Musical Experience, Sensorineural Auditory Processing, and Reading Subskills in Adults
Abstract
1. Introduction
2. Materials and Methods
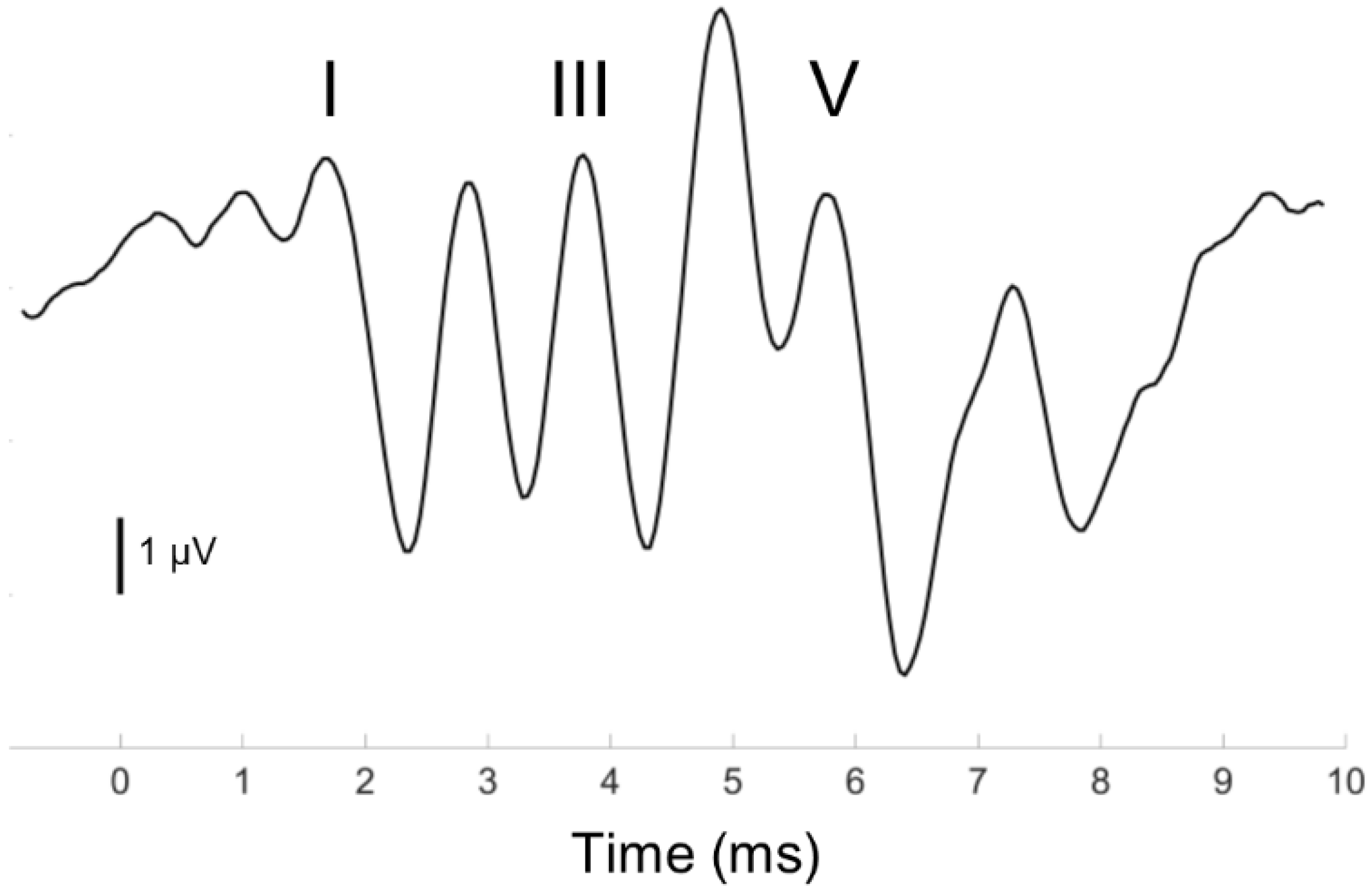
2.1. Auditory Brainstem Response (ABR) Protocol
2.2. Standardized Reading Battery
2.3. Music Questionnaire
3. Results
3.1. Statistical and Analysis Software
3.2. Participants
3.3. Reading Subskills and Non-Verbal IQ
3.4. Auditory Brainstem Response
3.5. Music Training Histories
3.6. ABR: Repeated-Measures Analyses
3.7. Principal Components Analysis: Dimensionality Reduction for Sensorineural Auditory Processing, Reading-Related Skills, and Musical Training Measures
