −0.2336 |
| 40 | 69 | 0.2185 | 13 | 80 | −0.2313 |
| 33 | 90 | 0.2181 | 50 | 89 | −0.2277 |
| 69 | 75 | 0.2172 | 58 | 59 | −0.2266 |
| 1 | 34 | 0.2154 | 53 | 67 | −0.2265 |
| 10 | 24 | 0.2154 | 56 | 62 | −0.2254 |
| 37 | 57 | 0.2141 | 60 | 82 | −0.2238 |
| 57 | 76 | 0.2139 | 59 | 80 | −0.2218 |
| 41 | 75 | 0.2137 | 36 | 46 | −0.2215 |
| 26 | 28 | 0.2132 | 2 | 88 | −0.2207 |
| 33 | 37 | 0.2118 | 15 | 60 | −0.2196 |
| 70 | 75 | 0.2115 | 50 | 85 | −0.2177 |
| 10 | 87 | 0.2115 | 51 | 63 | −0.2175 |
| 39 | 69 | 0.2113 | 67 | 80 | −0.2173 |
| 34 | 90 | 0.2092 | 51 | 82 | −0.2171 |
| 33 | 74 | 0.2089 | 13 | 52 | −0.2157 |
| 69 | 76 | 0.2083 | 35 | 65 | −0.2152 |
| 79 | 90 | 0.2076 | 19 | 46 | −0.215 |
| 15 | 41 | 0.2063 | 13 | 88 | −0.2134 |
| 33 | 40 | 0.2041 | 49 | 64 | −0.213 |
| 11 | 65 | 0.2014 | 67 | 79 | −0.2122 |
| 26 | 82 | −0.2122 | |||
| 18 | 31 | −0.2113 | |||
| 50 | 64 | −0.2113 | |||
| 55 | 88 | −0.2096 | |||
| 51 | 64 | −0.2092 | |||
| 25 | 82 | −0.2089 | |||
| 46 | 77 | −0.206 | |||
| 52 | 63 | −0.2053 | |||
| 18 | 71 | −0.2053 | |||
| 18 | 22 | −0.204 | |||
| 38 | 78 | −0.2036 | |||
| 38 | 47 | −0.2036 | |||
| 18 | 51 | −0.2025 | |||
| 30 | 88 | −0.2011 | |||
| 19 | 53 | −0.2004 | |||
| 13 | 86 | −0.2002 | |||
| 64 | 86 | −0.2001 | |||
| 19 | 68 | −0.2 | |||
References
- Flicker, C.; Ferris, S.H.; Reisberg, B. Mild cognitive impairment in the elderly: Predictors of dementia. Neurology 1991, 41, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment. Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Burhan, A.M.; Anazodo, U.C.; Chung, J.K.; Arena, A.; Graff-Guerrero, A.; Mitchell, D.G.V. The effect of task-irrelevant fearful-face distractor on working memory processing in mild cognitive impairment versus healthy controls: An exploratory fMRI study in female participants. Behav. Neurol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Machulda, M.M.; Ward, H.A.; Borowski, B.; Gunter, J.L.; Cha, R.H.; O’Brien, P.C.; Petersen, R.C.; Boeve, B.F.; Knopman, D.; Tang–Wai, D.F.; et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology 2003, 61, 500–506. [Google Scholar] [CrossRef]
- O’Sullivan, M.; Jones, D.K.; Summers, P.E.; Morris, R.G.; Williams, S.C.R.; Markus, H.S. AGE Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 2001, 57. [Google Scholar] [CrossRef]
- Celone, K.A.; Calhoun, V.D.; Dickerson, B.C.; Atri, A.; Chua, E.F.; Miller, S.L.; DePeau, K.; Rentz, D.M.; Selkoe, D.J.; Blacker, D.; et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. J. Neurosci. 2006, 26, 10222–10231. [Google Scholar] [CrossRef]
- Burhan, A.M.; Bartha, R.; Bocti, C.; Borrie, M.; Laforce, R.; Rosa-Neto, P.; Soucy, J.P. Role of emerging neuroimaging modalities in patients with cognitive impairment: A review from the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia 2012. Alzheimers Res. Ther. 2013, 5 (Suppl. 1), 1–14. [Google Scholar] [CrossRef]
- Farràs-Permanyer, L.; Guàrdia-Olmos, J.; Peró-Cebollero, M. Mild cognitive impairment and fMRI studies of brain functional connectivity: The state of the art. Front. Psychol. 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Faraco, C.C.; Puente, A.N.; Brown, C.; Terry, D.P.; Stephen Miller, L. Lateral temporal hyper-activation as a novel biomarker of mild cognitive impairment. Neuropsychologia 2013, 51, 2281–2293. [Google Scholar] [CrossRef]
- Miller, S.L.; Fenstermacher, E.; Bates, J.; Blacker, D.; Sperling, R.A.; Dickerson, B.C. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry 2008, 79, 630–635. [Google Scholar] [CrossRef]
