Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders
Abstract
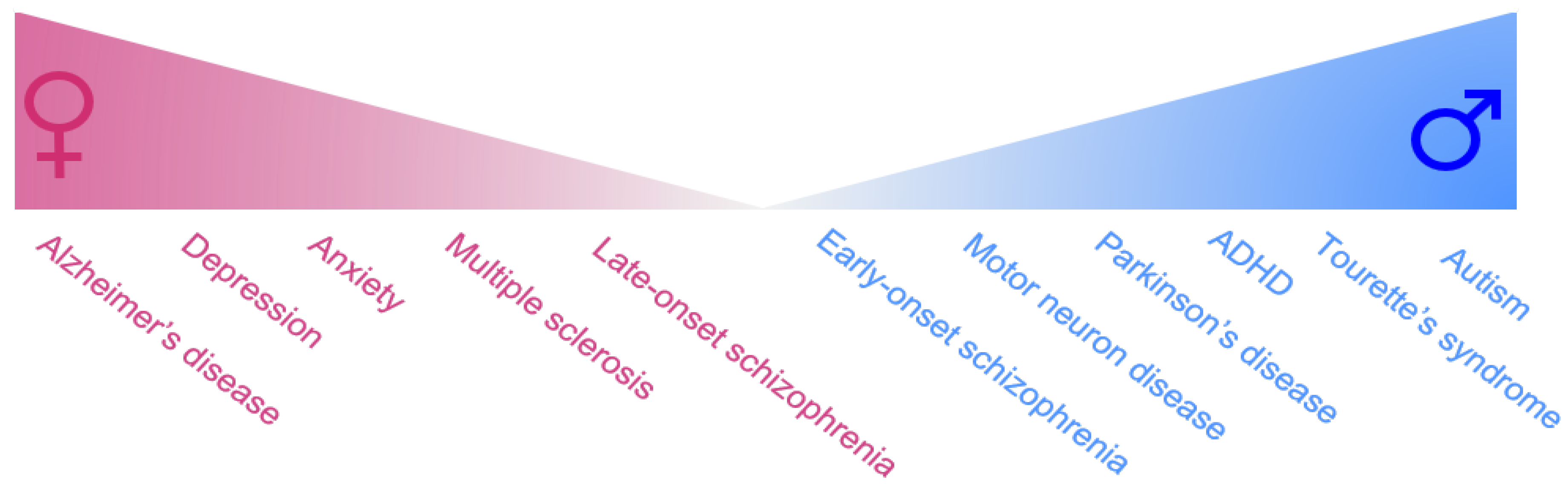
:1. Introduction
2. Female-Biased Brain Disorders
2.1. Female-Biased Neuropsychiatric Disorders
2.1.1. Anxiety Disorders
2.1.2. Depression
2.1.3. Late-Onset Schizophrenia
2.2. Female-Biased Neurodegenerative Disorders
2.2.1. Alzheimer’s Disease
2.2.2. Multiple Sclerosis
3. Male-Biased Disorders
3.1. Male-Biased Neuropsychiatric Disorders
3.1.1. Autism
3.1.2. Attention-Deficit Hyperactivity Disorder
3.1.3. Tourette’s Syndrome
3.1.4. Early-Onset Schizophrenia
3.2. Male-Biased Neurodegenerative Disorders
3.2.1. Parkinson’s Disease
3.2.2. Motor Neuron Disease
4. Role of Sex Hormones and Sex Chromosome Genes in Susceptibility to Neurological Disorders
4.1. Influence of Sex Hormones
4.1.1. Estrogen and Estrogen Signalling
4.1.2. Testosterone
4.2. Influence of Sex Chromosome Genes
4.2.1. X-Linked Dosage Effects
4.2.2. X-Linked Imprinting Effects
4.2.3. Y-Chromosome Effects
4.2.4. Y-Chromosome Gene SRY
5. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Loke, H.; Harley, V.; Lee, J. Biological factors underlying sex differences in neurological disorders. Int. J. Biochem. Cell. Boil. 2015, 65, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Young, L.J.; Pfaff, D.W. Sex differences in neurological and psychiatric disorders. Front. Neuroendocrinol. 2014, 35, 253–254. [Google Scholar] [CrossRef] [PubMed]
- Ngun, T.C.; Ghahramani, N.; Sánchez, F.J.; Bocklandt, S.; Vilain, E. The genetics of sex differences in brain and behavior. Front. Neuroendocr. 2011, 32, 227–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; De Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation during development. J. Steroid Biochem. Mol. Boil. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosgrove, K.P.; Mazure, C.M.; Staley, J.K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiatry 2007, 62, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; McArthur, S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol. Rev. 2010, 62, 155–198. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.-M.; Swaab, D.F. Sex differences in the brain, behavior, and neuropsychiatric disorders. Neuroscience 2010, 16, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.; Wilkinson, L.S. It is not all hormones: Alternative explanations for sexual differentiation of the brain. Brain Res. 2006, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.; Loisel, D.A.; Gilad, Y. Sex-specific genetic architecture of human disease. Nat. Rev. Genet. 2008, 9, 911–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, V.M. Why are sex and gender important to basic physiology and translational and individualized medicine? Am. J. Physiol.-Heart Circ. Physiol. 2014, 306, H781–H788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, P.M.; Rohde, P.; Seeley, J.R. Major depressive disorder in older adolescents: Prevalence, risk factors, and clinical implications. Clin. Psychol. Rev. 1998, 18, 765–794. [Google Scholar] [CrossRef]
- Biederman, J.; Mick, E.; Faraone, S.V.; Braaten, E.; Doyle, A.; Spencer, T.; Wilens, T.E.; Frazier, E.; Johnson, M.A. Influence of gender on attention deficit hyperactivity disorder in children referred to a psychiatric clinic. Am. J. Psychiatry 2002, 159, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in parkinson’s disease. Front. Neuroendocr. 2014, 35, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Haaxma, C.A.; Bloem, B.R.; Borm, G.F.; Oyen, W.J.; Leenders, K.L.; Eshuis, S.; Booij, J.; Dluzen, D.E.; Horstink, M.W. Gender differences in parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2007, 78, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- McCombe, P.A.; Henderson, R.D. Review article: Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010, 7, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P. Prevalence of amyotrophic lateral sclerosis—United States, 2010–2011. Am. J. Public Health 2015, 105, e7–e9. [Google Scholar]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocr. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Beeson, P.B. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 1994, 96, 457–462. [Google Scholar] [CrossRef]
- Alonso, A.; Hernán, M.A. Temporal trends in the incidence of multiple sclerosis. Neurology 2008, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.J.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Wetherington, C.L.; et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewing, P.; Chiang, C.W.; Sinchak, K.; Sim, H.; Fernagut, P.O.; Kelly, S.; Chesselet, M.F.; Micevych, P.E.; Albrecht, K.H.; Harley, V.R.; et al. Direct regulation of adult brain function by the male-specific factor SRY. Curr. Biol. 2006, 16, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Jazin, E.; Cahill, L. Sex differences in molecular neuroscience: From fruit flies to humans. Nat. Rev. Neurosci. 2010, 11, 9–17. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Milner, T.A. Understanding the broad influence of sex hormones and sex differences in the brain. J. Neurosci. Res. 2017, 95, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocr. 2005, 26, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Neufang, S.; Specht, K.; Hausmann, M.; Güntürkün, O.; Herpertz-Dahlmann, B.; Fink, G.R.; Konrad, K. Sex differences and the impact of steroid hormones on the developing human brain. Cereb. Cortex 2008, 19, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Gorski, R.A. Gonadal steroid induction of structural sex differences in the central nervous system. Annu. Rev. Neurosci. 1984, 7, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Dluzen, D.; Horstink, M. Estrogen as neuroprotectant of nigrostriatal dopaminergic system: Laboratory and clinical studies. Endocrine 2003, 21, 67–75. [Google Scholar] [CrossRef]
- Bourque, M.; Dluzen, D.E.; Di Paolo, T. Neuroprotective actions of sex steroids in parkinson’s disease. Front. Neuroendocr. 2009, 30, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.; de Castella, A.; Fitzgerald, P.B.; Gurvich, C.T.; Bailey, M.; Bartholomeusz, C.; Burger, H. Estrogen in severe mental illness: A potential new treatment approach. Arch. Gen. Psychiatry 2008, 65, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Martel, M.M.; Gobrogge, K.L.; Breedlove, S.M.; Nigg, J.T. Masculinized finger-length ratios of boys, but not girls, are associated with attention-deficit/hyperactivity disorder. Behav. Neurosci. 2008, 122, 273–281. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, E.I.; Verheij, F.; Wiegman, T.; Ferdinand, R.F. Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, adhd, and anxiety disorders. Dev. Med. Child. Neurol. 2006, 48, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocr. 2009, 30, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewing, P.; Shi, T.; Horvath, S.; Vilain, E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res. Mol. Brain Res. 2003, 118, 82–90. [Google Scholar] [CrossRef]
- Wittchen, H.U.; Zhao, S.; Kessler, R.C.; Eaton, W.W. Dsm-III-r generalized anxiety disorder in the national comorbidity survey. Arch. Gen. Psychiatry 1994, 51, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Breslau, N.; Roth, T.; Rosenthal, L.; Andreski, P. Sleep disturbance and psychiatric disorders: A longitudinal epidemiological study of young adults. Boil. Psychiatry 1996, 39, 411–418. [Google Scholar] [CrossRef]
- Koen, N.; Stein, D.J. Pharmacotherapy of anxiety disorders: A critical review. Dialogues Clin. Neurosci. 2011, 13, 423–437. [Google Scholar] [PubMed]
- Altemus, M.; Sarvaiya, N.; Neill Epperson, C. Sex differences in anxiety and depression clinical perspectives. Front. Neuroendocr. 2014, 35, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKinaw-Koons, B.; Vasey, M.W. Considering sex differences in anxiety and its disorders across the life span: A construct-validation approach. Appl. Prev. Psychol. 2000, 9, 191–209. [Google Scholar] [CrossRef]
- Bekker, M.H. Agoraphobia and gender: A review. Clin. Psychol. Rev. 1996, 16, 129–146. [Google Scholar] [CrossRef]
- Bekker, M.H.J. Anxiety disorders: Sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend. Med. 2007, 4, S178–S193. [Google Scholar] [CrossRef]
