How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study
Abstract
1. Introduction
2. Method
2.1. Participants
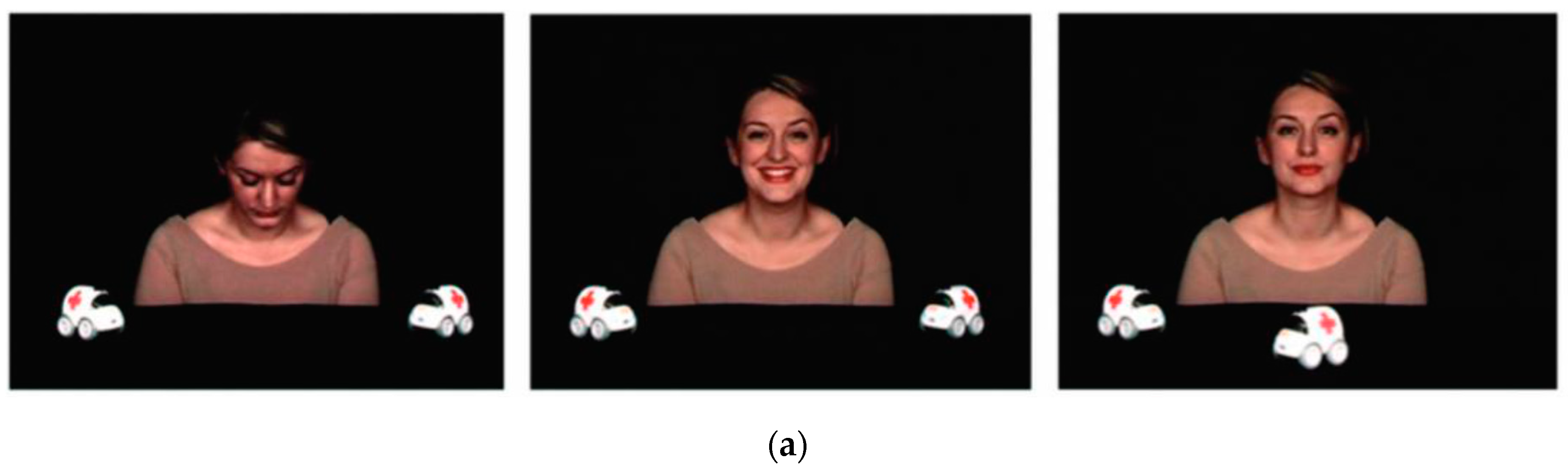
2.2. Procedure and Stimuli
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
3.1. ASD and TD Comparison at T1
3.2. Longitudinal Changes in ASD and Comparison with TD
3.3. ADOS Predictors of Eye Tracking Performance at T2
3.4. Correlations with Developmental Quotient
3.5. Longitudinal Modifications in Clinical Measures
4. Discussion
Availability of Data and Material
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narzisi, A.; Posada, M.; Barbieri, F.; Chericoni, N.; Ciuffolini, D.; Pinzino, M.; Romano, R.; Scattoni, M.L.; Tancredi, R.; Calderoni, S.; et al. Prevalence of autism spectrum disorder in a large Italian catchment area: A school-based population study within the ASDEU project. Epidemiol. Psychiatr. Sci. 2018. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Falck-Ytter, T.; Bölte, S.; Gredebäck, G. Eye tracking in early autism research. J. Neurodev. Disord. 2013, 5, 28. [Google Scholar] [CrossRef]
- Frazier, T.W.; Strauss, M.; Klingemier, E.W.; Zetzer, E.E.; Hardan, A.Y.; Eng, C.; Youngstrom, E.A. A meta-analysis of gaze differences to social and nonsocial information between individuals with and without autism. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Guillon, Q.; Hadjikhani, N.; Baduel, S.; Rogé, B. Visual social attention in autism spectrum disorder: Insights from eye tracking studies. Neurosci. Biobehav. Rev. 2014, 42, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Puce, A.; Bertenthal, B.I. New Frontiers of Investigation in Social Attention. In The Many Faces of Social Attention; Springer International Publishing: New York, NY, USA, 2015; pp. 1–19. [Google Scholar]
- Chawarska, K.; Macari, S.; Shic, F. Context modulates attention to social scenes in toddlers with autism. J. Child Psychol. Psychiatry 2012, 53, 903–913. [Google Scholar] [CrossRef]
- Chawarska, K.; Macari, S.; Shic, F. Decreased spontaneous attention to social scenes in 6-month-old infants later diagnosed with autism spectrum disorders. Biol. Psychiatry 2013, 74, 195–203. [Google Scholar] [CrossRef]
- Crawford, H.; Moss, J.; Oliver, C.; Elliott, N.; Anderson, G.M.; McCleery, J.P. Visual preference for social stimuli in individuals with autism or neurodevelopmental disorders: An eye-tracking study. Mol. Autism 2016, 7, 24. [Google Scholar] [CrossRef]