- Hojjati, S.H.; Ebrahimzadeh, A.; Khazaee, A.; Babajani-Feremi, A. Predicting conversion from MCI to AD using resting-state fMRI, graph theoretical approach and SVM. J. Neurosci. Methods 2017, 282, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Kochan, N.; Breakspear, M.; Slavin, M.J.; Valenzuela, M.; McCraw, S.; Brodaty, H.; Sachdev, P.S. Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge. Dement. Geriatr. Cogn. Disord. 2010, 30, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Sala-Llonch, R.; Bosch, B.; Arenaza-Urquijo, E.M.; Rami, L.; Bargalló, N.; Junqué, C.; Molinuevo, J.L.; Bartrés-Faz, D. Greater default-mode network abnormalities compared to high order visual processing systems in amnestic mild cognitive impairment: An integrated multi-modal MRI study. J. Alzheimers Dis. 2010, 22, 523–539. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, M.; Chen, X.; Pohmann, R.; Polimeni, J.R.; Scheffler, K.; Rosen, B.R.; Kleinfeld, D.; Yu, X. Ultra-slow single vessel BOLD and CBV-based fMRI spatiotemporal dynamics and their correlation with neuronal intracellular calcium signals. Neuron 2019, 97, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; He, Y.; Wang, M.; Merkle, H.; Dodd, S.J.; Silva, A.C.; Koretsky, A.P. Sensory and optogenetically driven single-vessel fMRI. Nat. Methods 2016, 13, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Zhang, Z.; Yu, H.; Shi, Y.; Yuan, Y.; Zhu, W.; Zhang, X.; Qian, Y. Default-mode network activity distinguishes amnestic type mild cognitive impairment from healthy aging: A combined structural and resting-state functional MRI study. Neurosci. Lett. 2008, 438, 111–115. [Google Scholar] [CrossRef]
- Gold, B.T.; Jiang, Y.; Jicha, G.A.; Smith, C.D. Functional response in ventral temporal cortex differentiates mild cognitive impairment from normal aging. Hum. Brain Mapp. 2010, 31, 1249–1259. [Google Scholar] [CrossRef]
- Krishnan, S.; Slavin, M.J.; Tran, T.T. Mild cognitive impairment: Evaluation with 4-T functional MR. Radiology 2006, 240, 177–186. [Google Scholar]
- Sperling, R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2007, 1097, 146–155. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Salat, D.H.; Greve, D.N.; Chua, E.F.; Rand-Giovannetti, E.; Rentz, D.M.; Bertram, L.; Mullin, K.; Tanzi, R.E.; Blacker, D.; et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005, 65, 404–411. [Google Scholar] [CrossRef]
- Mueller, S.; Keeser, D.; Reiser, M.F.; Teipel, S. Functional and structural MR imaging in neuropsychiatric disorders, Part 1: Imaging techniques and their application in mild cognitive impairment and Alzheimer disease. Am. J. Neuroradiol. 2012, 33, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- De Flores, R.; Mutlu, J.; Bejanin, A.; Gonneaud, J.; Landeau, B.; Tomadesso, C.; Mézenge, F.; de La Sayette, V.; Eustache, F.; Chételat, G. Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum. Brain Mapp. 2017, 38, 4922–4932. [Google Scholar] [CrossRef] [PubMed]
- Baglio, F.; Castelli, I.; Alberoni, M.; Blasi, V.; Griffanti, L.; Falini, A.; Nemni, R.; Marchetti, A. Theory of mind in amnestic mild cognitive impairment: An fMRI study. J. Alzheimers Dis. 2012, 29, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Staffen, W.; Ladurner, G.; Höller, Y.; Bergmann, J.; Aichhorn, M.; Golaszewski, S.; Kronbichler, M. Brain activation disturbance for target detection in patients with mild cognitive impairment: An fMRI study. Neurobiol. Aging 2012, 33, 1–16. [Google Scholar] [CrossRef]
