Weight Loss Maintenance: Have We Missed the Brain?
Abstract
1. Introduction
2. Materials and Methods
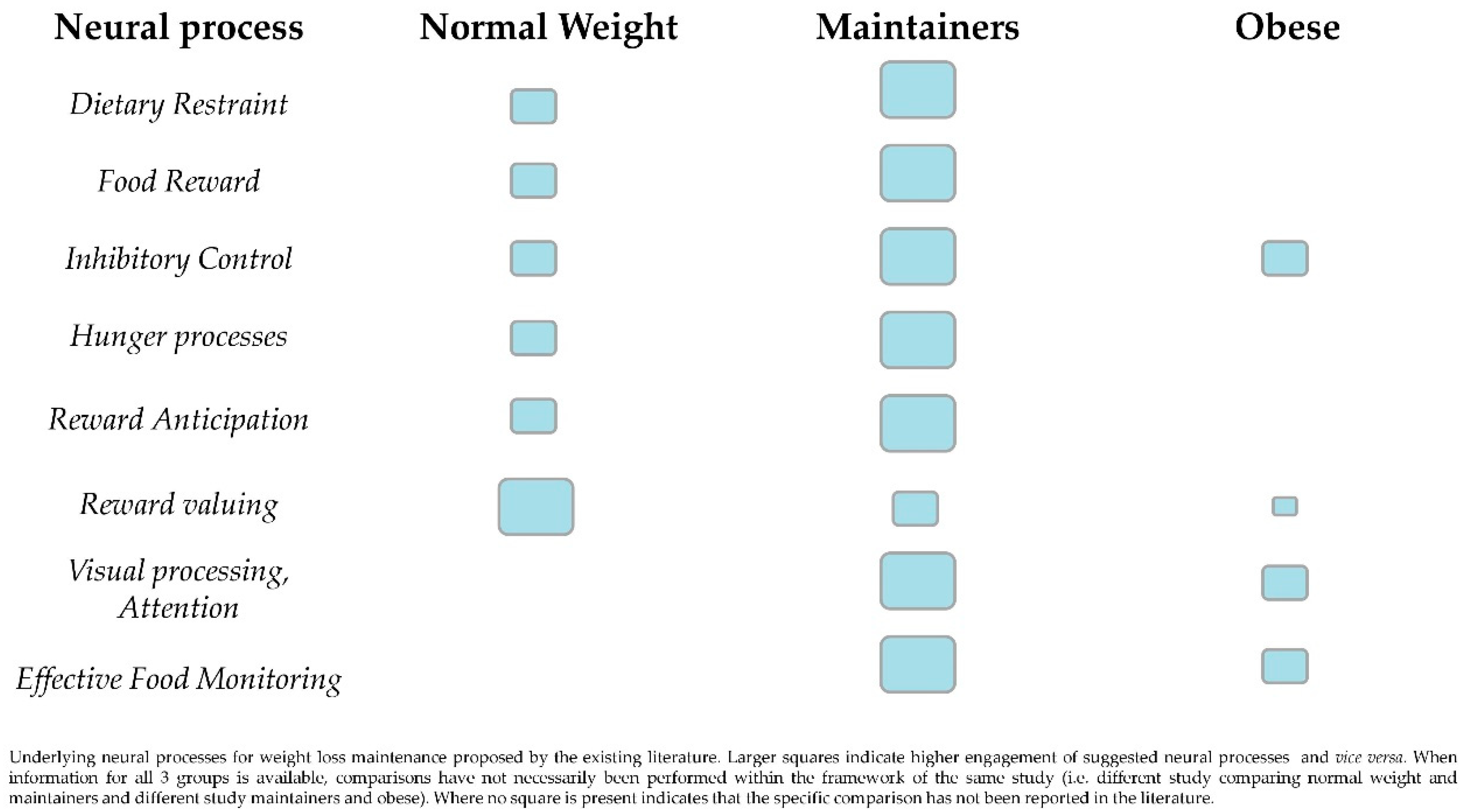
3. The Neural Background of Weight Loss Maintenance
4. The Interplay of Restraint and Reward Anticipation Brain Regions
5. Executive Functions Driving Maintenance
6. Clinical Implications and Future Possibilities
Author Contributions
Funding
Conflicts of Interest
References
- Ndumele, C.E.; Matsushita, K.; Lazo, M.; Bello, N.; Blumenthal, R.S.; Gerstenblith, G.; Nambi, V.; Ballantyne, C.M.; Solomon, S.D.; Selvin, E.; et al. Obesity and subtypes of incident cardiovascular disease. J. Am. Heart. Assoc. 2016, 5, e003921. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, C.; Sharp, S.J.; Schulze, M.B.; Rolandsson, O.; Overvad, K.; Forouhi, N.G.; Spranger, J.; Drogan, D.; Huerta, J.M.; Arriola, L.; et al. Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: The epic-interact case-cohort study. PLoS Med. 2012, 9, e1001230. [Google Scholar]
- Kyrgiou, M.; Kalliala, I.; Markozannes, G.; Gunter, M.J.; Paraskevaidis, E.; Gabra, H.; Martin-Hirsch, P.; Tsilidis, K.K. Adiposity and cancer at major anatomical sites: Umbrella review of the literature. BMJ 2017, 356, j477. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; La Marra, M.; Perrella, R.; Caviglia, G.; Iavarone, A.; Chieffi, S.; Messina, G.; Carotenuto, M.; Monda, M.; Messina, A. Obesity and brain illness: From cognitive and psychological evidences to obesity paradox. Diabetes Metab. Syndr. Obes. 2017, 10, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Sone, H.; Mizuno, S.; Yamada, N. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 1925–1927. [Google Scholar] [PubMed]
- Prickett, C.; Brennan, L.; Stolwyk, R. Examining the relationship between obesity and cognitive function: A systematic literature review. Obes. Res. Clin. Pract. 2015, 9, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Hazar, N.; Seddigh, L.; Rampisheh, Z.; Nojomi, M. Population attributable fraction of modifiable risk factors for Alzheimer disease: A systematic review of systematic reviews. Iran. J. Neurol. 2016, 15, 164–172. [Google Scholar] [PubMed]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.J.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement. (Amst.) 2017, 8, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Pedditzi, E.; Peters, R.; Beckett, N. Corrigenda: Corrigendum to the risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies’. Age Ageing 2016, 45, 740. [Google Scholar] [CrossRef] [PubMed]
- Behary, P.; Miras, A.D. Brain responses to food and weight loss. Exp. Physiol. 2014, 99, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M. Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 2012, 71, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Sargent-Cox, K.; Fraser, M.; Sachdev, P.; Anstey, K.J. Being overweight is associated with hippocampal atrophy: The path through life study. Int. J. Obes. (Lond.) 2015, 39, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship between body mass index and brain volume in healthy adults. Int. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mayer, E.A.; Labus, J.S.; Bhatt, R.R.; Ju, T.; Love, A.; Bal, A.; Tillisch, K.; Naliboff, B.; Sanmiguel, C.P.; et al. Sex commonalities and differences in obesity-related alterations in intrinsic brain activity and connectivity. Obesity (Silver Spring) 2018, 26, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Beilharz, J.E.; Maniam, J.; Reichelt, A.C.; Westbrook, R.F. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 2015, 58, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jokela, M.; Hintsanen, M.; Hakulinen, C.; Batty, G.D.; Nabi, H.; Singh-Manoux, A.; Kivimaki, M. Association of personality with the development and persistence of obesity: A meta-analysis based on individual-participant data. Obes. Rev. 2013, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Horstmann, A. It wasn’t me; it was my brain-obesity-associated characteristics of brain circuits governing decision-making. Physiol. Behav. 2017, 176, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Facchini, S.; Stubbs, B.; Luchini, C.; Solmi, M.; Manzato, E.; Sergi, G.; Maggi, S.; Cosco, T.; Fontana, L. Weight loss is associated with improvements in cognitive function among overweight and obese people: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 72, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Shah, K.; Waters, D.L.; Sinacore, D.R.; Qualls, C.; Villareal, D.T. Effect of weight loss, exercise, or both on cognition and quality of life in obese older adults. Am. J. Clin. Nutr. 2014, 100, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bruce, A.S.; Bruce, J.M.; Ness, A.R.; Lepping, R.J.; Malley, S.; Hancock, L.; Powell, J.; Patrician, T.M.; Breslin, F.J.; Martin, L.E.; et al. A comparison of functional brain changes associated with surgical versus behavioral weight loss. Obesity (Silver Spring) 2014, 22, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Montesi, L.; El Ghoch, M.; Brodosi, L.; Calugi, S.; Marchesini, G.; Dalle Grave, R. Long-term weight loss maintenance for obesity: A multidisciplinary approach. Diabetes Metab. Syndr. Obes. 2016, 9, 37–46. [Google Scholar] [PubMed]
- Franz, M.J.; VanWormer, J.J.; Crain, A.L.; Boucher, J.L.; Histon, T.; Caplan, W.; Bowman, J.D.; Pronk, N.P. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J. Am. Diet. Assoc. 2007, 107, 1755–1767. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araujo-Soares, V.; Sniehotta, F.F. Long term maintenance of weight loss with non-surgical interventions in obese adults: Systematic review and meta-analyses of randomised controlled trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef] [PubMed]
- Klem, M.L.; Wing, R.R.; McGuire, M.T.; Seagle, H.M.; Hill, J.O. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am. J. Clin. Nutr. 1997, 66, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.; Vieira, P.N.; Silva, M.N.; Sardinha, L.B.; Teixeira, P.J. Weight control behaviors of highly successful weight loss maintainers: The portuguese weight control registry. J. Behav. Med. 2017, 40, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Soini, S.; Mustajoki, P.; Eriksson, J.G. Weight loss methods and changes in eating habits among successful weight losers. Ann. Med. 2016, 48, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Karfopoulou, E.; Anastasiou, C.A.; Hill, J.O.; Yannakoulia, M. The medweight study: Design and preliminary results. Mediterr. J. Nutr. Metab. 2014, 7, 201–210. [Google Scholar]
- Shick, S.M.; Wing, R.R.; Klem, M.L.; McGuire, M.T.; Hill, J.O.; Seagle, H. Persons successful at long-term weight loss and maintenance continue to consume a low-energy, low-fat diet. J. Am. Diet. Assoc. 1998, 98, 408–413. [Google Scholar] [CrossRef]