3.7.1. PCA: Reading Tests
3.7.2. PCA: ABR Indices
3.7.3. PCA: Musical Training Variables
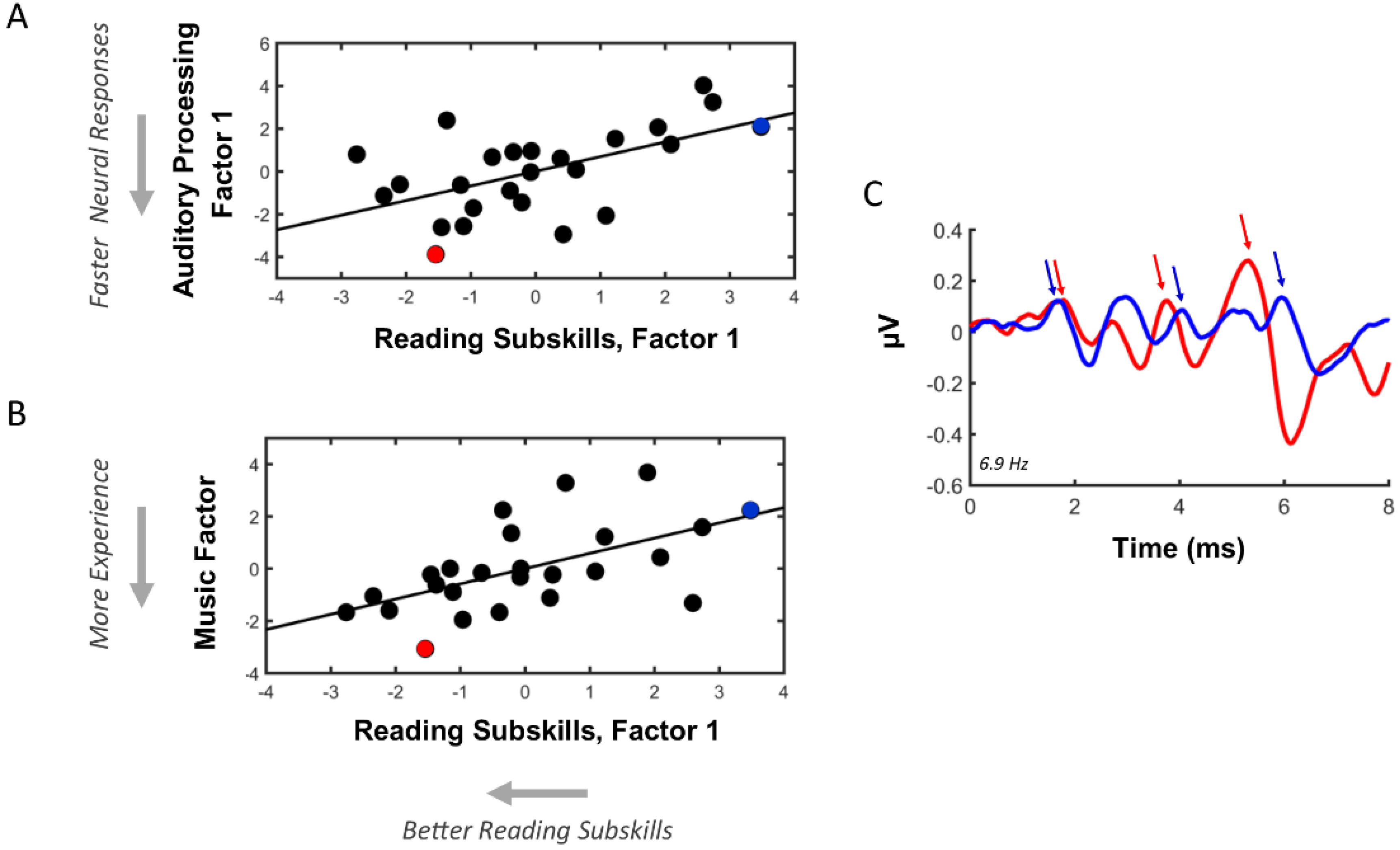
3.8. Correlation Analyses between Principal Components
3.8.1. Question 1: Is sensorineural Auditory Processing Related to Phonological and Rapid Naming Skills in Adulthood?
3.8.2. Question 2: Do Differences in Music-Training History Relate to Phonological and Rapid Naming Skills in Adulthood?
3.8.3. Question 3: Do Differences in Music-Training History Relate to Sensorineural Auditory Processing?
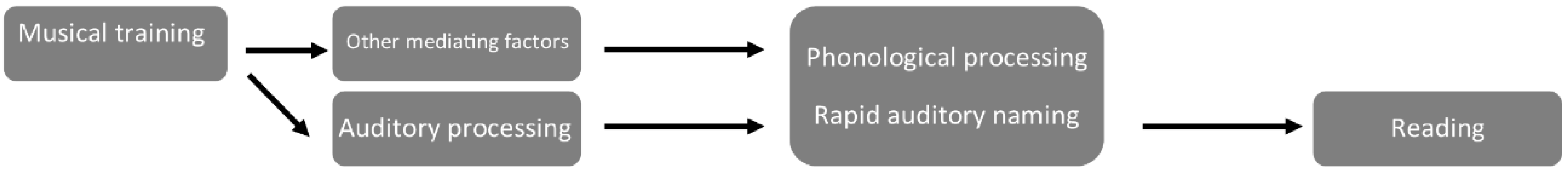
4. Discussion
4.1. Summary of Findings
4.2. Limitations and Future Work
4.3. Investigating Musical Training, Senorineural Processing, and Reading Across the Lifespan
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bradley, L.; Bryant, P.E. Categorizing sounds and learning to read—A causal connection. Nature 1983, 301, 419–421. [Google Scholar] [CrossRef]
- Bentin, S. Phonological Awareness, Reading, and Reading Acquisition: A Survey and Appraisal of Current Knowledge. Haskins Lab. Status Rep. Speech Res. 1992, 112, 167–180. [Google Scholar] [CrossRef]
- Manis, F.R.; Seidenberg, M.S.; Doi, L.M. See Dick RAN: Rapid Naming and the Longitudinal Prediction of Reading Subskills in First and Second Graders. Sci. Stud. Read. 1999, 3, 129–157. [Google Scholar] [CrossRef]
- Tallal, P.; Gaab, N. Dynamic auditory processing, musical experience and language development. Trends Neurosci. 2006, 29, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Huss, M.; Verney, J.P.; Fosker, T.; Mead, N.; Goswami, U. Music, rhythm, rise time perception and developmental dyslexia: Perception of musical meter predicts reading and phonology. Cortex 2011, 47, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F. Language discrimination by newborns: Teasing apart phonotactic, rhythmic, and intonational cues. Annu. Rev. Lang. Acquis. 2002, 2, 85–115. [Google Scholar] [CrossRef]
- Ramus, F.; Nespor, M.; Mehler, J. Correlates of linguistic rhythm in the speech signal. Cognition 1999, 73, 265–292. [Google Scholar] [CrossRef]
- Tallal, P.; Miller, S.; Fitch, R. Neuropsychological Bases of Speech: A case for the Preeminance of Temporal Processing. Ann. N. Y. Acad. Sci. 1993, 682, 37–41. [Google Scholar] [CrossRef]
- Protopapas, A. From temporal processing to developmental language disorders: Mind the gap. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2014, 369, 20130090. [Google Scholar] [CrossRef] [PubMed]
- Holliman, A.J.; Wood, C.; Sheehy, K. Does speech rhythm sensitivity predict children’s reading ability 1 year later? J. Educ. Psychol. 2010, 102, 356–366. [Google Scholar] [CrossRef]
- Tallal, P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980, 9, 182–198. [Google Scholar] [CrossRef]
- McAnally, K.I.; Stein, J.F. Auditory temporal coding in dyslexia. Proc. Biol. Sci. 1996, 263, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Boets, B.; Vandermosten, M.; Poelmans, H.; Luts, H.; Wouters, J.; Ghesquière, P. Preschool impairments in auditory processing and speech perception uniquely predict future reading problems. Res. Dev. Disabil. 2011, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Benasich, A.A.; Tallal, P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav. Brain Res. 2002, 136, 31–49. [Google Scholar] [CrossRef]
- Banai, K.; Ahissar, M. Musical Experience, Auditory Perception and Reading-Related Skills in Children. PLoS ONE 2013, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Anderson, S. For Reading Development, Auditory Processing Is Fundamental. Hear. J. 2013, 66, 40. [Google Scholar] [CrossRef]