- Beuker, K.T.; Rommelse, N.N.J.; Donders, R.; Buitelaar, J.K. Development of early communication skills in the first two years of life. Infant Behav. Dev. 2013, 36, 71–83. [Google Scholar] [CrossRef]
- Mundy, P.; Gomes, A. Individuals differences in joint attention skill development in the second year. Infant Behav. Dev. 1998, 21, 469–482. [Google Scholar] [CrossRef]
- Charman, T. Why is joint attention a pivotal skill in autism? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 315–324. [Google Scholar] [CrossRef]
- Landa, R.J.; Holman, K.C.; Garrett-Mayer, E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch. Gen. Psychiatry 2007, 64, 853–864. [Google Scholar] [CrossRef]
- Mundy, P.; Sigman, M.; Kasari, C. Joint attention, developmental level and symptom presentation in autism. Dev. Psychopathol. 1994, 6, 389–401. [Google Scholar] [CrossRef]
- Rozga, A.; Hutman, T.; Young, G.S.; Rogers, S.J.; Ozonoff, S.; Dapretto, M.; Sigman, M. Behavioral profiles of affected and unaffected siblings of children with autism: Contribution of measures of mother-infant interaction and nonverbal communication. J. Autism Dev. Disord. 2011, 41, 287–301. [Google Scholar] [CrossRef]
- Akechi, H.; Senju, A.; Kikuchi, Y.; Tojo, Y.; Osanai, H.; Hasegawa, T. Do children with ASD use referential gaze to learn the name of an object? An eye tracking study. Res. Autism Spectr. Disord. 2011, 5, 1230–1242. [Google Scholar] [CrossRef]
- Falck-Ytter, T.; Carlström, C.; Johansson, M. Eye contact modulates cognitive processing differently in children with autism. Child Dev. 2015, 86, 37–47. [Google Scholar] [CrossRef]
- Elsabbagh, M.; Fernandes, J.; Webb, S.J.; Dawson, G.; Charman, T.; Johnson, M.H.; British Autism Study of Infant Siblings Team. Disengagement of Visual Attention in Infancy is Associated with Emerging Autism in Toddlerhood. Biol. Psychiatry 2013, 74, 189–194. [Google Scholar] [CrossRef]
- Ibanez, L.V.; Grantz, C.J.; Messinger, D.S. The development of referential communication and autism symptomatology in high-risk infants. Infancy 2013, 18, 687–707. [Google Scholar] [CrossRef]
- Billeci, L.; Narzisi, A.; Campatelli, G.; Crifaci, G.; Calderoni, S.; Gagliano, A.; Calzone, C.; Colombi, C.; Pioggia, G.; Muratori, F.; et al. Disentangling the initiation from the response in joint attention: An eye-tracking study in toddlers with autism spectrum disorders. Transl. Psychiatry 2016, 6, e808. [Google Scholar] [CrossRef]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule, 2nd ed.; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Luiz, D.; Barnard, A.; Knoesen, N. Griffiths Mental Developmental Scales-Extended Revised: Two to Eight Years. Analysis Manual; Horgefe: Oxford, MS, UK, 2006. [Google Scholar]
