Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals
Abstract
:1. Introduction
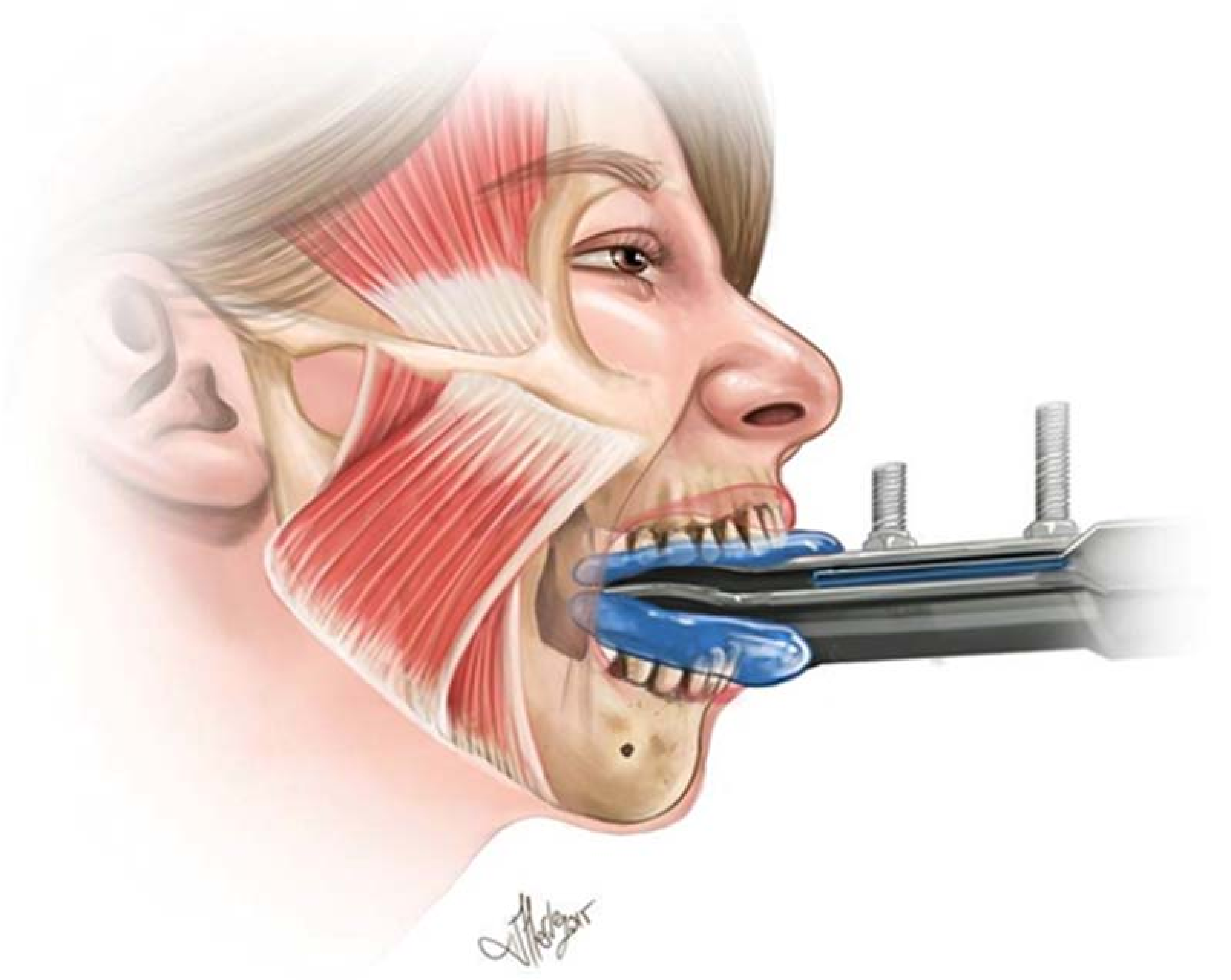
2. Materials and Methods
2.1. Intervention
2.1.1. Spinal Manipulation
2.1.2. Sham Intervention
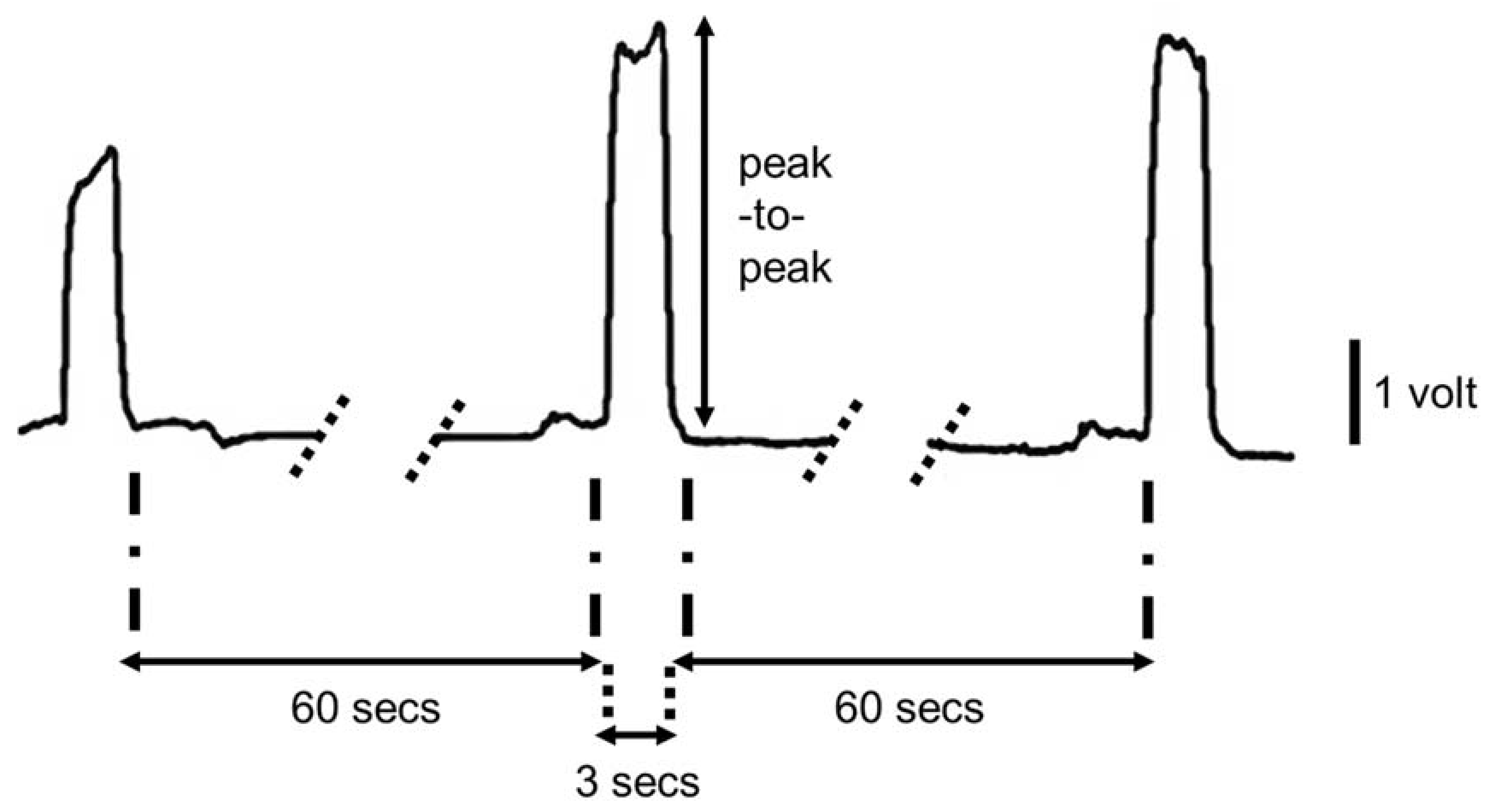
2.2. Data Analysis
2.3. Statistical Analysis
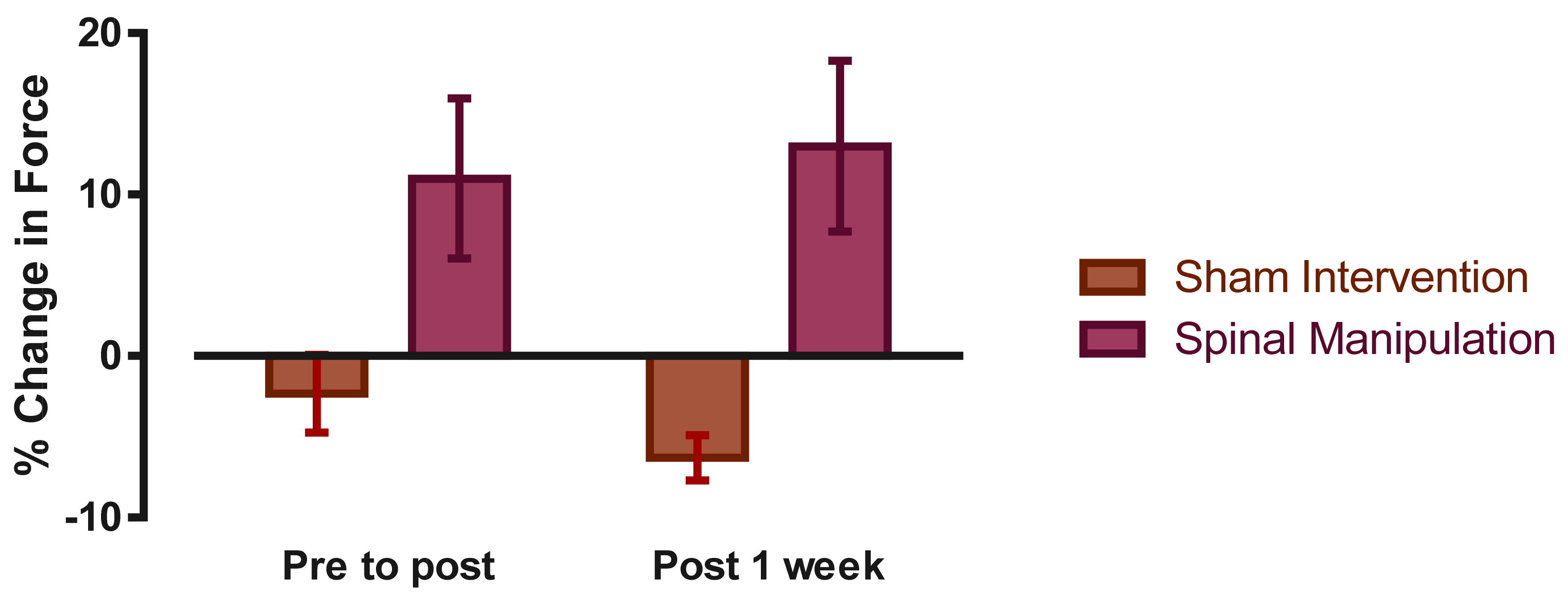
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Haavik, H.; Murphy, B. The role of spinal manipulation in addressing disordered sensorimotor integration and altered motor control. J. Electromyogr. Kinesiol. 2012, 22, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Niazi, I.K.; Jochumsen, M.; Sherwin, D.; Flavel, S.; Türker, K.S. Impact of spinal manipulation on cortical drive to upper and lower limb muscles. Brain Sci. 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Haavik Taylor, H.; Murphy, B. Cervical spine manipulation alters sensorimotor integration: A somatosensory evoked potential study. Clin. Neurophysiol. 2007, 118, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Niazi, I.; Türker, K.S.; Flavel, S.; Kinget, M.; Duehr, J.; Haavik, H. Changes in h-reflex and v waves following spinal manipulation. Exp. Brain Res. 2015, 233, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Lelic, D.; Niazi, I.K.; Holt, K.; Jochumsen, M.; Dremstrup, K.; Yielder, P.; Murphy, B.; Drewes, A.M.; Haavik, H. Manipulation of dysfunctional spinal joints affects sensorimotor integration in the prefrontal cortex: A brain source localization study. Neural Plast. 2016, 2016, 3704964. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, T.; Niazi, I.; Holt, K.; Nederggard, R.; Duehr, J.; Schlupp, V.; Marshal, P.; Türker, K.S.; Hartvigsen, J.; Haavik, H. The effects of a single session of spinal manipulation on strength and cortical drive in athletes. Eur. J. Appl. Physiol. 2018, 118, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Rodine, R.J.; Aker, P. Trigeminal neuralgia and chiropractic care: A case report. J. Can. Chiropr. Assoc. 2010, 54, 177–186. [Google Scholar] [PubMed]