- Hornickel, J.; Kraus, N. Unstable representation of sound: A biological marker of dyslexia. J. Neurosci. 2013, 33, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.; Kraus, N. Music Training for the Development of Reading Skills, 1st ed.; Elsevier B.V.: New York, NY, USA, 2013; Volume 207, ISBN 9780444633279. [Google Scholar]
- Gervain, J.; Mehler, J. Speech perception and language acquisition in the first year of life. Annu. Rev. Psychol. 2010, 61, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U. A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 2011, 15, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Boets, B.; Wouters, J.; van Wieringen, A.; De Smedt, B.; Ghesquière, P. Modelling relations between sensory processing, speech perception, orthographic and phonological ability, and literacy achievement. Brain Lang. 2008, 106, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Saffran, J.; Aslin, R.; Newport, E. Statistical learning by 8-month-old infants. Science 1996, 274, 1926–1928. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Anderson, S.; Skoe, E.; Yi, H.H.-G.; Kraus, N. Subcortical representation of speech fine structure relates to reading ability. Neuroreport 2012, 23, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Banai, K.; Hornickel, J.; Skoe, E.; Nicol, T.; Zecker, S.; Kraus, N. Reading and subcortical auditory function. Cereb. Cortex 2009, 19, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Strait, D.L.; Hornickel, J.; Kraus, N. Subcortical processing of speech regularities underlies reading and music aptitude in children. Behav. Brain Funct. 2011, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Chandrasekaran, B.; Zecker, S.; Kraus, N. Auditory brainstem measures predict reading and speech-in-noise perception in school-aged children. Behav. Brain Res. 2011, 216, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Knowles, E.; Kraus, N. Test-retest consistency of speech-evoked auditory brainstem responses in typically-developing children. Hear. Res. 2012, 284, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Skoe, E.; Nicol, T.; Zecker, S.; Kraus, N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc. Natl. Acad. Sci. USA 2009, 106, 13022–13027. [Google Scholar] [CrossRef] [PubMed]
- Neef, N.E.; Müller, B.; Liebig, J.; Schaadt, G.; Grigutsch, M.; Gunter, T.C.; Wilcke, A.; Kirsten, H.; Skeide, M.A.; Kraft, I.; et al. Dyslexia risk gene relates to representation of sound in the auditory brainstem. Dev. Cogn. Neurosci. 2017, 24, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Zecker, S.G.; Bradlow, A.R.; Kraus, N. Assistive listening devices drive neuroplasticity in children with dyslexia. Proc. Natl. Acad. Sci. USA 2012, 109, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Brody, L.; Theodore, R.M. Reading ability reflects individual differences in auditory brainstem function, even into adulthood. Brain Lang. 2017, 164, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Kraus, N. Musical training heightens auditory brainstem function during sensitive periods in development. Front. Psychol. 2013, 4, 622. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.; Krizman, J.; Skoe, E.; Johnston, K.; Kraus, N. High school music classes enhance the neural processing of speech. Front. Psychol. 2013, 4, 855. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.; Tierney, A.; Kraus, N. At-risk elementary school children with one year of classroom music instruction are better at keeping a beat. PLoS ONE 2013, 8, e77250. [Google Scholar] [CrossRef] [PubMed]
