Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
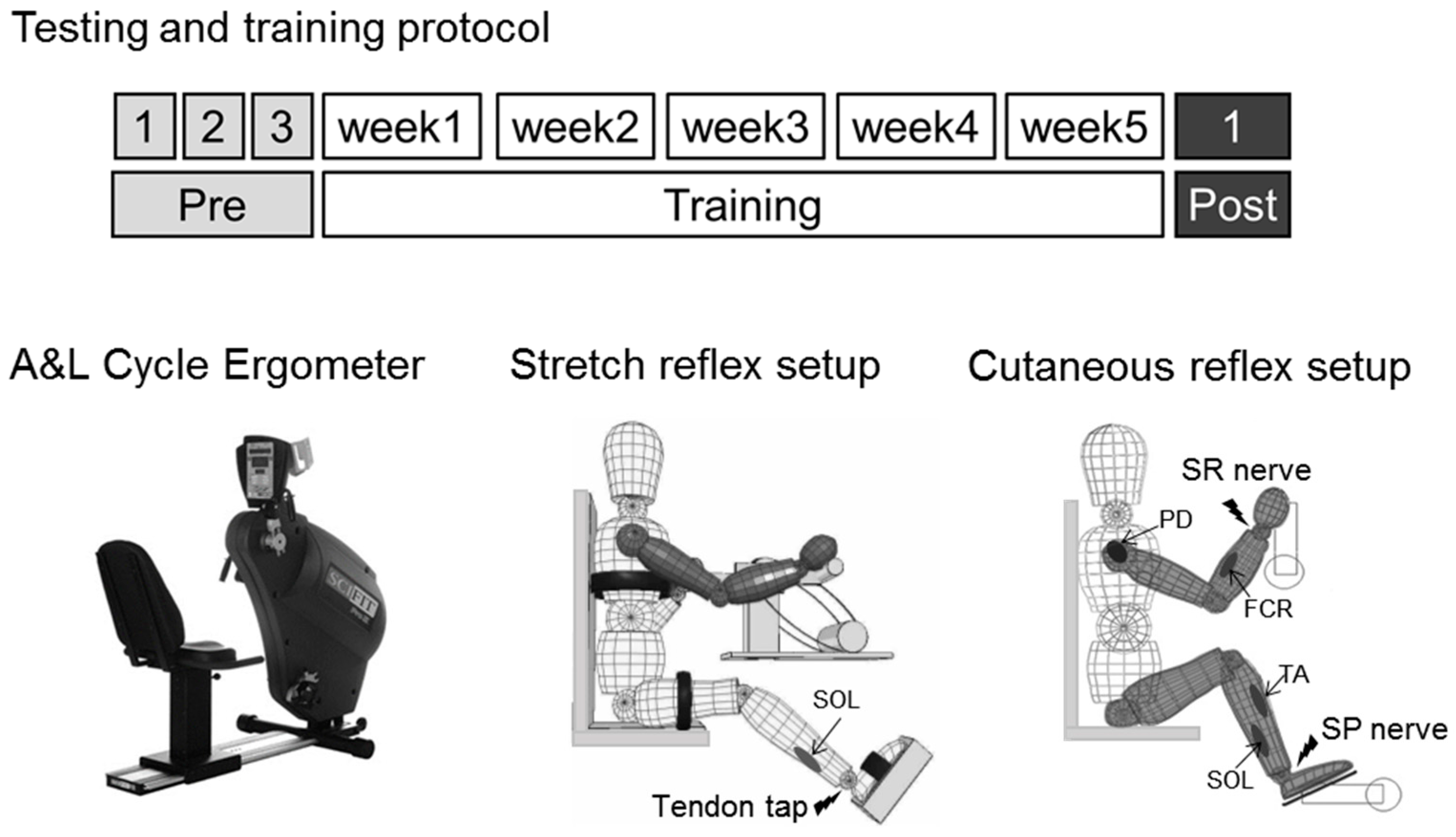
2.2. A & L Cycling Training
2.3. Multiple Baseline and Post-Test Measures
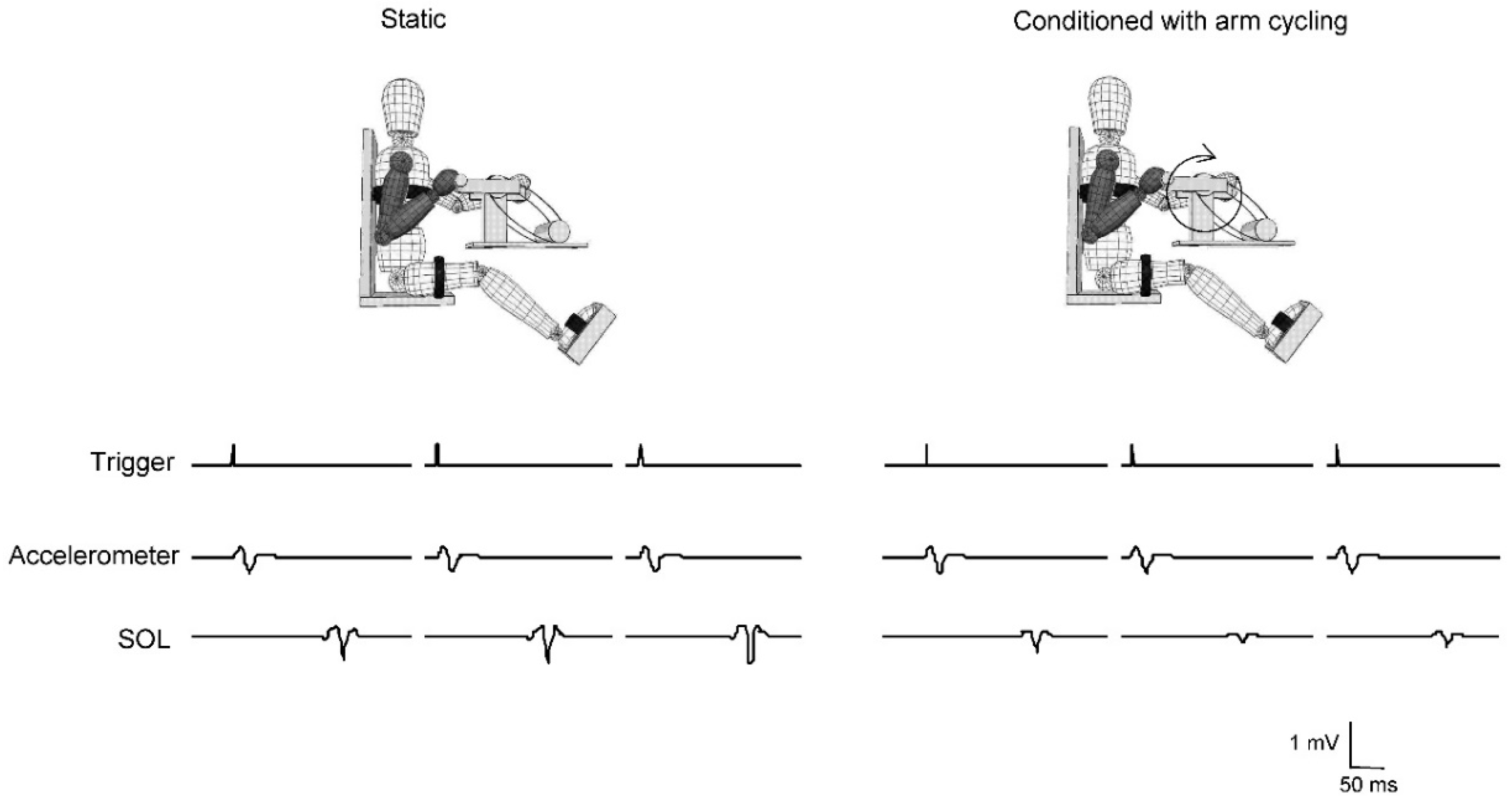
2.4. Stretch Reflexes
2.5. Cutaneous Reflexes
2.6. Statistics
3. Results
3.1. NOTE
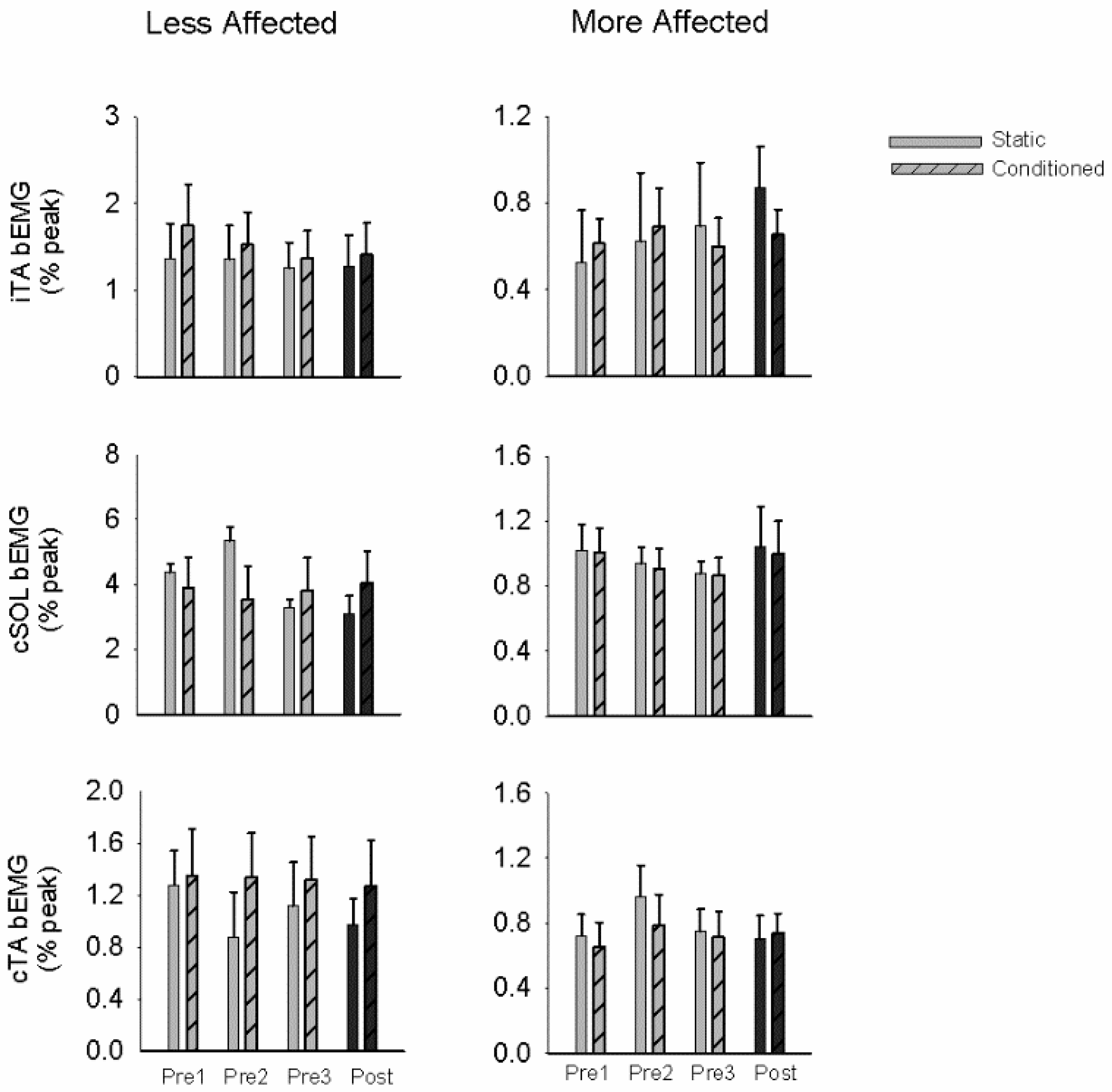
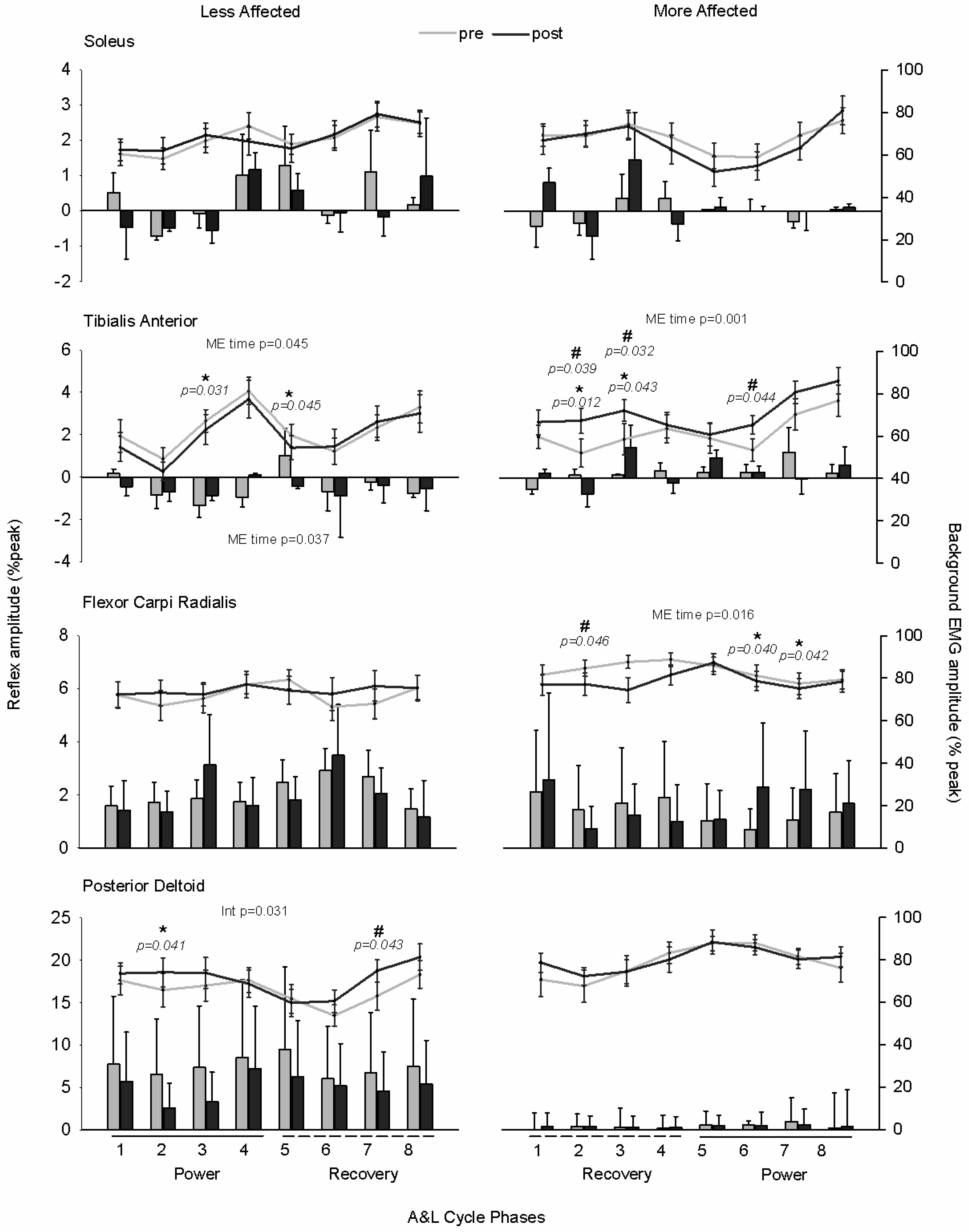
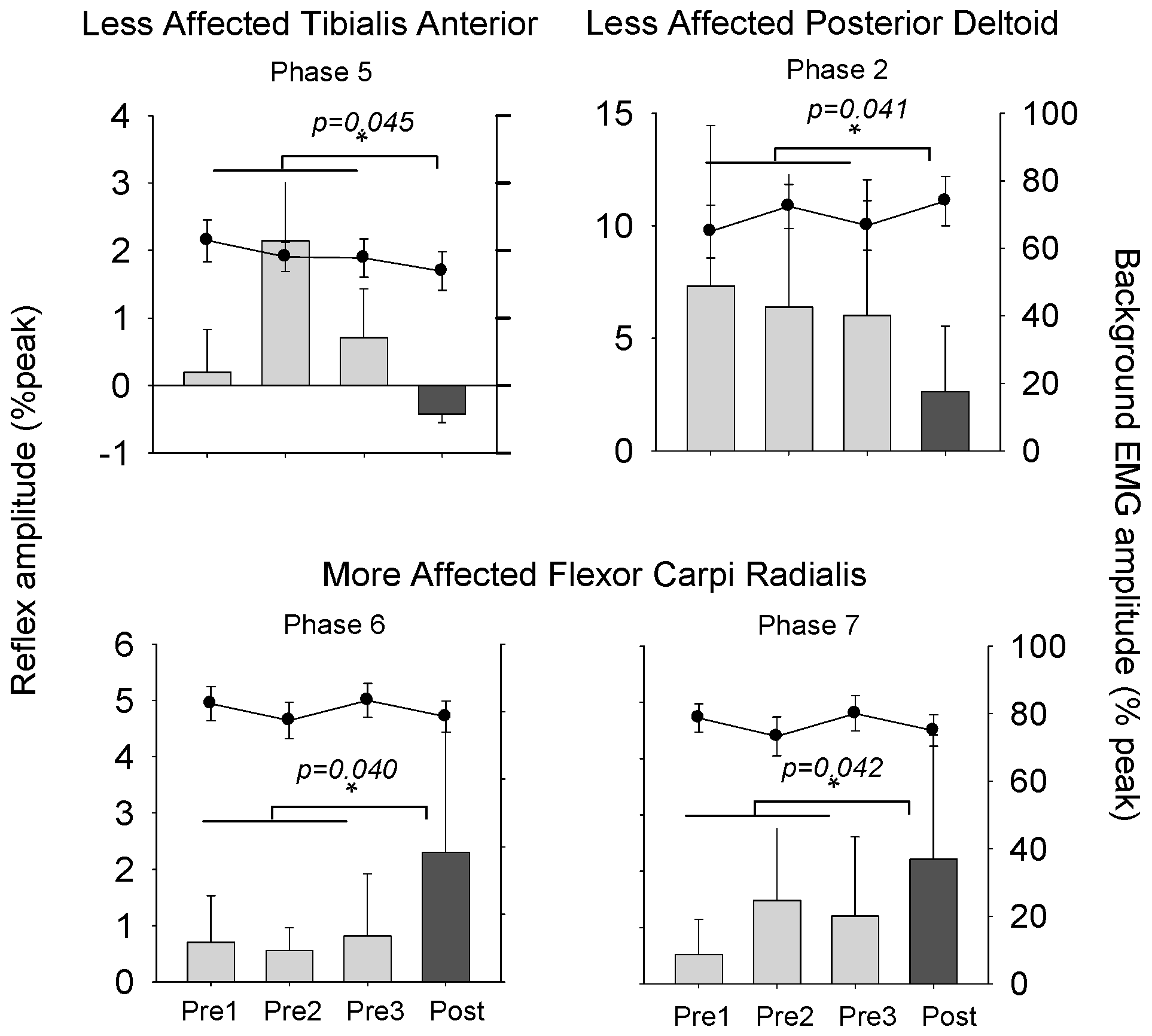
3.2. Stretch Reflexes
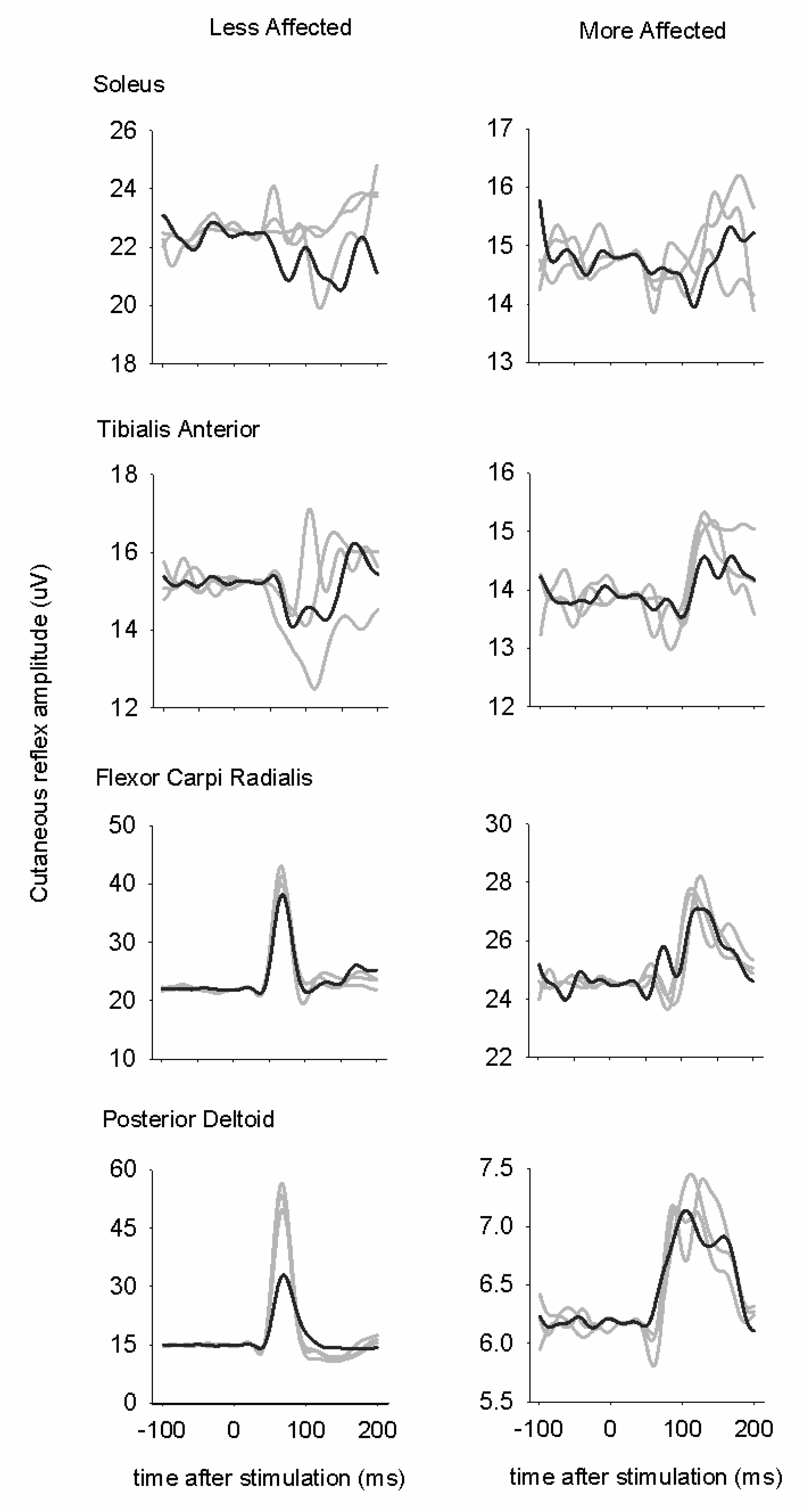
3.3. Cutaneous Reflexes
3.4. Modulation Index
4. Discussion
4.1. Plasticity in Stretch Reflex Modulation
4.2. Plasticity in Cutaneous Reflex Modulation
4.3. Methodological Considerations
4.4. Plasticity and Locomotor Rehabilitation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Skinner, R.D.D.; Adams, R.J.J.; Remmel, R.S.S. Responses of long descending propriospinal neurons to natural and electrical types of stimuli in cat. Brain Res. 1980, 196, 387–403. [Google Scholar] [CrossRef]
- Dietz, V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002, 25, 462–467. [Google Scholar] [CrossRef]
- Frigon, A.; Collins, D.F.; Zehr, E.P. Effect of rhythmic arm movement on reflexes in the legs: Modulation of soleus H-reflexes and somatosensory conditioning. J. Neurophysiol. 2004, 91, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Duysens, J. Regulation of arm and leg movement during human locomotion. Neuroscientist 2004, 10, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Hundza, S.R.; Vasudevan, E.V. The quadrupedal nature of human bipedal locomotion. Exerc. Sport Sci. Rev. 2009, 37, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Thibaudier, Y.; Hurteau, M.-F.; Telonio, A.; Frigon, A. Coordination between the fore- and hindlimbs is bidirectional, asymmetrically organized, and flexible during quadrupedal locomotion in the intact adult cat. Neuroscience 2013, 240, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Dickson, H.G.; Skuse, N.F. Task-dependent changes in the responses to low-threshold cutaneous afferent volleys in the human lower limb. J. Physiol. 1991, 432, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Klimstra, M.; Johnson, E.A.; Carroll, T.J. Rhythmic leg cycling modulates forearm muscle H-reflex amplitude and corticospinal tract excitability. Neurosci. Lett. 2007, 419, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Dragert, K.; Zehr, E.P. Rhythmic arm cycling modulates Hoffmann reflex excitability differentially in the ankle flexor and extensor muscles. Neurosci. Lett. 2009, 450, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Mezzarane, R.A.; Klimstra, M.; Lewis, A.; Hundza, S.R.; Zehr, E.P. Interlimb coupling from the arms to legs is differentially specified for populations of motor units comprising the compound H-reflex during “reduced” human locomotion. Exp. Brain Res. 2011, 208, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Collins, D.F.; Chua, R. Human interlimb reflexes evoked by electrical stimulation of cutaneous nerves innervating the hand and foot. Exp. Brain Res. 2001, 140, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Haridas, C.; Zehr, E.P. Coordinated interlimb compensatory responses to electrical stimulation of cutaneous nerves in the hand and foot during walking. J. Neurophysiol. 