- Marques, A.A.; Bevilaqua, M.C.; da Fonseca, A.M.; Nardi, A.E.; Thuret, S.; Dias, G.P. Gender differences in the neurobiology of anxiety: Focus on adult hippocampal neurogenesis. Neural. Plast. 2016, 5026713. [Google Scholar] [CrossRef] [PubMed]
- Cover, K.K.; Maeng, L.Y.; Lebron-Milad, K.; Milad, M.R. Mechanisms of estradiol in fear circuitry: Implications for sex differences in psychopathology. Transl. Psychiatry 2014, 4, e422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organisation. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Murray, C.J.; Lopez, A.D. Global mortality, disability, and the contribution of risk factors: Global burden of disease study. Lancet 1997, 349, 1436–1442. [Google Scholar] [CrossRef]
- Hammen, C.L.; Padesky, C.A. Sex differences in the expression of depressive responses on the beck depression inventory. J. Abnorm. Psychol. 1977, 86, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Steer, R.A.; Beck, A.T.; Brown, G. Sex differences on the revised beck depression inventory for outpatients with affective disorders. J. Pers. Assess. 1989, 53, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Kornstein, S.G.; Schatzberg, A.F.; Thase, M.E.; Yonkers, K.A.; McCullough, J.P.; Keitner, G.I.; Gelenberg, A.J.; Ryan, C.E.; Hess, A.L.; Harrison, W.; et al. Gender differences in chronic major and double depression. J. Affect. Disord. 2000, 60, 1–11. [Google Scholar] [CrossRef]
- Ressler, K.J.; Nemeroff, C.B. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depression Anxiety 2000, 12 (Suppl. 1), 2–19. [Google Scholar] [CrossRef]
- Rajkowska, G.; Miguel-Hidalgo, J.J. Gliogenesis and glial pathology in depression. CNS Neurol. Disord. Drug Targets 2007, 6, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Jiang, L.; De Feyter, H.M.; Fasula, M.; Krystal, J.H.; Rothman, D.L.; Mason, G.F.; Sanacora, G. Glutamate metabolism in major depressive disorder. Am. J. Psychiatry 2014, 171, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- DeRubeis, R.J.; Siegle, G.J.; Hollon, S.D. Cognitive therapy vs. Medications for depression: Treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008, 9, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; McGonagle, K.A.; Nelson, C.B.; Hughes, M.; Swartz, M.; Blazer, D.G. Sex and depression in the national comorbidity survey. II: Cohort effects. J. Affect. Disord. 1994, 30, 15–26. [Google Scholar] [CrossRef]
- Kessler, R.C.; McGonagle, K.A.; Swartz, M.; Blazer, D.G.; Nelson, C.B. Sex and depression in the national comorbidity survey. I: Lifetime prevalence, chronicity and recurrence. J. Affect. Disord. 1993, 29, 85–96. [Google Scholar] [CrossRef]
- Ross, C.E.; Mirowsky, J. Explaining the social patterns of depression: Control and problem solving—Or support and talking? J. Health Soc. Behav. 1989, 30, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Noble, R.E. Depression in women. Metab. Clin. Exp. 2005, 54, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Seeman, P.; Kapur, S. Schizophrenia: More dopamine, more d(2) receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 7673–7675. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Cherkerzian, S.; Tsuang, M.T.; Petryshen, T.L. Sex differences in the genetic risk for schizophrenia: History of the evidence for sex-specific and sex-dependent effects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013, 162B, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain A J. Neurol. 1999, 122 Pt 4, 593–624. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III—the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, G.K.; Marek, G.J. Serotonin and hallucinogens. Neuropsychopharmacology 1999, 21, 16S–23S. [Google Scholar] [CrossRef]
- Goff, D.C.; Coyle, J.T. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am. J. Psychiatry 2001, 158, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proc. Natl. Acad. Sci. USA 1996, 93, 9235–9240. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.N.; Meador-Woodruff, J.H. Linking the family of d2 receptors to neuronal circuits in human brain: Insights into schizophrenia. Neuropsychopharmacology 1997, 16, 375–384. [Google Scholar] [CrossRef]
- Kapur, S.; Arenovich, T.; Agid, O.; Zipursky, R.; Lindborg, S.; Jones, B. Evidence for onset of antipsychotic effects within the first 24 h of treatment. Am. J. Psychiatry 2005, 162, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V.; Seeman, P. Is schizophrenia a dopamine supersensitivity psychotic reaction? Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2014, 48, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Lavrestsky, H. History of Schizophrenia as a Psychiatric Disorder. In Clinical Handbook of Schizophrenia; Guilford Publications: New York, NY, USA, 2008. [Google Scholar]
- Ochoa, S.; Usall, J.; Cobo, J.; Labad, X.; Kulkarni, J. Gender differences in schizophrenia and first-episode psychosis: A comprehensive literature review. Schizophr. Res. Treat. 2012, 916198. [Google Scholar] [CrossRef] [PubMed]
- Kendell, R.E. Diagnosis and classification of functional psychoses. Br. Med Bull. 1987, 43, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Shtasel, D.L.; Gur, R.E.; Gallacher, F.; Heimberg, C.; Gur, R.C. Gender differences in the clinical expression of schizophrenia. Schizophr. Res. 1992, 7, 225–231. [Google Scholar] [CrossRef]
- Morgan, V.A.; Castle, D.J.; Jablensky, A.V. Do women express and experience psychosis differently from men? Epidemiological evidence from the australian national study of low prevalence (psychotic) disorders. Aust. N. Z. J. Psychiatry 2008, 42, 74–82. [Google Scholar] [CrossRef] [PubMed]
- McGlashan, T.H.; Bardenstein, K.K. Gender differences in affective, schizoaffective, and schizophrenic disorders. Schizophr. Bull. 1990, 16, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harbor Perspect. Med. 2012, 2, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Clark, A.W.; Coyle, J.T.; DeLong, M.R. Alzheimer disease: Evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child. Neurol. Soc. 1981, 10, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. The molecular pathology of alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef]
- Coyle, J.T.; Price, D.L.; Delong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Perl, D.P. Neuropathology of alzheimer’s disease. Mount Sinai J. Med. J. Transl. Personalized Med. J. Transl. Pers. Med. 2010, 77, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Wenk, G.L. Neuropathologic changes in alzheimer’s disease. J. Clin. Psychiatry 2003, 64, 7–10. [Google Scholar] [PubMed]
- Alzheimer’s Association. 2017 Alzheimer’s disease facts and figures. Alzheimers Dement. 2017, 13, 325–373. [Google Scholar] [CrossRef]
- Lobo, A.; Launer, L.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.; Copeland, J.; Dartigues, J.; Jagger, C.; Martinez-Lage, J. Prevalence of dementia and major subtypes in europe: A collaborative study of population-based cohorts. Neurology 2000, 54, S4–S9. [Google Scholar] [PubMed]