- Trivedi, M.; Murphy, C.M.; Goetz, C.; Shah, R.C.; Gabrieli, J.D.E.; Whitfield-Gabrieli, S.; Turner, D.A.; Stebbins, G.T. fMRI activation changes during successful episodic memory encoding and recognition in amnestic mild cognitive impairment relative to cognitively healthy older adults. Dement. Geriatr. Cogn. Disord. 2008, 26, 123–137. [Google Scholar] [CrossRef]
- Zhou, Y.; Dougherty, J.H.; Hubner, K.F.; Bai, B.; Cannon, R.L.; Hutson, R.K. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement J. Alzheimers Assoc. 2008, 4, 265–270. [Google Scholar] [CrossRef]
- Binnewijzend, M.A.; Schoonheim, M.M.; Sanz-Arigita, E.; Wink, A.M.; van der Flier, W.M.; Tolboom, N.; Adriaanse, S.M.; Damoiseaux, J.S.; Scheltens, P.; van Berckel, B.N.; et al. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2012, 33, 2018–2028. [Google Scholar] [CrossRef]
- Cai, S.; Chong, T.; Peng, Y.; Shen, W.; Li, J.; von Deneen, K.M.; Huang, L. Altered functional brain networks in amnestic mild cognitive impairment: A resting-state fMRI study. Brain Imaging Behav. 2017, 11, 619–631. [Google Scholar] [CrossRef]
- Yao, H.; Liu, Y.; Zhou, B.; Zhang, Z.; An, N.; Wang, P.; Zhang, X.; Jiang, T. Decreased functional connectivity of the amygdala in Alzheimer’s disease revealed by resting-state fMRI. Eur. J. Radiol. 2013, 82, 1531–1538. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Jiang, T.; Zhou, B.; An, N.; Dai, H.; Wang, P.; Niu, Y.; Wang, L.; Zhang, X. Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by Regional Homogeneity. NeuroImage 2012, 59, 1429–1440. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, F.; Duong, T.Q. White matter lesion load is associated with resting state functional MRI activity and amyloid pet but not FDG in mild cognitive impairment and early Alzheimer’s disease patients. J. Magn. Reson. Imaging 2013, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chand, G.B.; Wu, J.; Hajjar, I.; Qiu, D. Interactions of insula subdivisions-based networks with default-mode and central-executive networks in mild cognitive impairment. Front. Aging Neurosci. 2017, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chand, G.B.; Wu, J.; Hajjar, I.; Qiu, D. Interactions of the salience network and its subsystems with the default-mode and the central-executive networks in normal aging and mild cognitive impairment. Brain Connect. 2017, 7, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Geerligs, L.; Renken, R.J.; Saliasi, E.; Maurits, M.; Lorist, M.M. A brain-wide study of age-related changes in functional connectivity. Cereb. Cortex 2015, 25, 1987–1999. [Google Scholar] [CrossRef]
- He, X.; Qin, W.; Liu, Y.; Zhang, X.; Duan, Y.; Song, J.; Li, K.; Jiang, T.; Yu, C. Age-related decrease in functional connectivity of the right fronto-insular cortex with the central executive and default-mode networks in adults from young to middle age. Neurosci. Lett. 2013, 544, 74–79. [Google Scholar] [CrossRef]
- Onoda, K.; Ishihara, M.; Yamaguchi, S. Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 2012, 24, 2186–2198. [Google Scholar] [CrossRef]
- Farràs-Permanyer, L.; Mancho-Fora, N.; Montalà-Flaquer, M.; Bartrés-Faz, D.; Vaqué-Alcázar, L.; Peró-Cebollero, M.; Guàrdia-Olmos, J. Age-related changes in resting-state functional connectivity in older adults. Neural Regen. Res. 2019, 14, 1544–1555. [Google Scholar] [CrossRef]
- Siman-Tov, T.; Bosak, N.; Sprecher, E.; Paz, R.; Eran, A.; Aharon-Peretz, J.; Kahn, I. Early Age-related functional connectivity decline in high-order cognitive networks. Front. Aging Neurosci. 2017, 8, 330. [Google Scholar] [CrossRef]