- Balboni, G.; Belacchi, C.; Bonichini, S.; Coscarelli, A. Vineland-II, Survey Interview Form. Standardizzazione Italiana [Vineland-II, Survey Interview Form. Italian Standardization]; Giunti OS: Firenze, Italy, 2016. [Google Scholar]
- Achenbach, T.; Rescorla, L. Manual for ASEBA Preschool Forms and Profiles; Research Center for Children, Youth and Families, University of Vermont: Burlington, VT, USA, 2000. [Google Scholar]
- Gotham, K.; Risi, S.; Dawson, G.; Tager-Flusberg, H.; Joseph, R.; Carter, A.; Hepburn, S.; McMahon, W.; Rodier, P.; Hyman, S.L.; et al. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 642–651. [Google Scholar] [CrossRef]
- Andersson, R.; Nyström, M.; Holmqvist, K. Sampling frequency and eye-tracking measures: How speed affects durations, latencies, and more. J. Eye Mov. Res. 2010, 3. [Google Scholar] [CrossRef]
- Gotham, K.; Risi, S.; Pickles, A.; Lord, C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. J. Autism Dev. Disord. 2007, 37, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Mundy, P.; Jarrold, W. Infant joint attention, neural networks and social cognition. Neural Netw. 2010, 23, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Redcay, E.; Kleiner, M.; Saxe, R. Look at this: The neural correlates of initiating and responding to bids for joint attention. Front. Hum. Neurosci. 2012, 6, 169. [Google Scholar] [CrossRef] [PubMed]
- Schilbach, L.; Wilms, M.; Eickhoff, S.B.; Romanzetti, S.; Tepest, R.; Bente, G.; Shah, N.J.; Fink, G.R.; Vogeley, K. Minds made for sharing: Initiating joint attention recruits reward-related neurocircuitry. J. Cogn. Neurosci. 2010, 22, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G. Early behavioral intervention, brain plasticity, and the prevention of autism. Dev. Psychopathol. 2008, 20, 775–803. [Google Scholar] [CrossRef]
- Kasari, C.; Freeman, S.; Paparella, T. Joint attention and symbolic play in young children with autism: A randomized controlled intervention study. J. Child Psychol. Psychiatry 2006, 47, 611–620. [Google Scholar] [CrossRef]