- Brikou, D.; Zannidi, D.; Karfopoulou, E.; Anastasiou, C.A.; Yannakoulia, M. Breakfast consumption and weight-loss maintenance: Results from the medweight study. Br. J. Nutr. 2016, 115, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, H.R.; Grunwald, G.K.; Mosca, C.L.; Klem, M.L.; Wing, R.R.; Hill, J.O. Long-term weight loss and breakfast in subjects in the national weight control registry. Obes. Res. 2002, 10, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Karfopoulou, E.; Brikou, D.; Mamalaki, E.; Bersimis, F.; Anastasiou, C.A.; Hill, J.O.; Yannakoulia, M. Dietary patterns in weight loss maintenance: Results from the medweight study. Eur. J. Nutr. 2017, 56, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Ogden, L.G.; Stuht, J.; Phelan, S.; Wing, R.R.; Hill, J.O.; Wyatt, H.R. Physical activity patterns in the national weight control registry. Obesity (Silver Spring) 2008, 16, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, V.A.; Odgen, L.; Phelan, S.; Thomas, J.G.; Hill, J.; Wing, R.R.; Wyatt, H. Dietary habits and weight maintenance success in high versus low exercisers in the national weight control registry. J. Phys. Act. Health 2014, 11, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Phelan, S.; Wyatt, H.R.; Hill, J.O.; Wing, R.R. Are the eating and exercise habits of successful weight losers changing? Obesity (Silver Spring) 2006, 14, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Yannakoulia, M.; Anastasiou, C.A.; Karfopoulou, E.; Pehlivanidis, A.; Panagiotakos, D.B.; Vgontzas, A. Sleep quality is associated with weight loss maintenance status: The medweight study. Sleep Med. 2017, 34, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.M.; Graham Thomas, J.; Wing, R.R. Successful weight loss maintenance associated with morning chronotype and better sleep quality. J. Behav. Med. 2016, 39, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Klem, M.L.; Wing, R.R.; McGuire, M.T.; Seagle, H.M.; Hill, J.O. Psychological symptoms in individuals successful at long-term maintenance of weight loss. Health Psychol. 1998, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Karfopoulou, E.; Anastasiou, C.A.; Avgeraki, E.; Kosmidis, M.H.; Yannakoulia, M. The role of social support in weight loss maintenance: Results from the medweight study. J. Behav. Med. 2016, 39, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Fappa, E.; Karfopoulou, E.; Gkza, A.; Yannakoulia, M. Weight loss maintenance in relation to locus of control: The medweight study. Behav. Res. Ther. 2015, 71, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.J.; Silva, M.N.; Coutinho, S.R.; Palmeira, A.L.; Mata, J.; Vieira, P.N.; Carraca, E.V.; Santos, T.C.; Sardinha, L.B. Mediators of weight loss and weight loss maintenance in middle-aged women. Obesity (Silver Spring) 2010, 18, 725–735. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Chen, K.; Salbe, A.D.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 370–377. [Google Scholar] [CrossRef] [PubMed]
- DelParigi, A.; Chen, K.; Salbe, A.D.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int. J. Obes. (Lond.) 2007, 31, 440–448. [Google Scholar] [CrossRef] [PubMed]
- McCaffery, J.M.; Haley, A.P.; Sweet, L.H.; Phelan, S.; Raynor, H.A.; Del Parigi, A.; Cohen, R.; Wing, R.R. Differential functional magnetic resonance imaging response to food pictures in successful weight–loss maintainers relative to normal–weight and obese controls. Am. J. Clin. Nutr. 2009, 90, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Hassenstab, J.J.; Sweet, L.H.; Del Parigi, A.; McCaffery, J.M.; Haley, A.P.; Demos, K.E.; Cohen, R.A.; Wing, R.R. Cortical thickness of the cognitive control network in obesity and successful weight loss maintenance: A preliminary mri study. Psychiatry Res. 2012, 202, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Sweet, L.H.; Hassenstab, J.J.; McCaffery, J.M.; Raynor, H.A.; Bond, D.S.; Demos, K.E.; Haley, A.P.; Cohen, R.A.; Del Parigi, A.; Wing, R.R. Brain response to food stimulation in obese, normal weight, and successful weight loss maintainers. Obesity (Silver Spring) 2012, 20, 2220–2225. [Google Scholar] [CrossRef] [PubMed]