- Mansilla-Ferragut, P.; Fernández-de-las Peñas, C.; Alburquerque-Sendín, F.; Cleland, J.A.; Boscá-Gandía, J.J. Immediate effects of atlanto-occipital joint manipulation on active mouth opening and pressure pain sensitivity in women with mechanical neck pain. J. Manip. Physiol. Ther. 2009, 32, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.A.; Magee, D.J.; Parfitt, M.; Major, P.; Thie, N.M. The association between the cervical spine, the stomatognathic system, and craniofacial pain: A critical review. J. Orofac. Pain 2006, 20, 271–287. [Google Scholar]
- Palinkas, M.; Nassar, M.S.; Cecilio, F.A.; Siessere, S.; Semprini, M.; Machado-de-Sousa, J.P.; Hallak, J.E.; Regalo, S.C. Age and gender influence on maximal bite force and masticatory muscles thickness. Arch. Oral Biol. 2010, 55, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.J.; Pastore, M.G.; Bonjardim, L.R.; Castelo, P.M.; Gaviao, M.B. Molar bite force and its correlation with signs of temporomandibular dysfunction in mixed and permanent dentition. J. Oral Rehabil. 2007, 34, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Roldan, S.; Buschang, P.H.; Isaza Saldarriaga, J.F.; Throckmorton, G. Reliability of maximum bite force measurements in age-varying populations. J. Oral Rehabil. 2009, 36, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Türker, K.S. Reflex control of human jaw muscles. Crit. Rev. Oral Biol. Med. 2002, 13, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.; Kim, J.S.; Valiquette, C.; Lund, J.P. Evidence that periodontal pressoreceptors provide positive feedback to jaw closing muscles during mastication. J. Neurophysiol. 1987, 58, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Chinappi, A.S., Jr.; Getzoff, H. Chiropractic/dental cotreatment of lumbosacral pain with temporomandibular joint involvement. J. Manip. Physiol. Ther. 1996, 19, 607–612. [Google Scholar]
- Chinappi, A.S., Jr.; Getzoff, H. A new management model for treating structural-based disorders: Dental orthopedic and chiropractic co-treatment. J. Manip. Physiol. Ther. 1994, 17, 614–619. [Google Scholar]