- Samelli, A.; Carvallo, R.M.; de Beija, C.; Rabelo, C.; Matas, C.; Gomes, R.; Magliaro, F.L. Audiological and electrophysiological assessment of professional pop/rock musicians. Noise Health 2012, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Parbery-Clark, A.; Anderson, S.; Hittner, E.; Kraus, N. Musical experience strengthens the neural representation of sounds important for communication in middle-aged adults. Front. Aging Neurosci. 2012, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Kraus, N. A Little Goes a Long Way: How the Adult Brain Is Shaped by Musical Training in Childhood. J. Neurosci. 2012, 32, 11507–11510. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Hornickel, J.; Skoe, E. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: Implications for developmental dyslexia. Neuron 2009, 64, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Parbery-Clark, A.; Tierney, A.; Strait, D.L.; Kraus, N. Musicians have fine-tuned neural distinction of speech syllables. Neuroscience 2012, 219, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.; Skoe, E.; Russo, N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat. Neurosci. 2007, 10, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Bidelman, G.M.; Krishnan, A. Effects of reverberation on brainstem representation of speech in musicians and non-musicians. Brain Res. 2010, 1355, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Bidelman, G. Listening to the Brainstem: Musicianship Enhances Intelligibility of Subcortical Representations for Speech. J. Neurosci. 2015, 35, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Musacchia, G.; Sams, M.; Skoe, E.; Kraus, N. Musicians have enhanced subcortical auditory and audiovisual processing of speech and music. Proc. Natl. Acad. Sci. USA 2007, 104, 15894–15898. [Google Scholar] [CrossRef] [PubMed]
- Strait, D.L.; Parbery-Clark, A.; Hittner, E.; Kraus, N. Musical training during early childhood enhances the neural encoding of speech in noise. Brain Lang. 2012, 123, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D. Why would Musical Training Benefit the Neural Encoding of Speech? The OPERA Hypothesis. Front. Psychol. 2011, 2, 142. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D. Can nonlinguistic musical training change the way the brain processes speech? The expanded OPERA hypothesis. Hear. Res. 2014, 308, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Anvari, S.H.; Trainor, L.J.; Woodside, J.; Levy, B.A. Relations among musical skills, phonological processing, and early reading ability in preschool children. J. Exp. Child Psychol. 2002, 83, 111–130. [Google Scholar] [CrossRef]
- Forgeard, M.; Schlaug, G.; Norton, A.; Rosam, C.; Iyengar, U.; Winner, E. The relation between music and phonological processing in normal-reading children and children with dyslexia. Music Percept. Interdiscip. J. 2008, 25, 383–390. [Google Scholar] [CrossRef]
- David, D.; Wade-Woolley, L.; Kirby, J.R.; Smithrim, K. Rhythm and reading development in school-age children: A longitudinal study. J. Res. Read. 2007, 30, 169–183. [Google Scholar] [CrossRef]
- Peynircioglu, Z.F.; Durgunoglu, A.Y.; Uney-Kusefoglu, B. Phonological awareness and musical aptitude. J. Res. Read. 2002, 25, 68–80. [Google Scholar] [CrossRef]
- Degé, F.; Kubicek, C.; Schwarzer, G. Associations between musical abilities and precursors of reading in preschool aged children. Front. Psychol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ganschow, L.; Lloyd-Jones, J.; Miles, T.R. Dyslexia and musical notation. Ann. Dyslexia 1994, 44, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U.; Huss, M.; Mead, N.; Fosker, T.; Verney, J.P. Perception of patterns of musical beat distribution in phonological developmental dyslexia: Significant longitudinal relations with word reading and reading comprehension. Cortex 2013, 49, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Woodruff Carr, K.; White-Schwoch, T.; Tierney, A.T.; Strait, D.L.; Kraus, N. Beat synchronization predicts neural speech encoding and reading readiness in preschoolers. Proc. Natl. Acad. Sci. USA 2014, 111, 14559–14564. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.; Willatts, P. The relationship between musical ability and literacy skills. J. Res. Read. 1994, 17, 99–107. [Google Scholar] [CrossRef]