2003, 90, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.F.; Stein, R.B. Phase-dependent reflex reversal in human leg muscles during walking. J. Neurophysiol. 1990, 63, 1109–1117. [Google Scholar] [PubMed]
- Duysens, J.; Tax, A.A.M.; Trippel, M.; Dietz, V. Phase-dependent reversal of reflexly induced movements during human gait. Exp. Brain Res. 1992, 90. [Google Scholar] [CrossRef]
- Zehr, E.P.; Haridas, C.; Zehr, E.P.; Haridas, C.; Zehr, E.P.; Haridas, C. Modulation of cutaneous reflexes in arm muscles during walking: further evidence of similar control mechanisms for rhythmic human arm and leg movements. Exp. Brain Res. 2003, 149, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Sasada, S.; Tazoe, T.; Nakajima, T.; Futatsubashi, G.; Ohtsuka, H.; Suzuki, S.; Zehr, E.P.; Komiyama, T. A common neural element receiving rhythmic arm and leg activity as assessed by reflex modulation in arm muscles. J. Neurophysiol. 2016, 115, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Fouad, K.; Bastiaanse, C.M. Neuronal coordination of arm and leg movements during human locomotion. Eur. J. Neurosci. 2001, 14, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.V.; Zehr, E.P. Earth-referenced handrail contact facilitates interlimb cutaneous reflexes during locomotion. J. Neurophysiol. 2007, 98, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Balter, J.E.; Ferris, D.P.; Hundza, S.R.; Loadman, P.M.; Stoloff, R.H. Neural regulation of rhythmic arm and leg movement is conserved across human locomotor tasks. J. Physiol. 2007, 582, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Loadman, P.M.; Zehr, E.P. Rhythmic arm cycling produces a non-specific signal that suppresses Soleus H-reflex amplitude in stationary legs. Exp. Brain Res. 2007, 179, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Klimstra, M.; Dragert, K.; Barzi, Y.; Bowden, M.G.; Javan, B.; Phadke, C. Enhancement of arm and leg locomotor coupling with augmented cutaneous feedback from the hand. J. Neurophysiol. 2007, 98, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Mezzarane, R.A.; Klarner, T.; Barss, T.S.; Hundza, S.R.; Komiyama, T.; Zehr, E.P. Neural Mechanisms Influencing Interlimb Coordination during Locomotion in Humans: Presynaptic Modulation of Forearm H-Reflexes during Leg Cycling. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Huang, H.J.; Kao, P.C. Moving the arms to activate the legs. Exerc. Sport Sci. Rev. 2006, 34, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Klimstra, M.D.; Thomas, E.; Stoloff, R.H.; Ferris, D.P.; Zehr, E.P. Neuromechanical considerations for incorporating rhythmic arm movement in the rehabilitation of walking. Chaos 2009, 19, 026102. [Google Scholar] [CrossRef] [PubMed]
