3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus
Abstract
:1. Introduction
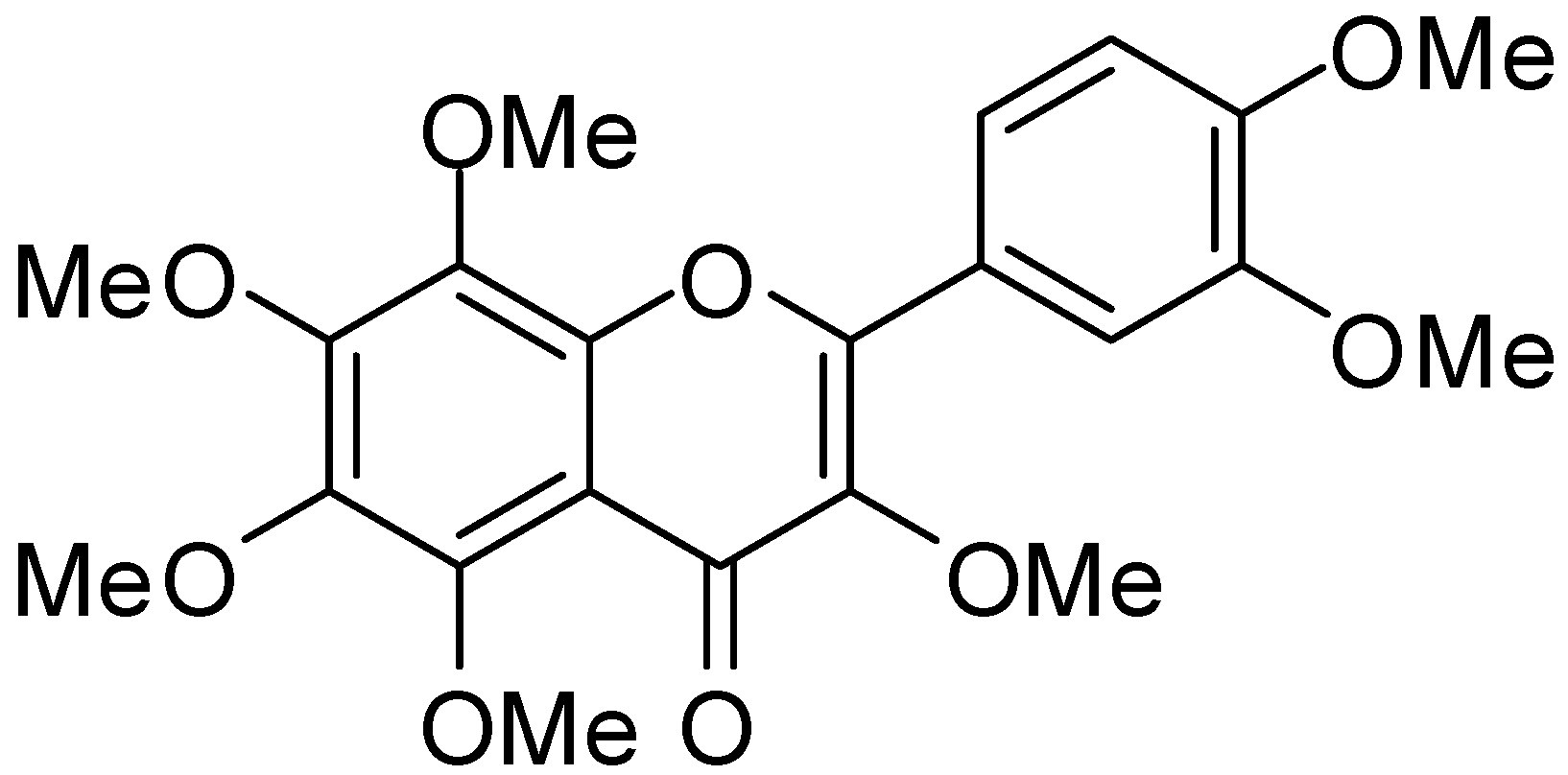
2. Experimental Section
2.1. Animals
2.2. HMF Treatment
2.3. LPS Intrahippocampal Challenge
2.4. Immunohistochemistry
2.5. RT-PCR Procedures
| Gene | Direction | Primer Sequence (5′ to 3′) | Cycles | Denaturation | Annealing | Elongation |
|---|---|---|---|---|---|---|