- Hämäläinen, J.A.; Salminen, H.K.; Leppänen, P.H.T. Basic Auditory Processing Deficits in Dyslexia: Systematic Review of the Behavioral and Event-Related Potential/Field Evidence. J. Learn. Disabil. 2013, 46, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Friesen, D.; Bialystok, E. Effect of music training on promoting preliteracy skills: Preliminary causal evidence. Music Percept. 2011, 29, 165–172. [Google Scholar] [CrossRef]
- Habib, M.; Lardy, C.; Desiles, T.; Commeiras, C.; Chobert, J.; Besson, M. Music and Dyslexia: A New Musical Training Method to Improve Reading and Related Disorders. Front. Psychol. 2016, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Bhide, A.; Power, A.; Goswami, U. A Rhythmic Musical Intervention for Poor Readers: A Comparison of Efficacy With a Letter-Based Intervention. Mind Brain Educ. 2013, 7, 113–123. [Google Scholar] [CrossRef]
- Cogo-Moreira, H.; Brandão de Ávila, C.R.; Ploubidis, G.B.; Mari, J.D.J. Effectiveness of music education for the improvement of reading skills and academic achievement in young poor readers: A pragmatic cluster-randomized, controlled clinical trial. PLoS ONE 2013, 8, e59984. [Google Scholar] [CrossRef] [PubMed]
- Overy, K. Dyslexia and Music: From Timing Deficits to Musical Intervention. Ann. N. Y. Acad. Sci. 2003, 29, 1–9. [Google Scholar] [CrossRef]
- Rautenberg, I. The effects of musical training on the decoding skills of German-speaking primary school children. J. Res. Read. 2015, 38, 1–17. [Google Scholar] [CrossRef]
- Register, D.; Darrow, A.A.; Swedberg, O.; Standley, J. The Use of Music to Enhance Reading Skills of Second Grade Students and Students with Reading Disabilities. J. Music Ther. 2007, 44, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Magne, C.; Besson, M. Musicians and the Metric Structure of Words. J. Cogn. Neurosci. 2011, 23, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Bishop-Liebler, P.; Welch, G.; Huss, M.; Thomson, J.M.; Goswami, U. Auditory temporal processing skills in musicians with dyslexia. Dyslexia 2014, 20, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Granot, R.; Ahissar, M. The enigma of dyslexic musicians. Neuropsychologia 2014, 54, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.L.; Fehd, H.M.; McCandliss, B.D. Does Music Training Enhance Literacy Skills? A Meta-Analysis. Front. Psychol. 2015, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.A.; Nicol, T.; Zecker, S.; Kraus, N. Abnormal Cortical Processing of the Syllable Rate of Speech in Poor Readers. J. Neurosci. 2009, 29, 7686–7693. [Google Scholar] [CrossRef] [PubMed]
- Leong, V.; Goswami, U. Assessment of rhythmic entrainment at multiple timescales in dyslexia: Evidence for disruption to syllable timing. Hear. Res. 2014, 308, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Rosen, S. Temporal Information in Speech: Acoustic, Auditory and Linguistic Aspects. Philos. Trans. R. Soc. B Biol. Sci. 1992, 336, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Slater, J.; Thompson, E.C.; Hornickel, J.; Strait, D.L.; Nicol, T.; White-Schwoch, T. Music Enrichment Programs Improve the Neural Encoding of Speech in At-Risk Children. J. Neurosci. 2014, 34, 11913–11918. [Google Scholar] [CrossRef] [PubMed]
- Tichko, P.; Skoe, E. Frequency-dependent fine structure in the frequency-following response: The byproduct of multiple generators. Hear. Res. 2017, 348, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Coffey, E.B.J.; Herholz, S.C.; Chepesiuk, A.M.P.; Baillet, S.; Zatorre, R.J. Cortical contributions to the auditory frequency-following response revealed by MEG. Nat. Commun. 2016, 7, 11070. [Google Scholar] [CrossRef] [PubMed]