- Zandi, P.P.; Carlson, M.C.; Plassman, B.L.; Welsh-Bohmer, K.A.; Mayer, L.S.; Steffens, D.C.; Breitner, J.C.; Investigators, C.C.M.S. Hormone replacement therapy and incidence of alzheimer disease in older women: The cache county study. JAMA 2002, 288, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.L.; Resnick, E.M.; Mallampalli, M.; Kalbarczyk, A. Sex and gender differences in alzheimer’s disease: Recommendations for future research. J. Womens Health 2012, 21, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Kelly-Hayes, M.; Kase, C.S.; Au, R.; Kannel, W.B.; Wolf, P.A. The lifetime risk of stroke: Estimates from the framingham study. Stroke J. Cereb. Circ. 2006, 37, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Toran-Allerand, C.D.; Greengard, P.; Gandy, S.E. Estrogen regulates metabolism of alzheimer amyloid beta precursor protein. J. Biol. Chem. 1994, 269, 13065–13068. [Google Scholar] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the united states: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Barnes, L.L.; Wilson, R.S.; Bienias, J.L.; Schneider, J.A.; Evans, D.A.; Bennett, D.A. Sex differences in the clinical manifestations of alzheimer disease pathology. Arch. Gen. Psychiatry 2005, 62, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B.A.; Convit, A.; Bachman, A.H. Analysis of the miriad data shows sex differences in hippocampal atrophy progression. J. Alzheimers Disease JAD 2016, 50, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Tschanz, J.T.; Corcoran, C.D.; Schwartz, S.; Treiber, K.; Green, R.C.; Norton, M.C.; Mielke, M.M.; Piercy, K.; Steinberg, M.; Rabins, P.V.; et al. Progression of cognitive, functional, and neuropsychiatric symptom domains in a population cohort with alzheimer dementia: The cache county dementia progression study. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 2011, 19, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Sinforiani, E.; Citterio, A.; Zucchella, C.; Bono, G.; Corbetta, S.; Merlo, P.; Mauri, M. Impact of gender differences on the outcome of alzheimer’s disease. Dement. Geriatr. Cogn. Disorders 2010, 30, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Henderson, V.W.; Buckwalter, J.G. Cognitive deficits of men and women with alzheimer’s disease. Neurology 1994, 44, 90. [Google Scholar] [CrossRef] [PubMed]
- Read, S.; Pedersen, N.L.; Gatz, M.; Berg, S.; Vuoksimaa, E.; Malmberg, B.; Johansson, B.; McClearn, G.E. Sex differences after all those years? Heritability of cognitive abilities in old age. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2006, 61, P137–P143. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Amieva, H.; Letenneur, L.; Orgogozo, J.-M.; Jacqmin-Gadda, H.; Dartigues, J.-F. Gender and education impact on brain aging: A general cognitive factor approach. Psychol. Aging 2008, 23, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Laws, K.R.; Irvine, K.; Gale, T.M. Sex differences in cognitive impairment in alzheimer’s disease. World J. Psychiatry 2016, 6, 54–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scacchi, R.; Gambina, G.; Broggio, E.; Corbo Rosa, M. Sex and esr1 genotype may influence the response to treatment with donepezil and rivastigmine in patients with alzheimer’s disease. Int. J. Geriatr. Psychiatry 2013, 29, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.H.; Bejar, C.; Weinstock, M. Gender differences in the effect of rivastigmine on brain cholinesterase activity and cognitive function in rats. Neuropharmacology 2000, 39, 497–506. [Google Scholar] [CrossRef]
- Canevelli, M.; Quarata, F.; Remiddi, F.; Lucchini, F.; Lacorte, E.; Vanacore, N.; Bruno, G.; Cesari, M. Sex and gender differences in the treatment of alzheimer’s disease: A systematic review of randomized controlled trials. Pharmacol. Res. 2017, 115, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2004, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T. Relapsing–remitting and primary progressive ms have the same cause(s)—The neuropathologist’s view: 2. Mult. Scler. J. 2013, 19, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Raffel, J.; Wakerley, B.; Nicholas, R. Multiple sclerosis. Medicine 2016, 44, 537–541. [Google Scholar] [CrossRef]
- Sánchez, M.P.; Nieto, A.; Barroso, J.; Martín, V.; Hernández, M.A. Brain atrophy as a marker of cognitive impairment in mildly disabling relapsing–remitting multiple sclerosis. Eur. J. Neurol. 2008, 15, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Agius, M.A. Current approved options for treating patients with multiple sclerosis. Neurology 2004, 63, S8–S14. [Google Scholar] [CrossRef] [PubMed]
- Ebers, G.C.; Sadovnick, A.D.; Dyment, D.A.; Yee, I.M.L.; Willer, C.J.; Risch, N. Parent-of-origin effect in multiple sclerosis: Observations in half-siblings. Lancet 2004, 363, 1773–1774. [Google Scholar] [CrossRef]
- Glad, S.B.; Nyland, H.I.; Aarseth, J.H.; Riise, T.; Myhr, K.M. Long-term follow-up of benign multiple sclerosis in hordaland county, western norway. Mult. Scler. 2009, 15, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, R. Prognostic factors in multiple sclerosis. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2007; Volume 79, pp. 423–447. [Google Scholar]
- Weinshenker, B.G.; Rice, G.P.A.; Noseworthy, J.H.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study3. Multivariate analysis of predictive factors and models of outcome. Brain 1991, 114, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, C.; Tomassini, V.; Marinelli, F.; Paolillo, A.; Gasperini, C.; Bastianello, S. ‘Gender gap’ in multiple sclerosis: Magnetic resonance imaging evidence. Eur. J. Neurol. 2003, 10, 95–97. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.M.; Csurhes, P.A.; Pender, M.P.; McCombe, P.A. Effect of gender on T-cell proliferative responses to myelin proteolipid protein antigens in patients with multiple sclerosis and controls. J. Autoimmun. 2004, 22, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luchetti, S.; van Eden, C.G.; Schuurman, K.; van Strien, M.E.; Swaab, D.F.; Huitinga, I. Gender differences in multiple sclerosis: Induction of estrogen signaling in male and progesterone signaling in female lesions. J. Neuropathol. Exp. Neurol. 2014, 73, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-P.; Landsborough, D.; Hsi, M.-S. Multiple sclerosis amongst Chinese in Taiwan. J. Neurol. Sci. 1976, 27, 459–484. [Google Scholar] [CrossRef]
- Kira, J.-I. Multiple sclerosis in the Japanese population. Lancet Neurol. 2003, 2, 117–127. [Google Scholar] [CrossRef]
- Orton, S.-M.; Herrera, B.M.; Yee, I.M.; Valdar, W.; Ramagopalan, S.V.; Sadovnick, A.D.; Ebers, G.C. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol. 2006, 5, 932–936. [Google Scholar] [CrossRef]
- Maghzi, A.H.; Ghazavi, H.; Ahsan, M.; Etemadifar, M.; Mousavi, S.A.; Khorvash, F.; Minagar, A. Increasing female preponderance of multiple sclerosis in Isfahan, Iran: A population-based study. Mult. Scler. J. 2010, 16, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Hirst, C.; Ingram, G.; Pickersgill, T.; Swingler, R.; Compston, D.A.S.; Robertson, N.P. Increasing prevalence and incidence of multiple sclerosis in south east wales. J. Neurol. Neurosurg. Psychiatry 2009, 80, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Celius, E.G.; Smestad, C. Change in sex ratio, disease course and age at diagnosis in Oslo MS patients through seven decades. Acta Neurol. Scand. Suppl. 2009, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, Y.; Hizem, Y.; Nasri, A.; Kacem, I.; Djebara, M.B.; Gargouri, A.; Gouider, R. A prospective study on the prognosis of multiple sclerosis in tunisia: Do we really have a distinct disease course in north africa? Mult. Scler. Relat. Disord. 2014, 3, 740–741. [Google Scholar] [CrossRef]
- Geschwind, D.H. Autism: Many genes, common pathways? Cell 2008, 135, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Baron-Cohen, S. Autism. Lancet 2014, 383, 896–910. [Google Scholar] [CrossRef]
- Webb, S.J.; Jones, E.J. Early identification of autism: Early characteristics, onset of symptoms, and diagnostic stability. Infants Young Child. 2009, 22, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Bespalova, I.N.; Buxbaum, J.D. Disease susceptibility genes for autism. Ann. Med. 2003, 35, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Barber, R.M.; Bhutta, Z.A.; Dandona, L.; Gething, P.W.; Hay, S.I.; Kinfu, Y.; Larson, H.J.; Liang, X.; Lim, S.S.; et al. Global, regional, and national levels of maternal mortality, 1990–2015: A systematic analysis for the global burden of disease study 2015. Lancet 2016, 388, 1775–1812. [Google Scholar] [CrossRef]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson Rosenberg, C.; White, T.; et al. Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, united states, 2014. MMWR Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Newschaffer, C.J.; Croen, L.A.; Daniels, J.; Giarelli, E.; Grether, J.K.; Levy, S.E.; Mandell, D.S.; Miller, L.A.; Pinto-Martin, J.; Reaven, J.; et al. The epidemiology of autism spectrum disorders. Annu. Rev. Public Health 2007, 28, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C.; Muzik, O.; Rothermel, R.; Behen, M.; Chakraborty, P.; Mangner, T.; da Silva, E.A.; Chugani, H.T. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann. Neurol. 1997, 42, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef]