- Franchini, M.; Armstrong, V.L.; Schaer, M.; Smith, I.M. Initiation of joint attention and related visual attention processes in infants with autism spectrum disorder: Literature review. Child Neuropsychol. 2019, 25, 287–317. [Google Scholar] [CrossRef]
- Paparella, T.; Freeman, S.F.N. Methods to improve joint attention in young children with autism: A review. Pediatric Health Med. Ther. 2015, 6, 65–78. [Google Scholar] [CrossRef]
- Gulsrud, A.C.; Hellemann, G.S.; Freeman, S.F.; Kasari, C. Two to ten years: Developmental trajectories of joint attention in children with ASD who received targeted social communication interventions. Autism Res. 2014, 7, 207–215. [Google Scholar] [CrossRef]
- Dawson, G.; Jones, E.J.; Merkle, K.; Venema, K.; Lowy, R.; Faja, S.; Kamara, D.; Murias, M.; Greenson, J.; Winter, J.; et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 1150–1159. [Google Scholar] [CrossRef]
- Billeci, L.; Narzisi, A.; Tonacci, A.; Sbriscia-Fioretti, B.; Serasini, L.; Fulceri, F.; Apicella, F.; Sicca, F.; Calderoni, S.; Muratori, F. An integrated EEG and eye-tracking approach for the study of responding and initiating joint attention in Autism Spectrum Disorders. Sci. Rep. 2017, 7, 13560. [Google Scholar] [CrossRef] [PubMed]
- Happé, F.; Frith, U. The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 2006, 36, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L.; Dawson, M.; Soulieres, I.; Hubert, B.; Burack, J. Enhanced perceptual functioning in autism: An update, and eight principles of autistic perception. J. Autism Dev. Disord. 2006, 36, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Elison, J.T.; Paterson, S.J.; Wolff, J.J.; Reznick, J.S.; Sasson, N.J.; Gu, H.; Botteron, K.N.; Dager, S.R.; Estes, A.M.; Evans, A.C.; et al. White matter microstructure and atypical visual orienting in 7-month-olds at risk for autism. Am. J. Psychiatry 2013, 170, 899–908. [Google Scholar] [CrossRef]
- Zwaigenbaum, L.; Bryson, S.; Rogers, T.; Roberts, W.; Brian, J.; Szatmari, P. Behavioral manifestations of autism in the first year of life. Int. J. Dev. Neurosci. 2005, 23, 143–152. [Google Scholar] [CrossRef]
- Sacrey, L.A.; Armstrong, V.L.; Bryson, S.E.; Zwaigenbaum, L. Impairments to visual disengagement in autism spectrum disorder: A review of experimental studies from infancy to adulthood. Neurosci. Biobehav. Rev. 2014, 47, 559–577. [Google Scholar] [CrossRef]
- Willyard, C. New efforts to design better tools to track autism therapy response. Nat. Med. 2016, 22, 570–571. [Google Scholar] [CrossRef]