- Damoiseaux, J.S. Effects of aging on functional and structural brain connectivity. NeuroImage 2017, 160, 32–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Chen, X.; Liu, M.; Zhu, X.; Lee, S.-W.; Shen, D. Strength and similarity guided group-level brain functional network construction for MCI diagnosis. Pattern Recognit. 2019, 88, 421–430. [Google Scholar] [CrossRef]
- Göthlin, M.; Eckerström, M.; Rolstad, S.; Wallin, A.; Nordlund, A. Prognostic accuracy of mild cognitive impairment subtypes at different cut-off levels. Dement. Geriatr. Cogn. Disord. 2017, 43, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokolowski, R.; Stemplowski, W.; Lakomski, M.; Zukow, W.; Kedziora-Kornatowska, K. Diagnosis of mild cognitive impairment. J. Health Sci. 2014, 4, 133–144. [Google Scholar]
- Guàrdia-Olmos, J.; Gudayol-Ferré, E.; Gallardo-Moreno, G.B.; Martínez-Ricart, M.; Peró-Cebollero, M.; González-Garrido, A.A. Complex systems representing effective connectivity in patients with Type One diabetes mellitus. PLoS ONE 2018, 13, e0208247. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Teipel, S.J.; Wohlert, A.; Metzger, C.; Grimmer, T.; Sorg, C.; Ewers, M.; Meisenzahl, E.; Klöppel, S.; Borchardt, V.; Grothe, M.J.; et al. Multicenter stability of resting state fMRI in the detection of Alzheimer’s disease and amnestic MCI. Neuroimage Clin. 2017, 14, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Solís, F.; Gómez-Pérez, M.E.; Matute, E.; Roselli, M.; Ardila, A.; Pineda, D. Neuropsi attention and memory: A neuropsychological test battery in spanish with norms by age and educational level. Appl. Neuropsychol. 2007, 14, 156–170. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef]
- Blesa, R.; Pujol, M.; Aguilar, M.; Santacruz, P.; Bertran-Serra, I.; Hernández, G.; Sol, J.M.; Pena-Casanova, J. and NORMACODEM Group. Clinical validity of the “mini-mental state” for Spanish speaking communities. Neuropsychologia 2001, 39, 1150–1157. [Google Scholar] [CrossRef]
- Villaseñor-Cabrera, T.; Guàrdia-Olmos, J.; Jiménez-Maldonado, M.; Rizo-Curiel, G.; Peró-Cebollero, M. Sensitivity and specificity of the mini-mental state examination in the Mexican population. Qual. Quant. 2010, 44, 1105–1112. [Google Scholar] [CrossRef]
- Smith, G.V.; Della Salla, S.; Logie, R.H.; Maylor, E.A.M. Prospective and retrospective memory in normal ageing and dementia: A questionnaire study. Memory 2000, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- González-Ramírez, M.T.; Mendoza-González, M.E. Spanish version of the Prospective and Retrospective Memory Questionnaire (PRMQ-S). Span. J. Psychol. 2011, 14, 385–391. [Google Scholar] [CrossRef]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current vision and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 1997, 9 (Suppl. 1), 173–178. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of functional activities in older adults in the community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef]
- Brink, T.L.; Yesavage, J.A.; Lum, O.; Heersema, P.; Adey, M.B.; Rose, T.L. Screening tests for geriatric depression. Clin. Gerontol. 1982, 1, 37–44. [Google Scholar] [CrossRef]
- Fernández-San Martín, M.I.; Andrade, C.; Molina, J.; Muñoz, P.E.; Carretero, B.; Rodríguez, M.; Silva, A. Validation of the Spanish version of the geriatric depression scale (GDS) in primary care. Int. J. Geriatr. Psychiatry 2002, 17, 279–287. [Google Scholar] [CrossRef]