- Morimoto, T.; Inoue, T.; Masuda, Y.; Nagashima, T. Sensory components facilitating jaw-closing muscle activities in the rabbit. Exp. Brain Res. 1989, 76, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, N.N.; Hellmann, D.; Schmitter, M.; Kruger, B.; Hauser, T.; Schindler, H.J. Neuromuscular interaction of jaw and neck muscles during jaw clenching. J. Orofac. Pain 2013, 27, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, J.F.; Debuse, T.; Duquesnoy, B. Chronic neck pain and masticatory dysfunction. Joint Bone Spine 2005, 72, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, C.; Celebisoy, N.; Uludag, B. Trigeminocervical reflexes elicited by stimulation of the infraorbital nerve: Head retraction reflex. J. Clin. Neurophysiol. 2001, 18, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, C. Emg versus force relationship in painful masseter muscles before and after intramuscular anesthetics and saline injections. Scand. J. Dent. Res. 1987, 95, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.W.; Yu, X.M.; Vernon, H.; Sessle, B.J. Excitatory effects on neck and jaw muscle activity of inflammatory irritant applied to cervical paraspinal tissues. Pain 1993, 55, 243–250. [Google Scholar] [CrossRef]
- Leandri, M.; Gottlieb, A.; Cruccu, G. Head extensor reflex evoked by trigeminal stimulation in humans. Clin. Neurophysiol. 2001, 112, 1828–1832. [Google Scholar] [CrossRef]
- Triano, J.J.; Budgell, B.; Bagnulo, A.; Roffey, B.; Bergmann, T.; Cooperstein, R.; Gleberzon, B.; Good, C.; Perron, J.; Tepe, R. Review of methods used by chiropractors to determine the site for applying manipulation. Chiropr. Man. Ther. 2013, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Hessell, B.W.; Herzog, W.; Conway, P.J.; McEwen, M.C. Experimental measurement of the force exerted during spinal manipulation using the Thompson technique. J. Manip. Physiol. Ther. 1990, 13, 448–453. [Google Scholar]
- Pickar, J.G.; Wheeler, J.D. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J. Manip. Physiol. Ther. 2001, 24, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Bonjardim, L.R.; Gaviao, M.B.; Pereira, L.J.; Castelo, P.M. Bite force determination in adolescents with and without temporomandibular dysfunction. J. Oral Rehabil. 2005, 32, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Van der Bilt, A.; Tekamp, A.; van der Glas, H.; Abbink, J. Bite force and electromyograpy during maximum unilateral and bilateral clenching. Euro. J. Oral Sci. 2008, 116, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Suter, E.; McMorland, G.; Herzog, W.; Bray, R. Decrease in quadriceps inhibition after sacroiliac joint manipulation in patients with anterior knee pain. J. Manip. Physiol. Ther. 1999, 22, 149–153. [Google Scholar] [CrossRef]