- Bidelman, G.M. Multichannel recordings of the human brainstem frequency-following response: Scalp topography, source generators, and distinctions from the transient ABR. Hear. Res. 2015, 323, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Bidelman, G.M. Subcortical sources dominate the neuroelectric auditory frequency-following response to speech. Neuroimage 2018. [Google Scholar] [CrossRef] [PubMed]
- Hall, J. Handbook of Auditory Evoked Responses; Allyn and Bacon: Needham, MA, USA, 1992. [Google Scholar]
- Eggermont, J.J.; Don, M. Mechanisms of Central Conduction Time Prolongation in Brain-Stem Auditory Evoked Potentials. Arch. Neurol. 1986, 43, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Poeppel, D.; Idsardi, W.J.; van Wassenhove, V.; Wassenhove, V. Van Speech perception at the interface of neurobiology and linguistics. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Tufts, J. Evidence of noise-induced subclinical hearing loss using auditory brainstem responses and objective measures of noise exposure in humans. Hear. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liberman, M.C.; Epstein, M.J.; Cleveland, S.S.; Wang, H.; Maison, S.F. Toward a differential diagnosis of hidden hearing loss in humans. PLoS ONE 2016, 11, e0162726. [Google Scholar] [CrossRef] [PubMed]
- Reiman, M.; Parkkola, R.; Johansson, R.; Jääskeläinen, S.K.; Kujari, H.; Lehtonen, L.; Haataja, L.; Lapinleimu, H. Diffusion tensor imaging of the inferior colliculus and brainstem auditory-evoked potentials in preterm infants. Pediatr. Radiol. 2009, 39, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Otto-Meyer, S.; Krizman, J.; White-Schwoch, T.; Kraus, N. Children with autism spectrum disorder have unstable neural responses to sound. Exp. Brain Res. 2018, 236, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Corrigall, K.; Trainor, L. Associations between length of music training and reading skills in children. Music Percept. 2011, 29, 147–155. [Google Scholar] [CrossRef]
- Slater, J.; Kraus, N. The role of rhythm in perceiving speech in noise: A comparison of percussionists, vocalists and non-musicians. Cogn. Process. 2016, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Krizman, J.; Anderson, S.; Kraus, N. Stability and plasticity of auditory brainstem function across the lifespan. Cereb. Cortex 2015, 25, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Chiappa, K.; Gladstone, K.; Young, R. Brain stem auditory evoked responses: Studies of waveform variations in 50 normal human subjects. Arch. Neurol. 1979, 36, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Don, M.; Allen, A.R.; Starr, A. Effect of Click Rate on the Latency of Auditory Brain Stem Responses in Humans. Ann. Otol. Rhinol. Laryngol. 1977, 86, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Moore, E.; Schaefer, R.; Bastin, M.; Roberts, N.; Overy, K. Can Musical Training Influence Brain Connectivity? Evidence from Diffusion Tensor MRI. Brain Sci. 2014, 4, 405–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Knösche, T.R. Intracortical myelination in musicians with absolute pitch: Quantitative morphometry using 7-T MRI. Hum. Brain Mapp. 2016, 37, 3486–3501. [Google Scholar] [CrossRef] [PubMed]