- Goodglass, H.; Kaplan, E.; Weintraub, S. Boston Naming Test; Lea & Febiger: Philadelphia, PA, USA, 1983. [Google Scholar]
- Fernández, A.L.; Fulbright, R.L. Construct and concurrent validity of the spanish adaptation of the Boston naming test. Appl. Neuropsychol. Adult 2015, 22, 355–362. [Google Scholar] [CrossRef]
- Diez, I.; Bonifazi, P.; Escudero, I.; Mateos, B.; Muñoz, M.A.; Stramaglia, S.; Cortes, J.M. A novel brain partition highlights the modular skeleton shared by structure and function. Sci. Rep. 2015, 5, 10532. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Nonlinear spatial normalization using basis functions. Hum. Brain Mapping 1999, 7, 254–266. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Huang, C.C.; Hsieh, W.J.; Lee, P.L.; Peng, L.N.; Liu, L.K.; Lee, W.J.; Huang, J.K.; Chen, L.K.; Lin, C.P. Age-related changes in resting-state networks of a large sample size of healthy elderly. CNS Neurosci. Ther. 2015, 21, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Shakil, S.; Magnuson, M.E.; Keilholz, S.D.; Lee, C.H. Cluster-based analysis for characterizing dynamic functional connectivity. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 982–985. [Google Scholar] [CrossRef]
- Dunn, J.C. Well-separated clusters and optimal fuzzy partitions. J. Cybern. 1974, 4, 95–104. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Zhao, Z.L.; Qi, Z.; Hu, Y.; Wang, Y.S.; Sheng, C.; Sun, Y.; Wang, X.; Jiang, L.L.; Yan, C.G.; et al. Local-to-remote cortical connectivity in amnestic mild cognitive impairment. Neurobiol. Aging 2017, 56, 138–149. [Google Scholar] [CrossRef]
| Groups/Variables | PAQ | BNT | GDS | MMSE | NEUROPSI | PRMQ |
|---|---|---|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | |
| Control | 0 (0) | 58 (2) | 1 (4) | 27.5 (3) | 111 (10) | 26.5 (6) |
| MCI | 1 (2) | 57 (8) | 5.5 (4) | 27.5 (3) | 95.5 (10) | 39.5 (16) |
| Mann–Whitney-Wilcoxon U test (p-value) | 23.5 (0.018) * | 65.00 (0.251) | 10.00 (0.0022) ** | 58.00 (0.537) | 85.5 (0.0072) ** | 13.00 (0.0048) ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farràs-Permanyer, L.; Mancho-Fora, N.; Montalà-Flaquer, M.; Gudayol-Ferré, E.; Gallardo-Moreno, G.B.; Zarabozo-Hurtado, D.; Villuendas-González, E.; Peró-Cebollero, M.; Guàrdia-Olmos, J. Estimation of Brain Functional Connectivity in Patients with Mild Cognitive Impairment. Brain Sci. 2019, 9, 350. https://doi.org/10.3390/brainsci9120350
Farràs-Permanyer L, Mancho-Fora N, Montalà-Flaquer M, Gudayol-Ferré E, Gallardo-Moreno GB, Zarabozo-Hurtado D, Villuendas-González E, Peró-Cebollero M, Guàrdia-Olmos J. Estimation of Brain Functional Connectivity in Patients with Mild Cognitive Impairment. Brain Sciences. 2019; 9(12):350. https://doi.org/10.3390/brainsci9120350
Chicago/Turabian StyleFarràs-Permanyer, Laia, Núria Mancho-Fora, Marc Montalà-Flaquer, Esteve Gudayol-Ferré, Geisa Bearitz Gallardo-Moreno, Daniel Zarabozo-Hurtado, Erwin Villuendas-González, Maribel Peró-Cebollero, and Joan Guàrdia-Olmos. 2019. "Estimation of Brain Functional Connectivity in Patients with Mild Cognitive Impairment" Brain Sciences 9, no. 12: 350. https://doi.org/10.3390/brainsci9120350
APA StyleFarràs-Permanyer, L., Mancho-Fora, N., Montalà-Flaquer, M., Gudayol-Ferré, E., Gallardo-Moreno, G. B., Zarabozo-Hurtado, D., Villuendas-González, E., Peró-Cebollero, M., & Guàrdia-Olmos, J. (2019). Estimation of Brain Functional Connectivity in Patients with Mild Cognitive Impairment. Brain Sciences, 9(12), 350. https://doi.org/10.3390/brainsci9120350