- Hillermann, B.; Gomes, A.N.; Korporaal, C.; Jackson, D. A pilot study comparing the effects of spinal manipulative therapy with those of extra-spinal manipulative therapy on quadriceps muscle strength. J. Manip. Physiol. Ther. 2006, 29, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Botelho, M.B.; Andrade, B.B. Effect of cervical spine manipulative therapy on judo athletes’ grip strength. J. Manip. Physiol. Ther. 2012, 35, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Humphries, K.M.; Ward, J.; Coats, J.; Nobert, J.; Amonette, W.; Dyess, S. Immediate effects of lower cervical spine manipulation on handgrip strength and free-throw accuracy of asymptomatic basketball players: A pilot study. J. Chiropr. Med. 2013, 12, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, P.D.; Cornish, S.M.; Schulte, A.; Jantz, N.; Magnus, C.R.A.; Schwanbeck, S.; Juurlink, B.H.J. The effect of spinal manipulation on imbalances in leg strength. J. Can. Chiropr. Assoc. 2011, 55, 183–192. [Google Scholar] [PubMed]
- Alkjaer, T.; Meyland, J.; Raffalt, P.C.; Lundbye-Jensen, J.; Simonsen, E.B. Neuromuscular adaptations to 4 weeks of intensive drop jump training in well-trained athletes. Physiol. Rep. 2013, 1, e00099. [Google Scholar] [CrossRef] [PubMed]
- Un, C.P.; Lin, K.H.; Shiang, T.Y.; Chang, E.C.; Su, S.C.; Wang, H.K. Comparative and reliability studies of neuromechanical leg muscle performances of volleyball athletes in different divisions. Eur. J. Appl. Physiol. 2013, 113, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. Altered sensorimotor integration with cervical spine manipulation. J. Manip. Physiol. Ther. 2008, 31, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.H.; Murphy, B. Altered central integration of dual somatosensory input after cervical spine manipulation. J. Manip. Physiol. Ther. 2010, 33, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Haavik Taylor, H.; Murphy, B. The effects of spinal manipulation on central