- Behrman, A.L.; Harkema, S.J. Locomotor training after human spinal cord injury: A series of case studies. Phys. Ther. 2000, 80, 688–700. [Google Scholar] [PubMed]
- Kawashima, N.; Nozaki, D.; Abe, M.O.; Nakazawa, K. Shaping appropriate locomotive motor output through interlimb neural pathway within spinal cord in humans. J. Neurophysiol. 2008, 99, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- De Kam, D.; Rijken, H.; Manintveld, T.; Nienhuis, B.; Dietz, V.; Duysens, J. Arm movements can increase leg muscle activity during submaximal recumbent stepping in neurologically intact individuals. J. Appl. Physiol. 2013, 115, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Clinical practice. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Dobkin, B.H. Strategies for stroke rehabilitation. Lancet Neurol. 2004, 3, 528–536. [Google Scholar] [CrossRef]
- Duysens, J.; Baken, B.C.M.; Burgers, L.; Plat, F.M.; Den Otter, A.R.; Kremer, H.P.H. Cutaneous reflexes from the foot during gait in hereditary spastic paraparesis. Clin. Neurophysiol. 2004, 115, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Thilmann, A.F.; Fellows, S.J.; Garms, E. Pathological stretch reflexes on the “good” side of hemiparetic patients. J. Neurol. Neurosurg. Psychiatry 1990, 53, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Loadman, P.M. Persistence of locomotor-related interlimb reflex networks during walking after stroke. Clin. Neurophysiol. 2012, 123, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Loadman, P.M.; Hundza, S.R. Neural control of rhythmic arm cycling after stroke. J. Neurophysiol. 2012, 108, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Barzi, Y.; Zehr, E.P. Rhythmic arm cycling suppresses hyperactive soleus H-reflex amplitude after stroke. Clin. Neurophysiol. 2008, 119, 1443–1452. [Google Scholar] [CrossRef] [PubMed]
- Mezzarane, R.A.; Nakajima, T.; Zehr, E.P. After stroke bidirectional modulation of soleus stretch reflex amplitude emerges during rhythmic arm cycling. Front. Hum. Neurosci. 2014, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Fujita, K.; Stein, R.B. Reflexes from the superficial peroneal nerve during walking in stroke subjects. J. Neurophysiol. 1998, 79, 848–858. [Google Scholar] [PubMed]
- Kline, T.L.; Schmit, B.D.; Kamper, D.G. Exaggerated interlimb neural coupling following stroke. Brain 2007, 130, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P. Training-induced adaptive plasticity in human somatosensory reflex pathways. J. Appl. Physiol. 2006, 101, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Klarner, T.; Barss, T.; Sun, Y.; Kaupp, C.; Loadman, P.M.; Zehr, E. Exploiting interlimb arm and leg connections for walking rehabilitation: A training intervention in stroke. Neural Plast. 2016, 2016, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [PubMed]