- Thorup, E.; Nyström, P.; Gredebäck, G.; Bölte, S.; Falck-Ytter, T.; EASE Team. Reduced alternating gaze during social interaction in infancy is associated with elevated symptoms of autism in toddlerhood. J. Abnorm. Child Psychol. 2018, 46, 1547–1561. [Google Scholar] [CrossRef]
- Campbell, D.J.; Shic, F.; Macari, S.; Chawarska, K. Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: A subtyping analysis. J. Autism Dev. Disord. 2014, 44, 431–442. [Google Scholar] [CrossRef]


| ASD T1 n = 12 | ASD T2 n = 12 | TD n = 15 | ASD T1 vs. ASD T2 p-Value | ASD T1 vs. TD T1 p-Value | ASD T2 vs. TD T1 p-Value | |
|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | ||||
| Age (months) | 25.1 (4.6) | 31.7 (4.7) | 26.5 (4.1) | − | t(24) = 0.86, p = 0.40 | − |
| Gender: M, F | 10, 2 | 10, 2 | 13, 2 | − | χ2 = 0.06, p = 0.81 | − |
| ADOS-2, total | 14.9 (4.5) | 11.0 (3.7) | − | t(22) = −2.31, p = 0.03 | − | − |
| GMDS, performance | 74.9 (25.0) | 83.5 (13.6) | 102.5 (11.7) | t(11) = −1.73, p = 0.11 | t(24) = 3.79, p = 0.001 | t(24) = 3.88, p = 0.001 |
| Vineland-II, total | 75.8 (4.4) | 79.6 (15.9) | − | t(4) = −0.86, p = 0.43 | − | − |
| ASD T1 n = 12 Mean (SD) | ASD T2 n = 12 Mean (SD) | TD n = 14 Mean (SD) | ASD T1 vs. ASD T2 p-Value | ASD T1 vs. TD T1 p-Value | ASD T2 vs. TD T1 p-Value | |
|---|---|---|---|---|---|---|
| Initiating JA-1 | ||||||
| FD: Face | 24.85 (20.11) | 19.34 (17.06) | 20.14 (14.61) | F = 0.86, p = 0.36, η2 = 0.03 | F = 0.53, p = 0.47, η2 = 0.02 | F = 0.07, p = 0.79, η2 = 0.03 |
| FD: Target object | 22.15 (13.69) | 34.54 (23.16) | 38.62 (20.62) | F = 2.17, p = 0.17, η2 = 0.18 | F = 2.22, p = 0.15, η2 = 0.08 | F = 2.15, p = 0.16, η2 = 0.08 |
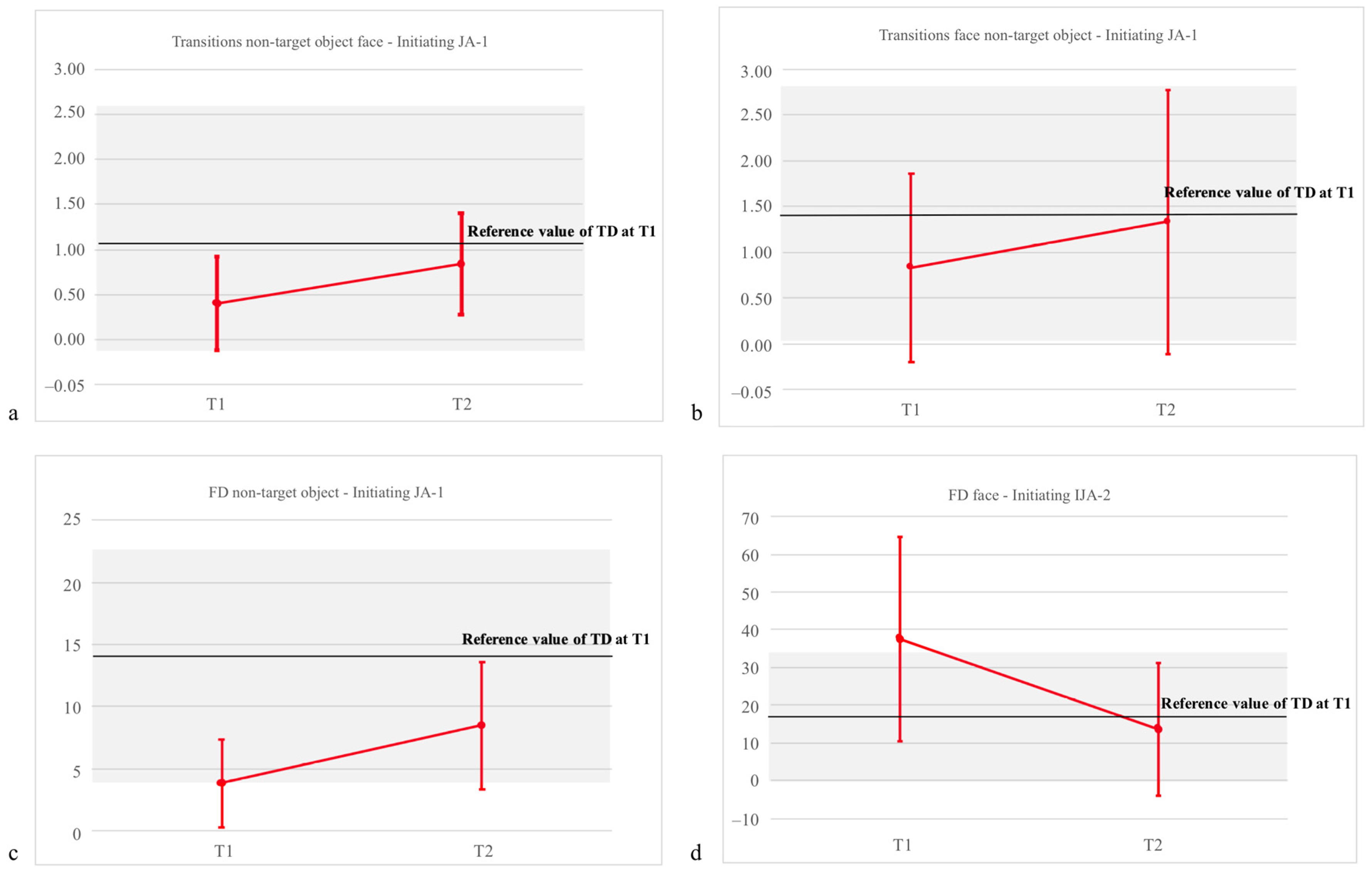
| FD: NT object | 3.84 (3.51) | 8.48 (5.11) | 13.56 (9.92) | F = 4.37, p = 0.038 *, η2 = 0.537 | F = 6.69, p = 0.016 *, η2 = 0.218 | F = 1.29, p = 0.27, η2 =0.05 |
| T to face from target object | 5.15 (4.11) | 4.58 (2.87) | 1.42 (1.13) | F = 0.89, p = 0.77, η2 = 0.01 | F = 5.781, p = 0.026 *, η2 = 0.216 | F = 2.48, p = 0.12, η2 = 0.09 |
| T to face from NT object | 0.40 (0.52) | 0.84 (0.56) | 1.38 (1.19) | F = 6.29, p = 0.024 *, η2 = 0.711 | F = 5.55, p = 0.029 *, η2 = 0.217 | F = 2.22, p = 0.18, η2 = 0.07 |
| Normalized transition score | 0.84 (0.20) | 0.77 (0.27) | 0.40 (0.48) | F = 0.80, p = 0.39, η2 = 0.08 | F = 4.99, p = 0.036 *, η2 = 0.185 | F = 3.49, p = 0.07, η2 = 0.132 |