integration of dual somatosensory input observed after motor training: A crossover study. J. Manip. Physiol. Ther. 2010, 33, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Haavik, H.; Murphy, B. Subclinical neck pain and the effects of cervical manipulation on elbow joint position sense. J. Manip. Physiol. Ther. 2011, 34, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Baarbe, J.K.; Holmes, M.W.; Murphy, H.E.; Haavik, H.; Murphy, B.A. Influence of subclinical neck pain on the ability to perform a mental rotation task: A 4-week longitudinal study with a healthy control group comparison. J. Manip. Physiol. Ther. 2016, 39, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Farid, B.; Yielder, P.; Holmes, M.; Haavik, H.; Murphy, B.A. Association of subclinical neck pain with altered multisensory integration at baseline and 4-week follow-up relative to asymptomatic controls. J. Manip. Physiol. Ther. 2018, 41, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Daligadu, J.; Haavik, H.; Yielder, P.C.; Baarbe, J.; Murphy, B. Alterations in cortical and cerebellar motor processing in subclinical neck pain patients following spinal manipulation. J. Manip. Physiol. Ther. 2013, 36, 527–537. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haavik, H.; Özyurt, M.G.; Niazi, I.K.; Holt, K.; Nedergaard, R.W.; Yilmaz, G.; Türker, K.S. Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals. Brain Sci. 2018, 8, 76. https://doi.org/10.3390/brainsci8050076
Haavik H, Özyurt MG, Niazi IK, Holt K, Nedergaard RW, Yilmaz G, Türker KS. Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals. Brain Sciences. 2018; 8(5):76. https://doi.org/10.3390/brainsci8050076
Chicago/Turabian StyleHaavik, Heidi, Mustafa Görkem Özyurt, Imran Khan Niazi, Kelly Holt, Rasmus Wiberg Nedergaard, Gizem Yilmaz, and Kemal Sitki Türker. 2018. "Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals" Brain Sciences 8, no. 5: 76. https://doi.org/10.3390/brainsci8050076
APA StyleHaavik, H., Özyurt, M. G., Niazi, I. K., Holt, K., Nedergaard, R. W., Yilmaz, G., & Türker, K. S. (2018). Chiropractic Manipulation Increases Maximal Bite Force in Healthy Individuals. Brain Sciences, 8(5), 76. https://doi.org/10.3390/brainsci8050076