- Lee, K.; Carson, L.; Kinnin, E.; Patterson, V. The Ashworth Scale: A reliable and reproducible method of measuring spasticity. Neurorehabil. Neural Repair 1989, 3, 205–209. [Google Scholar] [CrossRef]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical gait assessment in the neurologically impaired. Reliability and meaningfulness. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [PubMed]
- Gowland, C.; Stratford, P.; Ward, M.; Moreland, J.; Torresin, W.; van Hullenaar, S.; Sanford, J.; Barreca, S.; Vanspall, B.; Plews, N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993, 24, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Hage, J.J.; van der Steen, L.P.E.; de Groot, P.J.M. Difference in sensibility between the dominant and nondominant index finger as tested using the Semmes-Weinstein monofilaments pressure aesthesiometer. J. Hand Surg. Am. 1995, 20, 227–229. [Google Scholar] [CrossRef]
- Walker, H.K. Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Andersen, O.K.; Klimstra, M.; Thomas, E.; Loadman, P.M.; Hundza, S.R.; Zehr, E.P. Human cutaneous reflexes evoked with simultaneous multiple nerve stimulation during rhythmic locomotor-like arm and leg cycling in stroke subjects. Replace Repair Restor. Reli. Bridg. Clin. Eng. Solut. Neurorehabil. 2014, 1, 255–261. [Google Scholar]
- Zehr, E.P. Evidence-based risk assessment and recommendations for physical activity clearance: Stroke and spinal cord injury. Appl. Physiol. Nutr. Metab. 2011, 36, S214–S231. [Google Scholar] [CrossRef] [PubMed]
- Scherr, J.; Wolfarth, B.; Christle, J.W.; Pressler, A.; Wagenpfeil, S.; Halle, M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur. J. Appl. Physiol. 2013, 113, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Sibley, K.M.; Thomas, S.G.; McIlroy, W.E.; Brooks, D. Maximal exercise test results in subacute stroke. Arch. Phys. Med. Rehabil. 2006, 87, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Charlesworth, S.; Ivey, A.; Nettlefold, L.; Bredin, S.S. A systematic review of the evidence for canada’s physical activity guidelines. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Butefisch, C.; Hummelsheim, H.; Densler, P.; Mauritz, K. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J. Neurol. Sci. 1995, 130, 59–68. [Google Scholar] [CrossRef]
- Klarner, T.; Barss, T.; Sun, Y.; Kaupp, C.; Beattie, S.; Zehr, E.P. Reliability of multiple baseline measures for locomotor retraining after stroke. Replace Repair Restor. Reli. Bridg. Clin. Eng. Solut. Neurorehabil. 2014, 7, 479–486. [Google Scholar]
- Zehr, E.P. Considerations for use of the Hoffmann reflex in exercise studies. Eur. J. Appl. Physiol. 2002, 86, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lagerquist, O.; Zehr, E.P.; Baldwin, E.R.L.; Klakowicz, P.M.; Collins, D.F. Diurnal changes in the amplitude of the Hoffmann reflex in the human soleus but not in the flexor carpi radialis muscle. Exp. Brain Res. 2006, 170, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dragert, K.; Zehr, E.P. High-intensity unilateral dorsiflexor resistance training results in bilateral neuromuscular plasticity after stroke. Exp. Brain Res. 2013, 225, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Palomino, A.F.; Hundza, S.R.; Zehr, E.P. Rhythmic arm cycling differentially modulates stretch and H-reflex amplitudes in soleus muscle. Exp. Brain Res. 2011, 214, 529–537. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, G.C.; Hundza, S.R.; Zehr, E.P. Phase-dependent modulation of soleus H-reflex amplitude induced by rhythmic arm cycling. Neurosci. Lett. 2010, 475, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; McKeon, B.; Skuse, N.F. Dependence of the Achilles tendon reflex on the excitability of spinal reflex pathways. Ann. Neurol. 1981, 10, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Fornari, M.C.D.S.; Kohn, A.F. High frequency tendon reflexes in the human soleus muscle. Neurosci. Lett. 2008, 440, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Klarner, T.; Barss, T.; Sun, Y.; Kaupp, C.; Zehr, E.P. Preservation of common rhythmic locomotor control despite weakened supraspinal regulation after stroke. Front. Integr. Neurosci. 2014, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Hundza, S.R.S. Forward and backward arm cycling are regulated by equivalent neural mechanisms. J. Neurophysiol. 2005, 93, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, E.V.L.; Zehr, E.P. Multi-frequency arm cycling reveals bilateral locomotor coupling to increase movement symmetry. Exp. Brain Res. 2011, 211, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Zehr, E.P.; Komiyama, T.; Stein, R.B. Cutaneous reflexes during human gait: Electromyographic and kinematic responses to electrical stimulation. J. Neurophysiol. 1997, 77, 3311–3325. [Google Scholar] [PubMed]
- Zehr, E.P.; Stein, R.B.; Komiyama, T. Function of sural nerve reflexes during human walking. J. Physiol. 1998, 507 Pt 1, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, T.; Zehr, E.P.; Stein, R.B. Absence of nerve specificity in human cutaneous reflexes during standing. Exp. Brain Res. 2000, 133, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Duysens, J. Reflex control of locomotion as revealed by stimulation of cutaneous afferents in spontaneously walking premammillary cats. J. Neurophysiol. 1977, 40, 737–751. [Google Scholar] [PubMed]
- Zehr, E.; Nakajima, T.; Barss, T.; Klarner, T.; Miklosovic, S.; Mezzarane, R.A.; Nurse, M.; Komiyama, T. Cutaneous stimulation of discrete regions of the sole during locomotion produces “sensory steering” of the foot. BMC Sport Sci. Med. Rehabil. 2014, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Brooke, J.D.; Cheng, J.; Collins, D.F.; McIlroy, W.E.; Misiaszek, J.E.; Staines, W.R. Sensori-sensory afferent conditioning with leg movement: gain control in spinal reflex and ascending paths. Prog. Neurobiol. 1997, 51, 393–421. [Google Scholar] [CrossRef]