- Imfeld, A.; Oechslin, M.S.; Meyer, M.; Loenneker, T.; Jancke, L. White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage 2009, 46, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Giacosa, C.; Karpati, F.J.; Foster, N.E.V.; Penhune, V.B.; Hyde, K.L. Dance and music training have different effects on white matter diffusivity in sensorimotor pathways. Neuroimage 2016, 135, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Boebinger, D.; Evans, S.; Rosen, S.; Lima, C.F.; Manly, T.; Scott, S.K.; Lima, C.F.; Manly, T.; Scott, S.K. Musicians and non-musicians are equally adept at perceiving masked speech. J. Acoust. Soc. Am. 2015, 137, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.; Skoe, E.; Strait, D.L.; O’Connell, S.; Thompson, E.; Kraus, N.; O’Connell, S.; Thompson, E.; Kraus, N. Music training improves speech-in-noise perception: Longitudinal evidence from a community-based music program. Behav. Brain Res. 2015, 291, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Ruggles, D.R.; Freyman, R.L.; Oxenham, A.J. Influence of musical training on understanding voiced and whispered speech in noise. PLoS ONE 2014, 9, e86980. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U. Sensory theories of developmental dyslexia: Three challenges for research. Nat. Rev. Neurosci. 2015, 16, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Ho, Y.C.; Cheung, M.C. Music training improves verbal memory. Nature 1998, 396, 128. [Google Scholar] [CrossRef] [PubMed]
- Hanna-pladdy, B.; Mackay, A. The relation between instrumental musical activity and cognitive aging. Neuropsychology 2011, 25, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-C.; Cheung, M.-C.; Chan, A.S. Music training improves verbal but not visual memory: Cross-sectional and longitudinal explorations in children. Neuropsychology 2003, 17, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Parbery-Clark, A.; Skoe, E.; Kraus, N. Musical experience limits the degradative effects of background noise on the neural processing of sound. J. Neurosci. 2009, 29, 14100–14107. [Google Scholar] [CrossRef] [PubMed]
- Bigand, E.; Delbé, C.; Poulin-Charronnat, B.; Leman, M.; Tillmann, B. Empirical evidence for musical syntax processing? Computer simulations reveal the contribution of auditory short-term memory. Front. Syst. Neurosci. 2014, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.; Strait, D.L.; Parbery-Clark, A. Cognitive factors shape brain networks for auditory skills: Spotlight on auditory working memory. Ann. N. Y. Acad. Sci. 2012, 1252, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Parbery-Clark, A.; Skoe, E.; Lam, C.; Kraus, N. Musician enhancement for speech-in-noise. Ear Hear. 2009, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Law, L.N.C.; Zentner, M. Assessing Musical Abilities Objectively: Construction and Validation of the Profile of Music Perception Skills. PLoS ONE 2012, 7, e52508. [Google Scholar] [CrossRef] [PubMed]
- Skoe, E.; Krizman, J.; Kraus, N. The impoverished brain: Disparities in maternal education affect the neural response to sound. J. Neurosci. 2013, 33, 17221–17231. [Google Scholar] [CrossRef] [PubMed]
- Hille, K.; Gust, K.; Bitz, U.; Kammer, T. Associations between music education, intelligence, and spelling ability in elementary school. Adv. Cogn. Psychol. 2011, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]

| Test | Subtest | Description |
|---|---|---|
| Test of Non-Verbal Intelligence (TONI-3) | Non-Verbal IQ | Untimed task. Select an illustration from a set of illustrations to complete a visual puzzle |
| Comprehensive Test of Phonological Processing (CTOPP) | Elision | Untimed task. Say part of a word after saying the whole word (e.g., Say the word ‘spider’. Now say ‘spider’ without saying ‘der’) |
| Blending Words | Untimed task. Combine sounds of a word to form one word (e.g., ‘can’ + ‘dy’ = ‘candy’) | |
| Non-Word Repetition | Untimed task. Repeat back a list of non-words | |
| RAN (Rapid Automatized Naming) | RAN Numbers | Timed task. Read aloud a list of numbers as quickly as possible |
| RAN Letters | Timed task. Read aloud a list of letters as quickly as possible | |
| RAS 2-set | Timed task. Read aloud a list that contains both numbers and letters as quickly as possible |
| Test | Mean (SD) | Range of Standard Scores |