- Bachevalier, J.; Loveland, K.A. The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neurosci. Biobehav. Rev. 2006, 30, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Ring, H.A.; Bullmore, E.T.; Wheelwright, S.; Ashwin, C.; Williams, S.C.R. The amygdala theory of autism. Neurosci. Biobehav. Rev. 2000, 24, 355–364. [Google Scholar] [CrossRef]
- Saemundsen, E.; Magnusson, P.; Georgsdottir, I.; Egilsson, E.; Rafnsson, V. Prevalence of autism spectrum disorders in an icelandic birth cohort. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Leventhal, B.L.; Koh, Y.J.; Fombonne, E.; Laska, E.; Lim, E.C.; Cheon, K.A.; Kim, S.J.; Kim, Y.K.; Lee, H.; et al. Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 2011, 168, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Halladay, A.K.; Bishop, S.; Constantino, J.N.; Daniels, A.M.; Koenig, K.; Palmer, K.; Messinger, D.; Pelphrey, K.; Sanders, S.J.; Singer, A.T.; et al. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, C.; Cederlund, M.; Lamberg, K.; Zeijlon, L. Brief report: “The autism epidemic”. The registered prevalence of autism in a swedish urban area. J. Autism Dev. Disord. 2006, 36, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Auyeung, B.; Chakrabarti, B.; Baron-Cohen, S. Sex/gender differences and autism: Setting the scene for future research. J. Am. Acad. Child. Adolesc. Psychiatry 2015, 54, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Frazier, T.W.; Georgiades, S.; Bishop, S.L.; Hardan, A.Y. Behavioral and cognitive characteristics of females and males with autism in the simons simplex collection. J. Am. Acad. Child. Adolesc. Psychiatry 2014, 53, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Zwaigenbaum, L.; Bryson, S.E.; Szatmari, P.; Brian, J.; Smith, I.M.; Roberts, W.; Vaillancourt, T.; Roncadin, C. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. J. Autism Dev. Disord. 2012, 42, 2585–2596. [Google Scholar] [CrossRef] [PubMed]
- Gockley, J.; Willsey, A.J.; Dong, S.; Dougherty, J.D.; Constantino, J.N.; Sanders, S.J. The female protective effect in autism spectrum disorder is not mediated by a single genetic locus. Mol. Autism 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Baron-Cohen, S.; Buxbaum, J.D. Understanding autism in the light of sex/gender. Mol. Autism 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. The extreme male brain theory of autism. Trends Cogn. Sci. 2002, 6, 248–254. [Google Scholar] [CrossRef]
- Polanczyk, G.; de Lima, M.S.; Horta, B.L.; Biederman, J.; Rohde, L.A. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am. J. Psychiatry 2007, 164, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J. Attention-deficit hyperactivity disorder. Lancet 2005, 366, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Pliszka, S.R. The neuropsychopharmacology of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Pliszka, S.R. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol. Biochem. Behav. 2011, 99, 211–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biederman, J.; Arnsten, A.F.; Faraone, S.V.; Doyle, A.E.; Spencer, T.J.; Wilens, T.E.; Weiss, M.D.; Safren, S.A.; Culpepper, L. New developments in the treatment of adhd. J. Clin. Psychiatry 2006, 67, 148–159. [Google Scholar] [PubMed]
- Solanto, M.V.; Marks, D.J.; Wasserstein, J.; Mitchell, K.; Abikoff, H.; Alvir, J.M.; Kofman, M.D. Efficacy of meta-cognitive therapy for adult adhd. Am. J. Psychiatry 2010, 167, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Balint, S.; Czobor, P.; Komlosi, S.; Meszaros, A.; Simon, V.; Bitter, I. Attention deficit hyperactivity disorder (ADHD): Gender- and age-related differences in neurocognition. Psychol. Med. 2009, 39, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Spencer, T.; Wilens, T. Evidence-based pharmacotherapy for attention-deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. Off. Sci. J. Coll. Int. Neuropsychopharmacol. (CINP) 2004, 7, 77–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaub, M.; Carlson, C.L. Gender differences in adhd: A meta-analysis and critical review. J. Am. Acad. Child. Adolesc. Psychiatry 1997, 36, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Graetz, B.W.; Sawyer, M.G.; Baghurst, P. Gender differences among children with DSM-IV ADHD in Australia. J. Am. Acad Child. Adolesc Psychiatry 2005, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Rucklidge, J.J. Gender differences in ADHD: Implications for psychosocial treatments. Expert Rev. Neurother. 2008, 8, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: A meta-analytic review. Neurotherapeutics 2012, 9, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Arnett, A.B.; Pennington, B.F.; Willcutt, E.G.; DeFries, J.C.; Olson, R.K. Sex differences in ADHD symptom severity. J. Child. Psychol. Psychiatry 2015, 56, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Davies, W. Sex differences in attention deficit hyperactivity disorder: Candidate genetic and endocrine mechanisms. Front. Neuroendocrinol. 2014, 35, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Lahey, B.B.; Applegate, B.; McBurnett, K.; Biederman, J.; Greenhill, L.; Hynd, G.W.; Barkley, R.A.; Newcorn, J.; Jensen, P.; Richters, J.; et al. DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am. J. Psychiatry 1994, 151, 1673–1685. [Google Scholar] [PubMed]
- Willcutt, E.G.; Nigg, J.T.; Pennington, B.F.; Solanto, M.V.; Rohde, L.A.; Tannock, R.; Loo, S.K.; Carlson, C.L.; McBurnett, K.; Lahey, B.B. Validity of DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. J. Abnorm. Psychol. 2012, 121, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M. Age and gender effects in EEG coherence: III. Girls with attention-deficit/hyperactivity disorder. Clin. Neurophysiol. 2006, 117, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Hermens, D.F.; Soei, E.X.; Clarke, S.D.; Kohn, M.R.; Gordon, E.; Williams, L.M. Resting EEG theta activity predicts cognitive performance in attention-deficit hyperactivity disorder. Pediatr. Neurol. 2005, 32, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Hermens, D.F.; Williams, L.M.; Lazzaro, I.; Whitmont, S.; Melkonian, D.; Gordon, E. Sex differences in adult ADHD: A double dissociation in brain activity and autonomic arousal. Biol. Psychol. 2004, 66, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Walters, R.K.; Demontis, D.; Mattheisen, M.; Lee, S.H.; Robinson, E.; Brikell, I.; Ghirardi, L.; Larsson, H.; Lichtenstein, P.; et al. A genetic investigation of sex bias in the prevalence of attention-deficit/hyperactivity disorder. Biol. Psychiatry 2018, 83, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Mink, J.W. Recent advances in tourette syndrome research. Trends Neurosci. 2006, 29, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M. Gilles de la tourette syndrome: The complexities of phenotype and treatment. Br. J. Hosp. Med. 2011, 72, 100–107. [Google Scholar] [CrossRef]
- Singer, H.S.; Walkup, J.T. Tourette syndrome and other tic disorders. Diagnosis, pathophysiology, and treatment. Medicine 1991, 70, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, A.; Mosallaei, S. Psychiatric disorders and behavioral problems in children and adolescents with tourette syndrome. Brain Dev. 2009, 31, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.C.; Piedad, J.; Rickards, H.; Cavanna, A.E. The role of impulse control disorders in tourette syndrome: An exploratory study. J. Neurol. Sci. 2011, 310, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.; Silbersweig, D.A.; Chee, K.Y.; Holmes, A.; Robertson, M.M.; Trimble, M.; Frith, C.D.; Frackowiak, R.S.; Dolan, R.J. A functional neuroanatomy of tics in tourette syndrome. Arch. Gen. Psychiatry 2000, 57, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Minzer, K.; Lee, O.; Hong, J.J.; Singer, H.S. Increased prefrontal d2 protein in tourette syndrome: A postmortem analysis of frontal cortex and striatum. J. Neurol. Sci. 2004, 219, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, D.L.; Christian, B.T.; Gelfand, M.J.; Shi, B.; Mantil, J.; Sallee, F.R. Altered mesolimbocortical and thalamic dopamine in tourette syndrome. Neurology 2006, 67, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.D.; Ko, J.H.; Kideckel, D.M.; Rusjan, P.; Houle, S.; Sandor, P.; Lang, A.E.; Strafella, A.P. Extrastriatal dopaminergic dysfunction in tourette syndrome. Ann. Neurol. 2010, 67, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.; Schoenefeld, K.; Munchau, A.; Roessner, V. Neuromodulation in tourette syndrome: Dopamine and beyond. Neurosci. Biobehav. Rev. 2013, 37, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Shaw, Z.A.; Coffey, B.J. Tics and tourette syndrome. Psychiatr. Clin. N. Am. 2014, 37, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Egolf, A.; Coffey, B.J. Current pharmacotherapeutic approaches for the treatment of tourette syndrome. Drugs Today 2014, 50, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Cavanna, A.E. The effectiveness of habit reversal therapy in the treatment of tourette syndrome and other chronic tic disorders: A systematic review. Funct. Neurol. 2013, 28, 7–12. [Google Scholar] [PubMed]