- Cummings, G. Understanding the New Statistics. Effect Sizes, Confidence Intervals, and Meta-Analysis; Routledge: London, UK, 2013. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: London, UK, 2013. [Google Scholar]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of I a fibres: A study in man and the cat. J. Physiol. 1987, 389, 729–756. [Google Scholar] [CrossRef] [PubMed]
- Crone, C.; Nielsen, J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol. Scand. 1994, 152, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Petersen, N.; Christensen, L.O.; Sinkjaer, T.; Nielsen, J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J. Neurophysiol. 1998, 80, 610–620. [Google Scholar] [PubMed]
- Pierrot-Deseilligny, E.; Mazevet, D. The monosynaptic reflex: A tool to investigate motor control in humans. Interest and limits. Neurophysiol. Clin. 2000, 30, 67–80. [Google Scholar] [CrossRef]
- Morita, H.; Crone, C.; Christenhuis, D.; Petersen, N.T.; Nielsen, J.B. Modulation of presynaptic inhibition and disynaptic reciprocal Ia inhibition during voluntary movement in spasticity. Brain 2001, 124, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Kagamihara, Y.; Masakado, Y. Excitability of spinal inhibitory circuits in patients with spasticity. J. Clin. Neurophysiol. 2005, 22, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Petersen, N.T.; Crone, C.; Sinkjaer, T. Stretch reflex regulation in healthy subjects and patients with spasticity. Neuromodulation 2005, 8, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Durand, C. The influence of increased muscle spindle sensitivity on Achilles tendon jerk and H-reflex in relaxed human subjects. Somatosens. Mot. Res. 2002, 19, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Stein, R.B. Interaction of the Jendrássik maneuver with segmental presynaptic inhibition. Exp. Brain Res. 1999, 124, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Lamy, J.C.; Wargon, I.; Mazevet, D.; Ghanim, Z.; Pradat-Diehl, P.; Katz, R. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 2009, 132, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R.; Tennissen, A.M. Activity-dependent spinal cord plasticity in health and disease. Annu. Rev. Neurosci. 2001, 24, 807–843. [Google Scholar] [CrossRef] [PubMed]
- Adkins, D.L.; Boychuk, J.; Remple, M.S.; Kleim, J.A. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. 2006, 101, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol. 2007, 189, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Wolpaw, J.R. What can the spinal cord teach us about learning and memory? Neuroscientist 2010, 16, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Wirz, M.; Colombo, G.; Curt, A. Locomotor capacity and recovery of spinal cord function in paraplegic patients: A clinical and electrophysiological evaluation. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control 1998, 109, 140–153. [Google Scholar] [CrossRef]