- Murdaugh, D.L.; Cox, J.E.; Cook, E.W., 3rd; Weller, R.E. Fmri reactivity to high–calorie food pictures predicts short-and long-term outcome in a weight-loss program. Neuroimage 2012, 59, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.J.; Becker, A.; Sinno, M.H.; Skunde, M.; Bendszus, M.; Preissl, H.; Enck, P.; Herzog, W.; Friederich, H.C. Neural food reward processing in successful and unsuccessful weight maintenance. Obesity (Silver Spring) 2018, 26, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Perri, M.G.; Nezu, A.M.; McKelvey, W.F.; Shermer, R.L.; Renjilian, D.A.; Viegener, B.J. Relapse prevention training and problem–solving therapy in the long–term management of obesity. J. Consult. Clin. Psychol. 2001, 69, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Pursey, K.M.; Stanwell, P.; Callister, R.J.; Brain, K.; Collins, C.E.; Burrows, T.L. Neural responses to visual food cues according to weight status: A systematic review of functional magnetic resonance imaging studies. Front. Nutr. 2014, 1, 7. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.L.; Canterberry, M.; Borckardt, J.J.; Madan, A.; Byrne, T.K.; George, M.S.; O’Neil, P.M.; Hanlon, C.A. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring) 2013, 21, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.M.; Hancock, L.; Bruce, A.; Lepping, R.J.; Martin, L.; Lundgren, J.D.; Malley, S.; Holsen, L.M.; Savage, C.R. Changes in brain activation to food pictures after adjustable gastric banding. Surg. Obes. Relat. Dis. 2012, 8, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Le, D.S.; Pannacciulli, N.; Chen, K.; Salbe, A.D.; Del Parigi, A.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Krakoff, J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am. J. Clin. Nutr. 2007, 86, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Le, D.S.; Pannacciulli, N.; Chen, K.; Del Parigi, A.; Salbe, A.D.; Reiman, E.M.; Krakoff, J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am. J. Clin. Nutr. 2006, 84, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Weygandt, M.; Mai, K.; Dommes, E.; Ritter, K.; Leupelt, V.; Spranger, J.; Haynes, J.D. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage 2015, 109, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Hannay, H.J.; Fischer, J.S. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Nederkoorn, C.; Houben, K.; Hofmann, W.; Roefs, A.; Jansen, A. Control yourself or just eat what you like? Weight gain over a year is predicted by an interactive effect of response inhibition and implicit preference for snack foods. Health Psychol. 