- Wile, D.J.; Pringsheim, T.M. Behavior therapy for tourette syndrome: A systematic review and meta-analysis. Curr. Treat. Opt. Neurol. 2013, 15, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Kurlan, R. Tourette syndrome: Evolving concepts. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, S.L.; Pauls, D.L.; Goldstein, J.M.; Faraone, S.V.; Tsuang, M.T.; Leckman, J.F. Tourette’s syndrome: What are the influences of gender and comorbid obsessive-compulsive disorder? J. Am. Acad. Child. Adolesc. Psychiatry 1994, 33, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, T.L.; Singer, H.S.; Denckla, M.B.; Rubin, M.A.; Abrams, M.T.; Colli, M.J.; Reiss, A.L. Corpus callosum morphology in children with tourette syndrome and attention deficit hyperactivity disorder. Neurology 1996, 47, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Mostofsky, S.H.; Wendlandt, J.; Cutting, L.; Denckla, M.B.; Singer, H.S. Corpus callosum measurements in girls with tourette syndrome. Neurology 1999, 53, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Fahim, C.; Yoon, U.; Das, S.; Lyttelton, O.; Chen, J.; Arnaoutelis, R.; Rouleau, G.; Sandor, P.; Frey, K.; Brandner, C.; et al. Somatosensory-motor bodily representation cortical thinning in tourette: Effects of tic severity, age and gender. Cortex 2010, 46, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Lichter, D.G.; Finnegan, S.G. Influence of gender on tourette syndrome beyond adolescence. Eur. Psychiatry J. Assoc. Eur. Psychiatry 2015, 30, 334–340. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Welham, J.; El Saadi, O.; MacCauley, C.; Chant, D. A systematic review of the incidence of schizophrenia: The distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004, 2, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castle, D.J.; Wessely, S.; Murray, R.M. Sex and schizophrenia: Effects of diagnostic stringency, and associations with and premorbid variables. Br. J. Psychiatry 1993, 162, 658–664. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Van der Werf, M.; Hanssen, M.; Kohler, S.; Verkaaik, M.; Verhey, F.R.; van Winkel, R.; van Os, J.; Allardyce, J. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol. Med. 2014, 44, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Lozano, A.M. Parkinson’s disease. First of two parts. N. Engl. J. Med. 1998, 339, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Brotchie, J.M.; Lee, J.; Venderova, K. Levodopa-induced dyskinesia in parkinson’s disease. J. Neural Transm. 2005, 112, 359–391. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Lesage, S.; Brice, A. Parkinson’s disease: From monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009, 18, R48–R59. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.; Breteler, M.M. Epidemiology of parkinson’s disease. Lancet Neurol. 2006, 5, 525–535. [Google Scholar] [CrossRef]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. Ilsa working group. Italian longitudinal study on aging. Neurology 2000, 55, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Wooten, G.F.; Currie, L.J.; Bovbjerg, V.E.; Lee, J.K.; Patrie, J. Are men at greater risk for parkinson’s disease than women? J. Neurol. Neurosurg. Psychiatry 2004, 75, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Murray, H.E.; Pillai, A.V.; McArthur, S.R.; Razvi, N.; Datla, K.P.; Dexter, D.T.; Gillies, G.E. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: Differential actions of estrogen in males and females. Neuroscience 2003, 116, 213–222. [Google Scholar] [CrossRef]
- Leranth, C.; Roth, R.H.; Elsworth, J.D.; Naftolin, F.; Horvath, T.L.; Redmond, D.E., Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: Implications for parkinson’s disease and memory. J. Neurosci. 2000, 20, 8604–8609. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, I.; Keller-McGandy, C.; Bouzou, B.; Asteris, G.; Clark, T.W.; Frosch, M.P.; Standaert, D.G. Effects of gender on nigral gene expression and parkinson disease. Neurobiol. Dis. 2007, 26, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohjalainen, T.; Rinne, J.O.; Någren, K.; Syvälahti, E.; Hietala, J. Sex differences in the striatal dopamine d2 receptor binding characteristics in vivo. Am. J. Psychiatry 1998, 155, 768–773. [Google Scholar] [PubMed]
- Huisman, M.H.B.; de Jong, S.W.; van Doormaal, P.T.C.; Weinreich, S.S.; Schelhaas, H.J.; van der Kooi, A.J.; de Visser, M.; Veldink, J.H.; van den Berg, L.H. Population based epidemiology of amyotrophic lateral sclerosis using capture–recapture methodology. J. Neurol. Neurosurg. Psychiatry 2011, 82, 1165–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [Green Version]
- Ou, S.; Wu, F.; Harrich, D.; García-Martínez, L.F.; Gaynor, R.B. Cloning and characterization of a novel cellular protein, tdp-43, that binds to human immunodeficiency virus type 1 tar DNA sequence motifs. J. Virol. 1995, 69, 3584–3596. [Google Scholar] [PubMed]
- Buratti, E.; Baralle, F.E. Multiple roles of tdp-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008, 13, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated tdp-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Arai, T.; Hasegawa, M.; Akiyama, H.; Ikeda, K.; Nonaka, T.; Mori, H.; Mann, D.; Tsuchiya, K.; Yoshida, M.; Hashizume, Y. Tdp-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006, 351, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Buratti, E.; Baralle, F.E. The molecular links between tdp-43 dysfunction and neurodegeneration. Adv. Genet. 2009, 66, 1–34. [Google Scholar] [PubMed]
- Ravits, J.M.; La Spada, A.R. Als motor phenotype heterogeneity, focality, and spread. Neurology 2009, 73, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Chen, H.; Wirdefeldt, K.; Ronnevi, L.-O.; Al-Chalabi, A.; Peters, T.L.; Kamel, F.; Ye, W. Infection of the central nervous system, sepsis and amyotrophic lateral sclerosis. PLoS ONE 2011, 6, e29749. [Google Scholar] [CrossRef] [PubMed]
- Logroscino, G.; Traynor, B.J.; Hardiman, O.; Chiò, A.; Mitchell, D.; Swingler, R.J.; Millul, A.; Benn, E.; Beghi, E. Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 2009, 81, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worms, P.M. The epidemiology of motor neuron diseases: A review of recent studies. J. Neurol. Sci. 2001, 191, 3–9. [Google Scholar] [CrossRef]
- Haverkamp, L.J.; Appel, V.; Appel, S.H. Natural history of amyotrophic lateral sclerosis in a database population validation of a scoring system and a model for survival prediction. Brain 1995, 118, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Manjaly, Z.R.; Scott, K.M.; Abhinav, K.; Wijesekera, L.; Ganesalingam, J.; Goldstein, L.H.; Janssen, A.; Dougherty, A.; Willey, E.; Stanton, B.R. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotroph. Lateral Scler. 2010, 11, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Traynor, B.; Codd, M.; Corr, B.; Forde, C.; Frost, E.; Hardiman, O. Incidence and prevalence of als in ireland, 1995–1997 a population-based study. Neurology 1999, 52, 504. [Google Scholar] [CrossRef] [PubMed]