- Field-Fote, E.C. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys. Med. Rehabil. 2001, 82, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Stark, A.; Cameron, I.D.; Pollock, A. Treadmill training and body weight support for walking after stroke. Stroke 2003, 34, 3006. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Sullivan, K.J.; Behrman, A.L.; Azen, S.P.; Wu, S.S.; Nadeau, S.E.; Dobkin, B.H.; Rose, D.K.; Tilson, J.K.; Cen, S.; et al. Body-weight-supported treadmill rehabilitation after stroke. N. Engl. J. Med. 2011, 364, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M.; Mummidisetty, C.K. Locomotor training improves premotoneuronal control after chronic spinal cord injury. J. Neurophysiol. 2014, 111, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Rymer, W.; Knikou, M. Locomotor training modifies soleus monosynaptic motoneuron responses in human spinal cord injury. Exp. Brain Res. 2015, 233, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Knikou, M. Functional reorganization of soleus H-reflex modulation during stepping after robotic-assisted step training in people with complete and incomplete spinal cord injury. Exp. Brain Res. 2013, 228, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, M.; Vry, J.; Mall, V.; Faist, M. Changes in soleus H-reflex modulation after treadmill training in children with cerebral palsy. Brain 2009, 132, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.K.; Pomerantz, F.R.; Wolpaw, J.R. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J. Neurosci. 2013, 33, 2365–2375. [Google Scholar] [CrossRef] [PubMed]


| N | Sex/Age/MA Side/Years Since Stroke | Modified Ashworth (ankle/knee) | FAC (/6) | Chedoke-McMaster (A/H/L/F) | Monofilament (hand/foot) | Reflexes (L1/S1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| 1 | M/74/R/5 | 3/1+ | 3/1+ | 4 | 4 | 2/2/3/2 | 2/2/2/3 | J4.31/J4.31 | J4.31/J4.31 | 3+/1+ | 3+/1+ |
| 2 | F/70/R/2 | 0/0 | 0/0 | 5 | 5 | 7/5/7/7 | 7/7/7/7 | J4.31/J4.31 | J4.31/J4.31 | 2+/2+ | 2+/2+ |
| 3 | F/45/R/7 | 1/0 | 1/0 | 5 | 5 | 5/5/6/5 | 5/5/6/4 | F3.61/J4.31 | D2.83/F3.61 | 0/0 | 1+/1+ |
| 4 | M/59/R/3 | 2/0 | 2/0 | 5 | 5 | 2/2/4/2 | 2/2/4/2 | T6.65/J4.31 | K4.56/J4.31 | 3+/3+ | 3+/3+ |
| 5 | M/82/R/3 | 0/1 | 0/1 | 3 | 3 | 4/6/6/5 | 5/6/6/5 | UTF/UTF | UTF/UTF | 3+/0 | 3+/0 |
| 6 | M/86/L/4 | 1+/0 | 1+/0 | 5 | 5 | 7/7/6/5 | 7/7/6/6 | J4.31/T6.65 | J4.31/T6.65 | 0/0 | 0/0 |
| 7 | F/80/R/6 | 0/0 | 0/0 | 5 | 5 | 3/5/5/5 | 3/5/5/6 | J4.31/J4.31 | F3.61/J4.31 | 0/0 | 0/0 |
| 8 | M/59/R/11 | 1/1 | 2/1 | 5 | 5 | 5/5/5/4 | 5/6/6/4 | T6.65/T6.65 | T6.65/T6.65 | 3+/4+ | 3+/3+ |
| 9 | M/74/R/6 | 1/0 | 1/1 | 5 | 5 | 6/5/6/5 | 7/7/6/5 | J4.31/F3.61 | F3.61/D2.83 | 3+/2+ | 3+/2+ |
| 10 | M/47/L/6 | 4/2 | 2/2 | 4 | 4 | 2/1/2/2 | 2/2/2/2 | T6.65/T6.65 | T6.65/T6.65 | 4+/3+ | 4+/3+ |
| 11 | M/69/L/5 | 2/3 | 1+/2 | 4 | 4 | 2/2/3/2 | 2/2/3/3 | T6.65/T6.65 | T6.65/T6.65 | 3+/3+ | 3+/3+ |
| 12 | F/72/R/6 | 2/2 | 2/2 | 3 | 6 | 2/3/2/3 | 3/3/3/3 | UTF/J4.31 | T6.65/J4.31 | 1+/3+ | 2+/2+ |
| 13 | M/59/L/5 | 1/1 | 1/0 | 6 | 5 | 6/6/6/4 | 7/6/6/6 | J4.31/J4.31 | J4.31/J4.31 | 3+/2+ | 3+/2+ |
| 14 | M/56/L/8 | 1/1 | 0/1 | 5 | 4 | 1/1/4/2 | 1/1/4/2 | T6.65/T6.65 | D2.83/K4.56 | 3+/3+ | 3+/3+ |