2010, 29, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, M.B.; Alosco, M.; Strain, G.; Devlin, M.; Cohen, R.; Paul, R.; Crosby, R.D.; Mitchell, J.E.; Gunstad, J. Cognitive function predicts 24-month weight loss success following bariatric surgery. Surg. Obes. Relat. Dis. 2013, 9, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Gettens, K.M.; Gorin, A.A. Executive function in weight loss and weight loss maintenance: A conceptual review and novel neuropsychological model of weight control. J. Behav. Med. 2017, 40, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Hardman, C.A.; Lawrence, N.; Field, M. Cognitive training as a potential treatment for overweight and obesity: A critical review of the evidence. Appetite 2018, 124, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Sartirana, M.; El Ghoch, M.; Calugi, S. Personalized multistep cognitive behavioral therapy for obesity. Diabetes Metab. Syndr. Obes. 2017, 10, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, M.; Zhang, Y.; Song, H.; Von Deneen, K.M.; Shi, Y.; Liu, Y.; He, D. Intrinsic brain subsystem associated with dietary restraint, disinhibition and hunger: An fmri study. Brain Imaging Behav. 2017, 11, 264–277. [Google Scholar] [CrossRef] [PubMed]
- Olivo, G.; Zhou, W.; Sundbom, M.; Zhukovsky, C.; Hogenkamp, P.; Nikontovic, L.; Stark, J.; Wiemerslage, L.; Larsson, E.-M.; Benedict, C.; et al. Resting-state brain connectivity changes in obese women after roux-en-y gastric bypass surgery: A longitudinal study. Sci. Rep. 2017, 7, 6616. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Mercer, J.G.; Overduin, J.; La Fleur, S.E.; Adan, R.A. Dietary factors affect food reward and motivation to eat. Obes. Facts 2012, 5, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Dalle Grave, R.; Calugi, S.; El Ghoch, M. Are personality characteristics as measured by the temperament and character inventory (TCI) associated with obesity treatment outcomes? A systematic review. Curr. Obes. Rep. 2018, 7, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Monda, V.; Salerno, M.; Sessa, F.; Bernardini, R.; Valenzano, A.; Marsala, G.; Zammit, C.; Avola, R.; Carotenuto, M.; Messina, G.; et al. Functional changes of orexinergic reaction to psychoactive substances. Mol. Neurobiol. 2018, 55, 6362–6368. [Google Scholar] [CrossRef] [PubMed]
- Chieffi, S.; Carotenuto, M.; Monda, V.; Valenzano, A.; Villano, I.; Precenzano, F.; Tafuri, D.; Salerno, M.; Filippi, N.; Nuccio, F.; et al. Orexin system: The key for a healthy life. Front. Physiol. 2017, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Boughton, C.K.; Murphy, K.G. Can neuropeptides treat obesity? A review of neuropeptides and their potential role in the treatment of obesity. Br. J. Pharmacol. 2013, 170, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of cardiology/American heart association task force on practice guidelines and the obesity society. Circulation 2014, 129, S102–S138. [Google Scholar] [CrossRef] [PubMed]
- Hinkle, W.; Cordell, M.; Leibel, R.; Rosenbaum, M.; Hirsch, J. Effects of reduced weight maintenance and leptin repletion on functional connectivity of the hypothalamus in obese humans. PLoS ONE 2013, 8, e59114. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. (Lond.) 2015, 39, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Maccani, J.Z.J.; Hawley, N.L.; Wing, R.R.; Kelsey, K.T.; McCaffery, J.M. Epigenetic patterns in successful weight loss maintainers: A pilot study. Int. J. Obes. (Lond.) 2015, 39, 865–868. [Google Scholar] [CrossRef] [PubMed]
| Study, Study Design | Study Population | Weight Loss Maintenance Definition | Imaging Method | Exclusion Criteria | Measures |
|---|---|---|---|---|---|
| Del Parigi et al., 2004 [41] Observational Case-Control | 11 Maintainers 23 OB 21 NW | Stable weight for ≥3 months, after intentional weight reduction from a BMI ≥ 35 kg/m2 to <25 kg/m2, through diet and exercise | PET-scan | Not reported | Regional cerebral blood flow at baseline (after a 36-h fast), after tasting and after consuming a satiating liquid meal, in 4 brain regions |