- Abhinav, K.; Stanton, B.; Johnston, C.; Hardstaff, J.; Orrell, R.; Howard, R.; Clarke, J.; Sakel, M.; Ampong, M.-A.; Shaw, C. Amyotrophic lateral sclerosis in south-east england: A population-based study. Neuroepidemiology 2007, 29, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Chio, A.; Meineri, P.; Tribolo, A.; Schiffer, D. Risk factors in motor neuron disease: A case-control study. Neuroepidemiology 1991, 10, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.A.; Jordan, C.L.; Breedlove, S.M. Sexual differentiation of the vertebrate nervous system. Nat. Neurosci. 2004, 7, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blekhman, R.; Marioni, J.C.; Zumbo, P.; Stephens, M.; Gilad, Y. Sex-specific and lineage-specific alternative splicing in primates. Genome Res. 2010, 20, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lahr, G.; Maxson, S.C.; Mayer, A.; Just, W.; Pilgrim, C.; Reisert, I. Transcription of the y chromosomal gene, sry, in adult mouse brain. Mol. Brain Res. 1995, 33, 179–182. [Google Scholar] [CrossRef]
- Mayer, A.; Lahr, G.; Swaab, D.F.; Pilgrim, C.; Reisert, I. The y-chromosomal genes sry and zfy are transcribed in adult human brain. Neurogenetics 1998, 1, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Reinius, B.; Saetre, P.; Leonard, J.A.; Blekhman, R.; Merino-Martinez, R.; Gilad, Y.; Jazin, E. An evolutionarily conserved sexual signature in the primate brain. PLoS Genet. 2008, 4, e1000100. [Google Scholar] [CrossRef] [PubMed]
- Vawter, M.P.; Evans, S.; Choudary, P.; Tomita, H.; Meador-Woodruff, J.; Molnar, M.; Li, J.; Lopez, J.F.; Myers, R.; Cox, D. Gender-specific gene expression in post-mortem human brain: Localization to sex chromosomes. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004, 29, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Burgoyne, P.S.; Arnold, A.P. Sex differences in sex chromosome gene expression in mouse brain. Hum. Mol. Genet. 2002, 11, 1409–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Schadt, E.E.; Wang, S.; Wang, H.; Arnold, A.P.; Ingram-Drake, L.; Drake, T.A.; Lusis, A.J. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006, 16, 995–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galfalvy, H.C.; Erraji-Benchekroun, L.; Smyrniotopoulos, P.; Pavlidis, P.; Ellis, S.P.; Mann, J.J.; Sibille, E.; Arango, V. Sex genes for genomic analysis in human brain: Internal controls for comparison of probe level data extraction. BMC Bioinform. 2003, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Quinn, N.P.; Marsden, C.D. Menstrual-related fluctuations in parkinson’s disease. Mov. Disord. 1986, 1, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C.; Reingold, S.C.; Looney, P.A. A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef] [PubMed]
- Merlo, S.; Spampinato, S.F.; Sortino, M.A. Estrogen and alzheimer’s disease: Still an attractive topic despite disappointment from early clinical results. Eur. J. Pharmacol. 2017, 817, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Currie, L.J.; Harrison, M.B.; Trugman, J.M.; Bennett, J.P.; Wooten, G.F. Postmenopausal estrogen use affects risk for parkinson disease. Arch. Neurol. 2004, 61, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Tsang, K.L.; Ho, S.L.; Lo, S.K. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 2000, 54, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Saunders-Pullman, R.; Gordon-Elliott, J.; Parides, M.; Fahn, S.; Saunders, H.R.; Bressman, S. The effect of estrogen replacement on early parkinson’s disease. Neurology 1999, 52, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Finkelsztejn, A.; Brooks, J.; Paschoal, F., Jr.; Fragoso, Y. What can we really tell women with multiple sclerosis regarding pregnancy? A systematic review and meta-analysis of the literature. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 790–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillies, G.E.; Murray, H.E.; Dexter, D.; McArthur, S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of parkinson’s disease. Pharmacol. Biochem. Behav. 2004, 78, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Bebo, B.F.; Fyfe-Johnson, A.; Adlard, K.; Beam, A.G.; Vandenbark, A.A.; Offner, H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 2001, 166, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.C.; Rosario, E.R.; Chang, L.; Stanczyk, F.Z.; Oddo, S.; LaFerla, F.M.; Pike, C.J. Progesterone and estrogen regulate alzheimer-like neuropathology in female 3xtg-ad mice. J. Neurosci. 2007, 27, 13357–13365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, H.; Uljon, S.N.; Gross, R.; Hardy, K.; Gaynor, J.; Lafrancois, J.; Simpkins, J.; Refolo, L.M.; Petanceska, S.; et al. Modulation of a(beta) peptides by estrogen in mouse models. J. Neurochem. 2002, 80, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, J.; Chen, S.; Irwin, R.W.; Iwamoto, S.; Brinton, R.D. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Green, P.S.; Gridley, K.E.; Simpkins, J.W. Estradiol protects against β-amyloid (25–35)-induced toxicity in sk-n-sh human neuroblastoma cells. Neurosci. Lett. 1996, 218, 165–168. [Google Scholar] [CrossRef]
- Hickey, M.; Bryant, C.; Judd, F. Evaluation and management of depressive and anxiety symptoms in midlife. Climacteric J. Int. Menopause Soc. 2012, 15, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pigott, T.A. Anxiety disorders in women. Psychiatr. Clin. N. Am. 2003, 26, 621–672. [Google Scholar] [CrossRef]
- Ross, L.E.; McLean, L.M. Anxiety disorders during pregnancy and the postpartum period: A systematic review. J. Clin. Psychiatry 2006, 67, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Van Veen, J.F.; Jonker, B.W.; van Vliet, I.M.; Zitman, F.G. The effects of female reproductive hormones in generalized social anxiety disorder. Int. J. Psychiatry Med. 2009, 39, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Vesga-Lopez, O.; Blanco, C.; Keyes, K.; Olfson, M.; Grant, B.F.; Hasin, D.S. Psychiatric disorders in pregnant and postpartum women in the united states. Arch. Gen. Psychiatry 2008, 65, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Walf, A.A.; Frye, C.A. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2006, 31, 1097–1111. [Google Scholar] [CrossRef] [PubMed]
- Weissman, M.M. Treatment of depression: Men and women are different? Am. J. Psychiatry 2014, 171, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Riecher Rossler, A. Can estradiol modulate schizophrenic symptomatology? Schizophr. Bull. 1994, 20, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Gattaz, W.F.; Vogel, P.; Riecher-Rössler, A.; Soddu, G. Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biol. Psychiatry 1994, 36, 137–139. [Google Scholar] [CrossRef]
- Hallonquist, J.D.; Seeman, M.V.; Lang, M.; Rector, N.A. Variation in symptom severity over the menstrual cycle of schizophrenics. Biol. Psychiatry 1993, 33, 207–209. [Google Scholar] [CrossRef]
- Kulkarni, J.; Riedel, A.; de Castella, A.R.; Fitzgerald, P.B.; Rolfe, T.J.; Taffe, J.; Burger, H. Estrogen—A potential treatment for schizophrenia. Schizophr. Res. 2001, 48, 137–144. [Google Scholar] [CrossRef]
- Riecher-Rossler, A.; Kulkarni, J. Estrogens and gonadal function in schizophrenia and related psychoses. Curr. Top. Behav. Neurosci. 2011, 8, 155–171. [Google Scholar] [PubMed]
- Österlund, M.K.; Gustafsson, J.-A.K.; Keller, E.; Hurd, Y.L. Estrogen receptor β (erβ) messenger ribonucleic acid (mRNA) expression within the human forebrain: Distinct distribution pattern to erα mRNA. J. Clin. Endocrinol. Metab. 2000, 85, 3840–3846. [Google Scholar] [PubMed]
- Österlund, M.K.; Hurd, Y.L. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog. Neurobiol. 2001, 64, 251–267. [Google Scholar] [CrossRef]
- Östlund, H.; Keller, E.; Hurd, Y.L. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann. N. Y. Acad. Sci. 2003, 54–63. [Google Scholar] [CrossRef]
- Rissman, E.F.; Heck, A.L.; Leonard, J.E.; Shupnik, M.A.; Gustafsson, J.-Å. Disruption of estrogen receptor β gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. USA 2002, 99, 3996–4001. [Google Scholar] [CrossRef] [PubMed]
- Rissman, E.F.; Wersinger, S.R.; Fugger, H.N.; Foster, T.C. Sex with knockout models: Behavioral studies of estrogen receptor α1. Brain Res. 1999, 835, 80–90. [Google Scholar] [CrossRef]