| 15 | M/77/L/8 | 2/2 | 2/2 | 3 | 5 | 4/5/5/3 | 5/5/5/3 | UTF/T6.65 | T6.65/T6.65 | 3+/3+ | 3+/3+ |
| 16 | F/63/L/13 | 1/2 | 1/2 | 5 | 4 | 2/2/3/4 | 2/2/5/5 | T6.65/K4.56 | D2.83/D2.83 | 3+/1+ | 3+/1+ |
| 17 | M/71/R/6 | 1/2 | 1/2 | 4 | 4 | 3/2/4/4 | 4/2/4/4 | F3.61/J4.31 | F3.61/F3.61 | 2+/3+ | 2+/2+ |
| 18 | M/62/R/8 | 1+/2 | 1/2 | 4 | 5 | 4/3/4/5 | 4/3/5/5 | D2.83/D2.83 | D2.83/D2.83 | 3+/3+ | 3+/2+ |
| 19 | M/78/L/29 | 3/1 | 2/1+ | 4 | 4 | 3/3/4/4 | 3/4/4/4 | T6.65/T6.65 | J4.31/F3.61 | 0/0 | 0/1 |
| Measure | Participants (/19) with Significant Changes after Training | Average Pretest with 95% Confidence Interval and Post-Test Score |
|---|---|---|
| Stretch Reflex | ||
| LA SOL | 10 |  |
| MA SOL * | 15 |  |
| Ratio * | 10 |  |
| bEMG with arm cycling | ||
| LA iTA | 2 |  |
| LA cSOL | 3 |  |
| LA cTA | 1 |  |
| MA iTA | 3 |  |
| MA cSOL | 4 |  |
| MA cTA | 1 |  |
| Cutaneous Reflex Modulation Index | ||
| MA SOL | 7 | 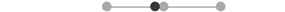 |
| MA TA | 8 |  |
| LA SOL | 5 | 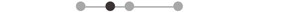 |
| LA TA * | 8 |  |
| MA FCR * | 11 | 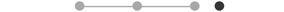 |
| MA PD | 8 | 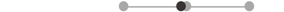 |
| LA FCR | 10 | 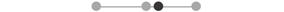 |
| LA PD | 10 | 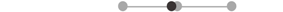 |
| Cutaneous Reflex Modulation Index Ratio | ||
| SOL * | 12 | 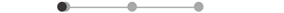 |
| TA * | 10 | 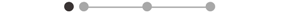 |
| FCR | 5 | 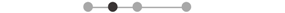 |
| PD | 8 |  |
| bEMG Modulation Index | ||
| MA SOL | 7 | 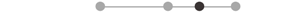 |
| MA TA * | 9 |  |
| LA SOL | 9 | 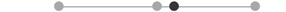 |
| LA TA | 10 | 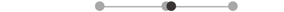 |
| MA FCR * | 8 |  |
| MA PD | 7 | 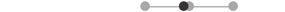 |
| LA FCR | 10 |  |
| LA PD * | 8 |  |
| bEMG Modulation Index Ratio | ||
| SOL | 4 |  |
| TA * | 7 | 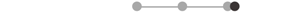 |
| FCR * | 10 | 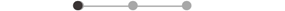 |
| PD | 8 |  |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klarner, T.; Barss, T.S.; Sun, Y.; Kaupp, C.; Loadman, P.M.; Zehr, E.P. Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke. Brain Sci. 2016, 6, 54. https://doi.org/10.3390/brainsci6040054
Klarner T, Barss TS, Sun Y, Kaupp C, Loadman PM, Zehr EP. Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke. Brain Sciences. 2016; 6(4):54. https://doi.org/10.3390/brainsci6040054
Chicago/Turabian StyleKlarner, Taryn, Trevor S. Barss, Yao Sun, Chelsea Kaupp, Pamela M. Loadman, and E. Paul Zehr. 2016. "Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke" Brain Sciences 6, no. 4: 54. https://doi.org/10.3390/brainsci6040054
APA StyleKlarner, T., Barss, T. S., Sun, Y., Kaupp, C., Loadman, P. M., & Zehr, E. P. (2016). Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke. Brain Sciences, 6(4), 54. https://doi.org/10.3390/brainsci6040054