|---|---|---|
| TONI | 102.3 (11.69) | 77–130 |
| CTOPP Elision | 10.08 (1.35) | 7–12 |
| CTOPP Blending Words | 11.16 (1.72) | 8–13 |
| CTOPP Nonword Repetition | 9.8 (1.94) | 7–14 |
| RAN Numbers | 110.1 (4.53) | 102–117 |
| RAN Letters | 108.6 (4.97) | 98–117 |
| RAN 2 Set | 112.1 (6.11) | 100–125 |
| Rate | Wave III | Wave V | Response Consistency (RC) |
|---|---|---|---|
| 6.9 Hz | 3.85 (0.18) ms | 5.69 (0.25) ms | 0.80 (0.18) Pearson’s r |
| 31.25 Hz | 3.94 (0.17) ms | 5.75 (0.21)ms | 0.73 (0.20) Pearson’s r |
| 61.5 Hz | 4.04 (0.18) ms | 5.96 (0.24) ms | 0.71 (0.27) Pearson’s r |
| Rate | I–III IPL | I–V IPL |
|---|---|---|
| 6.9 Hz | 2.21 (0.18) ms | 4.04 (0.23) ms |
| 31.25 Hz | 2.26 (0.17) ms | 4.07 (0.20) ms |
| 61.5 Hz | 2.30 (0.15) ms | 4.22 (0.20) ms |
| Musical Training (MT) Variable | Mean (SD) | Range |
|---|---|---|
| Minimum Age of MT | 8.17 (3.30) Years | 2–15 Years |
| Total Years of MT | 8.36 (4.91) Years | 0–20 Years |
| Max Proficiency of MT | 7.78 (2.02) | 3–10 |
| Years Since MT | 1.17 (1.75) Years | 0–5 Years |
| Factor I | Factor II | |
|---|---|---|
| CTOPP Blending Words | −0.30 | −0.64 |
| CTOPP Elision | −0.25 | −0.36 |
| CTOPP Nonword Repetition | −0.21 | −0.52 |
| RAN Letters | −0.53 | 0.28 |
| RAN Numbers | −0.51 | 0.26 |
| RAN 2 Set | −0.51 | 0.21 |
| Proportion of Variance | 0.46 | 0.23 |
| Culmulative Variance | 0.46 | 0.68 |
| Factor I | Factor II | |
|---|---|---|
| RC (6.9 Hz) | 0.02 | −0.65 |
| RC (31.25 Hz) | −0.03 | −0.42 |
| RC (61.5 Hz) | −0.22 | −0.49 |
| I–V IPL (6.9 Hz) | 0.44 | 0.05 |
| I–V IPL (31.25 Hz) | 0.39 | 0.14 |
| I–V IPL (61.5 Hz) | 0.41 | 0.10 |
| I–III IPL (6.9 Hz) | 0.38 | −0.23 |
| I–III IPL (31.25 Hz) | 0.42 | −0.20 |
| I–III IPL (61.5 Hz) | 0.34 | −0.16 |
| Proportion of Variance | 0.45 | 0.20 |
| Cumulative Variance | 0.45 | 0.65 |
| Factor 1 | |
|---|---|
| Max Profiency | −0.53 |
| Total Years of Musical Training | −0.52 |
| Minum Age of Musical Training | 0.47 |
| Years Since Musical Training | 0.48 |
| Proportion of Variance | 0.76 |
| Reading Subtests (Factor 1) | |||
|---|---|---|---|
| Pearson’s R | p-Value | Corrected p-Value | |
| ABR (Factor 1) | 0.56 | 0.003 | 0.014 |
| ABR (Factor 2) | 0.25 | 0.231 | 0.40 |
| Reading Subtests (Factor 2) | |||
| Pearson’s R | p-Value | Corrected p-Value | |
| ABR (Factor 1) | −0.01 | 0.96 | 0.96 |
| ABR (Factor 2) | −0.24 | 0.25 | 0.40 |
| Reading Subtests (Factor 1) | |||
|---|---|---|---|
| Pearson’s R | p-Value | Corrected p-Value | |
| Musical Training (Factor 1) | 0.58 | 0.003 | 0.014 |
| Reading Subtests (Factor 2) | |||
| Pearson’s R | p-Value | Corrected p-Value | |
| Musical Training (Factor 1) | 0.21 | 0.33 | 0.44 |
| ABR (Factor 1) | |||
|---|---|---|---|
| Pearson’s R | p-Value | Corrected p-Value | |
| Musical Training (Factor 1) | 0.42 | 0.044 | 0.119 |
| ABR (Factor 2) | |||
| Pearson’s R | p-Value | Corrected p-Value | |
| Musical Training (Factor 1) | −0.18 | 0.42 | 0.48 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tichko, P.; Skoe, E. Musical Experience, Sensorineural Auditory Processing, and Reading Subskills in Adults. Brain Sci. 2018, 8, 77. https://doi.org/10.3390/brainsci8050077
Tichko P, Skoe E. Musical Experience, Sensorineural Auditory Processing, and Reading Subskills in Adults. Brain Sciences. 2018; 8(5):77. https://doi.org/10.3390/brainsci8050077
Chicago/Turabian StyleTichko, Parker, and Erika Skoe. 2018. "Musical Experience, Sensorineural Auditory Processing, and Reading Subskills in Adults" Brain Sciences 8, no. 5: 77. https://doi.org/10.3390/brainsci8050077
APA StyleTichko, P., & Skoe, E. (2018). Musical Experience, Sensorineural Auditory Processing, and Reading Subskills in Adults. Brain Sciences, 8(5), 77. https://doi.org/10.3390/brainsci8050077