- Liu, F.; Day, M.; Muniz, L.C.; Bitran, D.; Arias, R.; Revilla-Sanchez, R.; Grauer, S.; Zhang, G.; Kelley, C.; Pulito, V. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat. Neurosci. 2008, 11, 334–343. [Google Scholar] [CrossRef] [PubMed]
- D’Astous, M.; Morissette, M.; Di Paolo, T. Effect of estrogen receptor agonists treatment in mptp mice: Evidence of neuroprotection by an er alpha agonist. Neuropharmacology 2004, 47, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Morissette, M.; Jourdain, S.; Al Sweidi, S.; Menniti, F.S.; Ramirez, A.D.; Di Paolo, T. Role of estrogen receptors in neuroprotection by estradiol against mptp toxicity. Neuropharmacology 2007, 52, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Honda, S.; Harada, N. Alteration in sex-specific behaviors in male mice lacking the aromatase gene. Neuroendocrinology 2003, 77, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Matsumoto, T.; Kawano, H.; Watanabe, T.; Uematsu, Y.; Sekine, K.; Fukuda, T.; Aihara, K.; Krust, A.; Yamada, T.; et al. Brain masculinization requires androgen receptor function. Proc. Natl. Acad. Sci. USA 2004, 101, 1673–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, M. Early androgen influences on human neural and behavioural development. Early Hum. Dev. 2008, 84, 805–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G.; Hines, M. Fetal testosterone predicts sexually differentiated childhood behavior in girls and in boys. Psychol. Sci. 2009, 20, 144–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, B.; Baron-Cohen, S.; Ashwin, E.; Knickmeyer, R.; Taylor, K.; Hackett, G. Fetal testosterone and autistic traits. Br. J. Psychol. 2009, 100, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, J.T.; Scutt, D.; Wilson, J.; Lewis-Jones, D.I. The ratio of 2nd to 4th digit length: A predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum. Reprod. 1998, 13, 3000–3004. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Koonce, C.J.; Edinger, K.L.; Osborne, D.M.; Walf, A.A. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm. Behav. 2008, 54, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodosy, J.; Zelmanova, D.; Majzunova, M.; Filova, B.; Malinova, M.; Ostatnikova, D.; Celec, P. The anxiolytic effect of testosterone in the rat is mediated via the androgen receptor. Pharmacol. Biochem. Behav. 2012, 102, 191–195. [Google Scholar] [CrossRef] [PubMed]
- McDermott, C.M.; Liu, D.; Schrader, L.A. Role of gonadal hormones in anxiety and fear memory formation and inhibition in male mice. Physiol. Behav. 2012, 105, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Leckman, J.F.; Scahill, L. Possible exacerbation of tics by androgenic steroids. N. Engl. J. Med. 1990, 322, 1674. [Google Scholar] [CrossRef] [PubMed]
- Muroni, A.; Paba, S.; Puligheddu, M.; Marrosu, F.; Bortolato, M. A preliminary study of finasteride in tourette syndrome. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 2146–2147. [Google Scholar] [CrossRef] [PubMed]
- Bebo, B.F.; Zelinka-Vincent, E.; Adamus, G.; Amundson, D.; Vandenbark, A.A.; Offner, H. Gonadal hormones influence the immune response to plp 139–151 and the clinical course of relapsing experimental autoimmmune encephalomyelitis. J. Neuroimmunol. 1998, 84, 122–130. [Google Scholar] [CrossRef]
- Khasnavis, S.; Ghosh, A.; Roy, A.; Pahan, K. Castration induces parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J. Biol. Chem. 2013, 288, 20843–20855. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.; Isles, A.R.; Wilkinson, L.S. Imprinted gene expression in the brain. Neurosci. Biobehav. Rev. 2005, 29, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kopsida, E.; Stergiakouli, E.; Lynn, P.M.; Wilkinson, L.S.; Davies, W. The role of the y chromosome in brain function. Open Neuroendocrinol. J. 2009, 2, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, L.; Li, H.M.; Tang, Y.L.; Wu, Z.M.; Chen, Y.; Wang, Y.F.; Qian, Q.J. Interactions between maoa and syp polymorphisms were associated with symptoms of attention-deficit/hyperactivity disorder in chinese han subjects. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168B, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Carriere, I.; Ritchie, K.; Ancelin, M.L. Involvement of gpr50 polymorphisms in depression: Independent replication in a prospective elderly cohort. Brain Behav. 2015, 5, e00313. [Google Scholar] [CrossRef] [PubMed]
- Czech, D.P.; Lee, J.; Sim, H.; Parish, C.L.; Vilain, E.; Harley, V.R. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J. Neurochem. 2012, 122, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Piton, A.; Gauthier, J.; Hamdan, F.F.; Lafreniere, R.G.; Yang, Y.; Henrion, E.; Laurent, S.; Noreau, A.; Thibodeau, P.; Karemera, L.; et al. Systematic resequencing of x-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol. Psychiatry 2011, 16, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, S.; Swaab, H. Executive dysfunction and the relation with behavioral problems in children with 47,xxy and 47,xxx. Genes Brain Behav. 2015, 14, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Lyon, M.F. Gene action in the x-chromosome of the mouse (mus musculus l.). Nature 1961, 190, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in x-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Taya, S.; Kaibuchi, K.; Arnold, A.P. Sexually dimorphic expression of usp9x is related to sex chromosome complement in adult mouse brain. Eur. J. Neurosci. 2005, 21, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Deng, X.; Watkins, R.; Disteche, C.M. Sex-specific differences in expression of histone demethylases utx and uty in mouse brain and neurons. J. Neurosci. 2008, 28, 4521–4527. [Google Scholar] [CrossRef] [PubMed]
- Russell, H.F.; Wallis, D.; Mazzocco, M.M.; Moshang, T.; Zackai, E.; Zinn, A.R.; Ross, J.L.; Muenke, M. Increased prevalence of adhd in turner syndrome with no evidence of imprinting effects. J. Pediatr. Psychol. 2006, 31, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Skuse, D.H. Imprinting, the x-chromosome, and the male brain: Explaining sex differences in the liability to autism. Pediatr. Res. 2000, 47, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.I.; Chue, P.S.; Tibbo, P. Investigation of turner syndrome in schizophrenia. Am. J. Med. Genet. 2000, 96, 373–378. [Google Scholar] [CrossRef]
- Davies, W.; Humby, T.; Isles, A.R.; Burgoyne, P.S.; Wilkinson, L.S. X-monosomy effects on visuospatial attention in mice: A candidate gene and implications for turner syndrome and attention deficit hyperactivity disorder. Biol. Psychiatry 2007, 61, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Laumonnier, F.; Bonnet-Brilhault, F.; Gomot, M.; Blanc, R.; David, A.; Moizard, M.P.; Raynaud, M.; Ronce, N.; Lemonnier, E.; Calvas, P.; et al. X-linked mental retardation and autism are associated with a mutation in the nlgn4 gene, a member of the neuroligin family. Am. J. Hum. Genet. 2004, 74, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the x-linked genes encoding neuroligins nlgn3 and nlgn4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, S.M.; Pfaff, D.W. Etiologies underlying sex differences in autism spectrum disorders. Front. Neuroendocrinol. 2014, 35, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.L.; Liu, X.; Schutz, C.; White, B.N.; Jenkins, E.C.; Brown, W.T.; Holden, J.J. Association of autism severity with a monoamine oxidase a functional polymorphism. Clin. Genet. 2003, 64, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.L.; Liu, X.; Lewis, M.E.; Chudley, A.; Forster-Gibson, C.; Gonzalez, M.; Jenkins, E.C.; Brown, W.T.; Holden, J.J. Autism severity is associated with child and maternal maoa genotypes. Clin. Genet. 2011, 79, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tassone, F.; Qi, L.; Zhang, W.; Hansen, R.L.; Pessah, I.N.; Hertz-Picciotto, I. Maoa, dbh, and slc6a4 variants in charge: A case-control study of autism spectrum disorders. Autism Res. Off. J. Int. Soc. Autism Res. 2011, 4, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Bhowmik, A.D.; Sinha, S.; Chattopadhyay, A.; Chaudhuri, K.; Singh, M.; Mukhopadhyay, K. Maoa promoter polymorphism and attention deficit hyperactivity disorder (adhd) in indian children. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Warwick, M.M.; Doody, G.A.; Lawrie, S.M.; Kestelman, J.N.; Best, J.J.; Johnstone, E.C. Volumetric magnetic resonance imaging study of the brain in subjects with sex chromosome aneuploidies. J. Neurol. Neurosurg. Psychiatry 1999, 66, 628–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartaglia, N.R.; Ayari, N.; Hutaff-Lee, C.; Boada, R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: Xxy, xxx, xyy, and xxyy. J. Dev. Behav. Pediatr. JDBP 2012, 33, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Lynn, P.M.; Davies, W. The 39,xo mouse as a model for the neurobiology of turner syndrome and sex-biased neuropsychiatric disorders. Behav. Brain Res. 2007, 179, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Reik, W.; Walter, J. Genomic imprinting: Parental influence on the genome. Nat. Rev. Genet. 2001, 2, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Skuse, D.H.; James, R.S.; Bishop, D.V.; Coppin, B.; Dalton, P.; Aamodt-Leeper, G.; Bacarese-Hamilton, M.; Creswell, C.; McGurk, R.; Jacobs, P.A. Evidence from turner’s syndrome of an imprinted x-linked locus affecting cognitive function. Nature 1997, 387, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Canning, E.; Elgar, K.; Morris, E.; Jacobs, P.; Skuse, D. Distinctive patterns of memory function in subgroups of females with turner syndrome: Evidence for imprinted loci on the x-chromosome affecting neurodevelopment. Neuropsychologia 2000, 38, 712–721. [Google Scholar] [CrossRef]
- Davies, W.; Isles, A.; Smith, R.; Karunadasa, D.; Burrmann, D.; Humby, T.; Ojarikre, O.; Biggin, C.; Skuse, D.; Burgoyne, P.; et al. Xlr3b is a new imprinted candidate for x-linked parent-of-origin effects on cognitive function in mice. Nat. Genet. 2005, 37, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.; Jentsch, J.D. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology 2012, 219, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Jobling, M.A.; Tyler-Smith, C. The human y chromosome: An evolutionary marker comes of age. Nat. Rev. Genet. 2003, 4, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. Y chromosome’s roles in sex differences in disease. Proc. Natl. Acad. Sci. USA 2017, 114, 3787–3789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgoyne, P.S. The role of the mammalian y chromosome in spermatogenesis. Development 1987, 101, 133–141. [Google Scholar] [PubMed]
- Goodfellow, P.N.; Lovell-Badge, R. Sry and sex determination in mammals. Annu. Rev. Genet. 1993, 27, 71–92. [Google Scholar] [CrossRef] [PubMed]
- Lahn, B.T.; Page, D.C. Functional coherence of the human y chromosome. Science 1997, 278, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Zeger, M.P.; Kushner, H.; Zinn, A.R.; Roeltgen, D.P. An extra x or y chromosome: Contrasting the cognitive and motor phenotypes in childhood in boys with 47,xyy syndrome or 47,xxy klinefelter syndrome. Dev. Disabil. Res. Rev. 2009, 15, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ruud, A.; Arnesen, P.; Stray, L.L.; Vildalen, S.; Vesterhus, P. Stimulant medication in 47,xyy syndrome: A report of two cases. Dev. Med. Child Neurol. 2005, 47, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.-M.; Lovell-Badge, R.; Goodfellow, P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Koopman, P.; Munsterberg, A.; Capel, B.; Vivian, N.; Lovell-Badge, R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature 1990, 348, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Mosler, G.; Just, W.; Pilgrim, C.; Reisert, I. Developmental profile of sry transcripts in mouse brain. Neurogenetics 2000, 3, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Milsted, A.; Serova, L.; Sabban, E.L.; Dunphy, G.; Turner, M.E.; Ely, D.L. Regulation of tyrosine hydroxylase gene transcription by SRY. Neurosci. Lett. 2004, 369, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Chen, K.; Li, Y.; Lau, Y.-F.C.; Shih, J.C. Regulation of monoamine oxidase a by the sry gene on the y chromosome. FASEB J. 2009, 23, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Ely, D.; Underwood, A.; Dunphy, G.; Boehme, S.; Turner, M.; Milsted, A. Review of the y chromosome, sry and hypertension. Steroids 2010, 75, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.E.; Farkas, J.; Dunmire, J.; Ely, D.; Milsted, A. Which SRY locus is the hypertensive y chromosome locus? Hypertension 2009, 53, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Czech, D.P.; Lee, J.; Correia, J.; Loke, H.; Moller, E.; Vilain, E.; Harley, V.R. Transient neuroprotection by sry up-regulation in dopamine cells following injury in males. Endocrinology 2014, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.L.; Tartaglia, N.; Merry, D.E.; Dalva, M.; Zinn, A.R. Behavioral phenotypes in males with xyy and possible role of increased nlgn4y expression in autism features. Genes Brain Behav. 2015, 14, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Williams-Burris, S.M.; McClusky, R.; Ngun, T.C.; Ghahramani, N.; Barseghyan, H.; Reue, K.; Vilain, E.; Arnold, A.P. The sex chromosome trisomy mouse model of xxy and xyy: Metabolism and motor performance. Biol. Sex. Differ. 2013, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Fillit, H.; Weinreb, H.; Cholst, I.; Luine, V.; McEwen, B.; Amador, R.; Zabriskie, J. Observations in a preliminary open trial of estradiol therapy for senile dementia-alzheimer’s type. Psychoneuroendocrinology 1986, 11, 337–345. [Google Scholar] [CrossRef]
- Ohkura, T.; Isse, K.; Akazawa, K.; Hamamoto, M.; Yaoi, Y.; Hagino, N. Evaluation of estrogen treatment in female patients with dementia of the alzheimer type. Endocr. J. 1994, 41, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Asthana, S.; Craft, S.; Baker, L.D.; Raskind, M.A.; Birnbaum, R.S.; Lofgreen, C.P.; Veith, R.C.; Plymate, S.R. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with alzheimer’s disease: Results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology 1999, 24, 657–678. [Google Scholar] [CrossRef]
- Tang, M.-X.; Jacobs, D.; Stern, Y.; Marder, K.; Schofield, P.; Gurland, B.; Andrews, H.; Mayeux, R. Effect of oestrogen during menopause on risk and age at onset of alzheimer’s disease. Lancet 1996, 348, 429–432. [Google Scholar] [CrossRef]
- Sicotte, N.L.; Liva, S.M.; Klutch, R.; Pfeiffer, P.; Bouvier, S.; Odesa, S.; Wu, T.J.; Voskuhl, R.R. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2002, 52, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, A.; Kumar, R.; Everitt, B.; Studd, J. Transdermal oestrogen for treatment of severe postnatal depression. Lancet 1996, 347, 930–933. [Google Scholar] [CrossRef]
- Tan, R.; Pu, S. A pilot study on the effects of testosterone in hypogonadal aging male patients with alzheimer’s disease. Aging Male 2003, 6, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sicotte, N.L.; Giesser, B.S.; Tandon, V.; Klutch, R.; Steiner, B.; Drain, A.E.; Shattuck, D.W.; Hull, L.; Wang, H.-J.; Elashoff, R.M. Testosterone treatment in multiple sclerosis: A pilot study. Arch. Neurol. 2007, 64, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Okun, M.S.; Walter, B.L.; McDonald, W.M.; Tenover, J.L.; Green, J.; Juncos, J.L.; DeLong, M.R. Beneficial effects of testosterone replacement for the nonmotor symptoms of parkinson disease. Arch. Neurol. 2002, 59, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinares-Garcia, P.; Stratikopoulos, M.; Zagato, A.; Loke, H.; Lee, J. Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sci. 2018, 8, 154. https://doi.org/10.3390/brainsci8080154
Pinares-Garcia P, Stratikopoulos M, Zagato A, Loke H, Lee J. Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sciences. 2018; 8(8):154. https://doi.org/10.3390/brainsci8080154
Chicago/Turabian StylePinares-Garcia, Paulo, Marielle Stratikopoulos, Alice Zagato, Hannah Loke, and Joohyung Lee. 2018. "Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders" Brain Sciences 8, no. 8: 154. https://doi.org/10.3390/brainsci8080154
APA StylePinares-Garcia, P., Stratikopoulos, M., Zagato, A., Loke, H., & Lee, J. (2018). Sex: A Significant Risk Factor for Neurodevelopmental and Neurodegenerative Disorders. Brain Sciences, 8(8), 154. https://doi.org/10.3390/brainsci8080154