| Del Parigi et al., 2007 [42] Observational Case-Control | 9 Maintainers 20 NW | Stable weight for ≥3 months, after intentional weight reduction from a BMI ≥ 35 kg/m2 to <25 kg/m2, through diet and exercise | PET-scan | Not reported | Brain response to the sensory experience of food and meal consumption |
| McCaffery et al., 2009 [43] Observational Case-Control | 17 Maintainers 16 OB 18 NW | Maintenance of intentional weight loss ≥13.6 kg, from a maximum BMI ≥ 30 kg/m2 to normal BMI, for at least 3 years | fMRI | Medication Left-handedness Neuropathology Psychopathology Standard MRI contradictions | Visual stimuli of low and high calorie foods and non-foods, in a single 8-min run, after a 4-h fast |
| Hassenstab et al., 2012 [44] Observational Case-Control | 17 Maintainers 17 OB 19 NW | Maintenance of intentional weight loss ≥13.6 kg, from a maximum BMI ≥ 30 kg/m2 to normal BMI, for at least 3 years | MRI | Medication Neuropathology Psychopathology Standard MRI contradictions | Cortical thickness in 4 a-priori set brain regions of the cognitive control network |
| Sweet et al., 2012 [45] Observational Case-Control | 17 Maintainers 14 OB 18 NW | Maintenance of intentional weight loss ≥13.6 kg, from a maximum BMI ≥ 30 kg/m2 to a BMI≥18.5 and <27 kg/m2, for at least 3 years | fMRI | Medication Left-handedness Neuropathology Psychopathology Standard MRI contradictions | Neurological response during an 1-min orosensory paradigm, after a 4-h fast |
| Murdaugh et al., 2012 [46] Prospective observation | 25 OB, scanned prior and after a 12-week dietary intervention, and on 9-month follow up | Maintenance of weight loss achieved through a 3-month behavioural intervention, 9 months post intervention | fMRI | Left-handedness IQ < 80 Chronic conditions Neuropathology Psychopathology Standard MRI contradictions | Visual stimuli of high-quality color food or non-food photographs |
| Weygandt et al., 2015 Prospective observation | 23 OW and OB, scanned after a 12-week dietary intervention, and on 12-month follow up | Maintenance of weight loss achieved through the dietary intervention | fMRI | Psychopathology Neuropathology | Food related delay-discounting task |
| Simon et al., 2018 [47] Cross-sectional crossover | 17 Maintainers 16 Regainers | Maintenance of weight loss ≥10% of initial body weight, 6 months after a dietary intervention | fMRI | Medication Left-handedness Psychopathology Standard MRI contradictions | Neural processing during two types of incentive delay tasks, during the anticipation and receipt of monetary and/or food-related reward |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulimeneas, D.; Yannakoulia, M.; Anastasiou, C.A.; Scarmeas, N. Weight Loss Maintenance: Have We Missed the Brain? Brain Sci. 2018, 8, 174. https://doi.org/10.3390/brainsci8090174
Poulimeneas D, Yannakoulia M, Anastasiou CA, Scarmeas N. Weight Loss Maintenance: Have We Missed the Brain? Brain Sciences. 2018; 8(9):174. https://doi.org/10.3390/brainsci8090174
Chicago/Turabian StylePoulimeneas, Dimitrios, Mary Yannakoulia, Costas A. Anastasiou, and Nikolaos Scarmeas. 2018. "Weight Loss Maintenance: Have We Missed the Brain?" Brain Sciences 8, no. 9: 174. https://doi.org/10.3390/brainsci8090174
APA StylePoulimeneas, D., Yannakoulia, M., Anastasiou, C. A., & Scarmeas, N. (2018). Weight Loss Maintenance: Have We Missed the Brain? Brain Sciences, 8(9), 174. https://doi.org/10.3390/brainsci8090174