| T from face to target object | 4.08 (2.27) | 4.75 (2.73) | 3.27 (2.08) | F = 1.08, p = 0.25, η2 = 0.13 | F = 0.99, p = 0.33, η2 = 0.04 | F = 0.64, p = 0.43, η2 = 0.03 |
| T from face to NT object | 0.83 (1.03) | 1.33 (1.43) | 1.33 (1.44) | F = 5.67, p = 0.04 *, η2 = 0.36 | F = 1.07, p = 0.31, η2 = 0.04 | F = 0.35, p = 0.56, η2 = 0.02 |
| Between object transitions | 3.89 (3.43) | 4.42 (3.11) | 5.07 (3.91) | F = 4.55, p = 0.06, η2 = 0.29 | F = 0.21, p = 0.15, η2 = 0.08 | F = 2.08, p = 0.16, η2 = 0.08 |
| Initiating JA-2 | ||||||
| FD: Face | 37.57 (27.03) | 13.65 (17.52) | 16.76 (16.86) | F = 6.00, p = 0.03 *, η2 = 0.375 | F = 8.02, p = 0.01 *, η2 = 0.276 | F = 0.001, p = 0.97, η2 = 0.0001 |
| FD: Target object | 28.52 (22.12) | 34.54 (23.16) | 31.84 (22.30) | F = 0.62, p = 0.45, η2 = 0.06 | F = 0.07, p = 0.79, η2 = 0.003 | F = 0.12, p = 0.73, η2 = 0.006 |
| T from target object to face | 4.42 (1.78) | 4.00 (1.79) | 1.71 (1.37) | F = 1.93, p = 0.19, η2 = 0.18 | F = 7.65, p = 0.011 *, η2 = 0.242 | F = 6.21, p = 0.15, η2 = 0.20 |
| T from face to target object | 4.0 (2.0) | 4.79 (2.75) | 1.31 (1.37) | F = 2.62, p = 0.14, η2 = 0.23 | F = 7.47, p = 0.012 *, η2 = 0.237 | F = 4.57, p = 0.04 *, η2 = 0.222 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muratori, F.; Billeci, L.; Calderoni, S.; Boncoddo, M.; Lattarulo, C.; Costanzo, V.; Turi, M.; Colombi, C.; Narzisi, A. How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study. Brain Sci. 2019, 9, 344. https://doi.org/10.3390/brainsci9120344
Muratori F, Billeci L, Calderoni S, Boncoddo M, Lattarulo C, Costanzo V, Turi M, Colombi C, Narzisi A. How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study. Brain Sciences. 2019; 9(12):344. https://doi.org/10.3390/brainsci9120344
Chicago/Turabian StyleMuratori, Filippo, Lucia Billeci, Sara Calderoni, Maria Boncoddo, Caterina Lattarulo, Valeria Costanzo, Marco Turi, Costanza Colombi, and Antonio Narzisi. 2019. "How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study" Brain Sciences 9, no. 12: 344. https://doi.org/10.3390/brainsci9120344
APA StyleMuratori, F., Billeci, L., Calderoni, S., Boncoddo, M., Lattarulo, C., Costanzo, V., Turi, M., Colombi, C., & Narzisi, A. (2019). How Attention to Faces and Objects Changes Over Time in Toddlers with Autism Spectrum Disorders: Preliminary Evidence from An Eye Tracking Study. Brain Sciences, 9(12), 344. https://doi.org/10.3390/brainsci9120344