| COX-2 | forward | 5′-AAGGCCTCCATTGACCAG-3′ | 32 | 94 °C | 56 °C | 72 °C |
| reverse | 5′-TCTTACAGCTCAGTTGAACGC-3′ | |||||
| iNOS | forward | 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ | 35 | 94 °C | 65 °C | 72 °C |
| reverse | 5′-GGCTGTCAGAGAGCCTCGTGGCTTTGG-3′ | |||||
| IL-1β | forward | 5′-CTTGGGCTGTCCAGATGAGAGCAT-3′ | 33 | 94 °C | 63 °C | 72 °C |
| reverse | 5′-GAAGACACGGGTTCCATGGTGAAG-3′ | |||||
| TNF-α | forward | 5′-ATGAGCACAGAAAGCATGAT-3′ | 35 | 94 °C | 50 °C | 72 °C |
| reverse | 5′-TGACTTTCTCCTGGTATGA-3′ | |||||
| β-Actin | forward | 5′-CATGTTGAGACCTTCAACACCCC-3′ | 37 | 94 °C | 60 °C | 72 °C |
| reverse | 5′-GCCATCTCCTGCTCGAAGTCTAG-3′ |
2.6. Statistical Analysis
3. Results
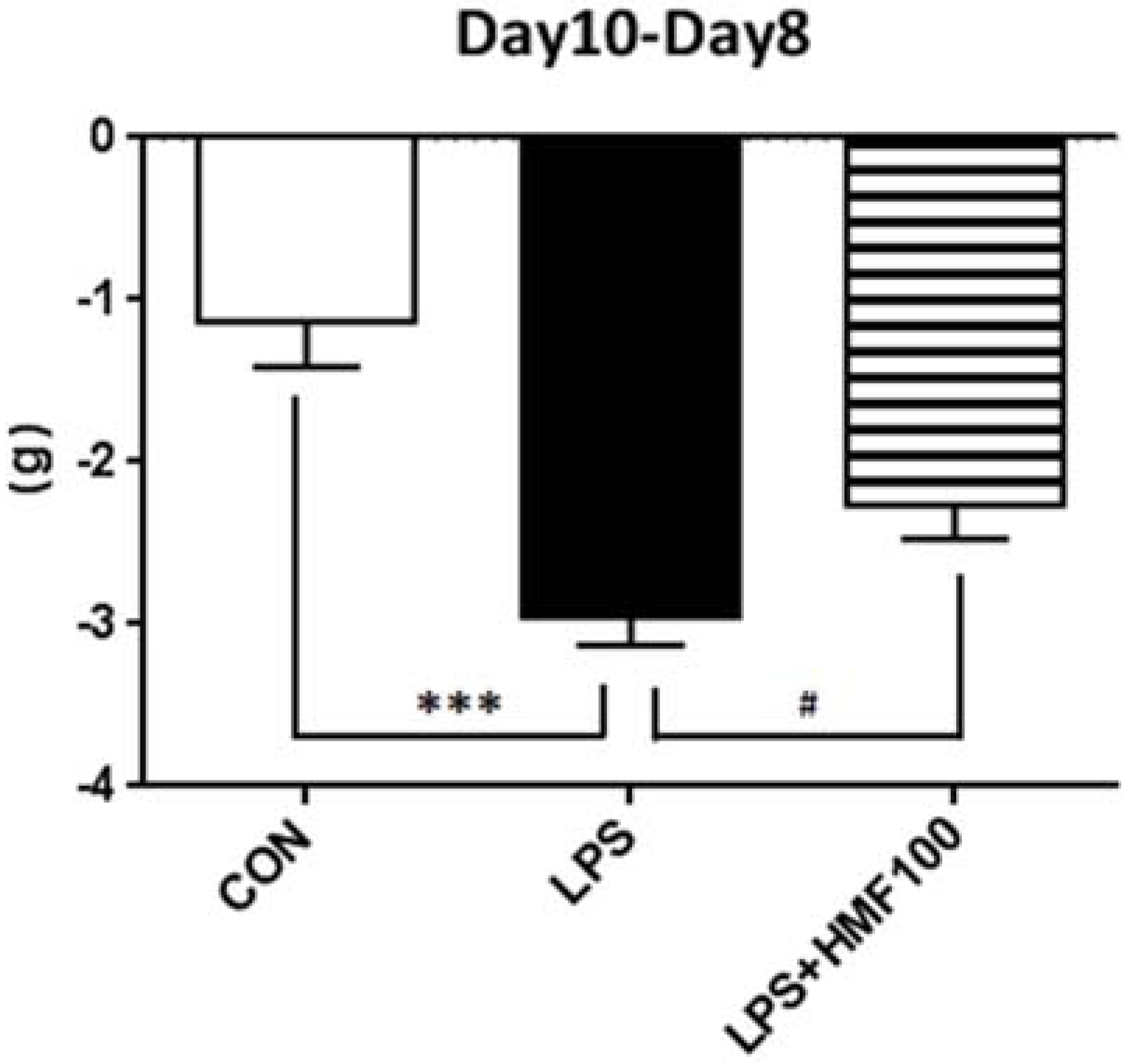
3.1. HMF Suppressed Body Weight Loss
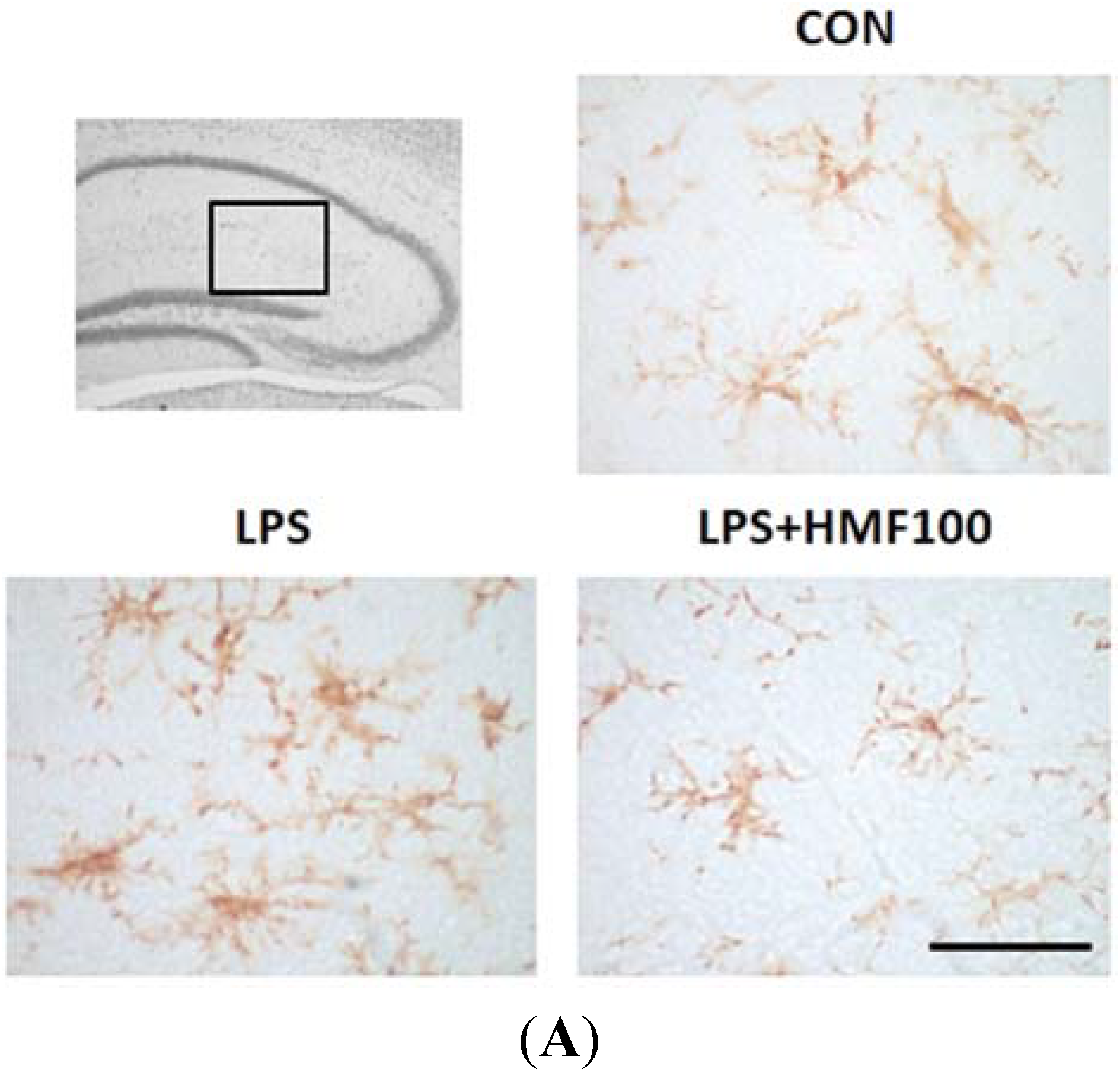
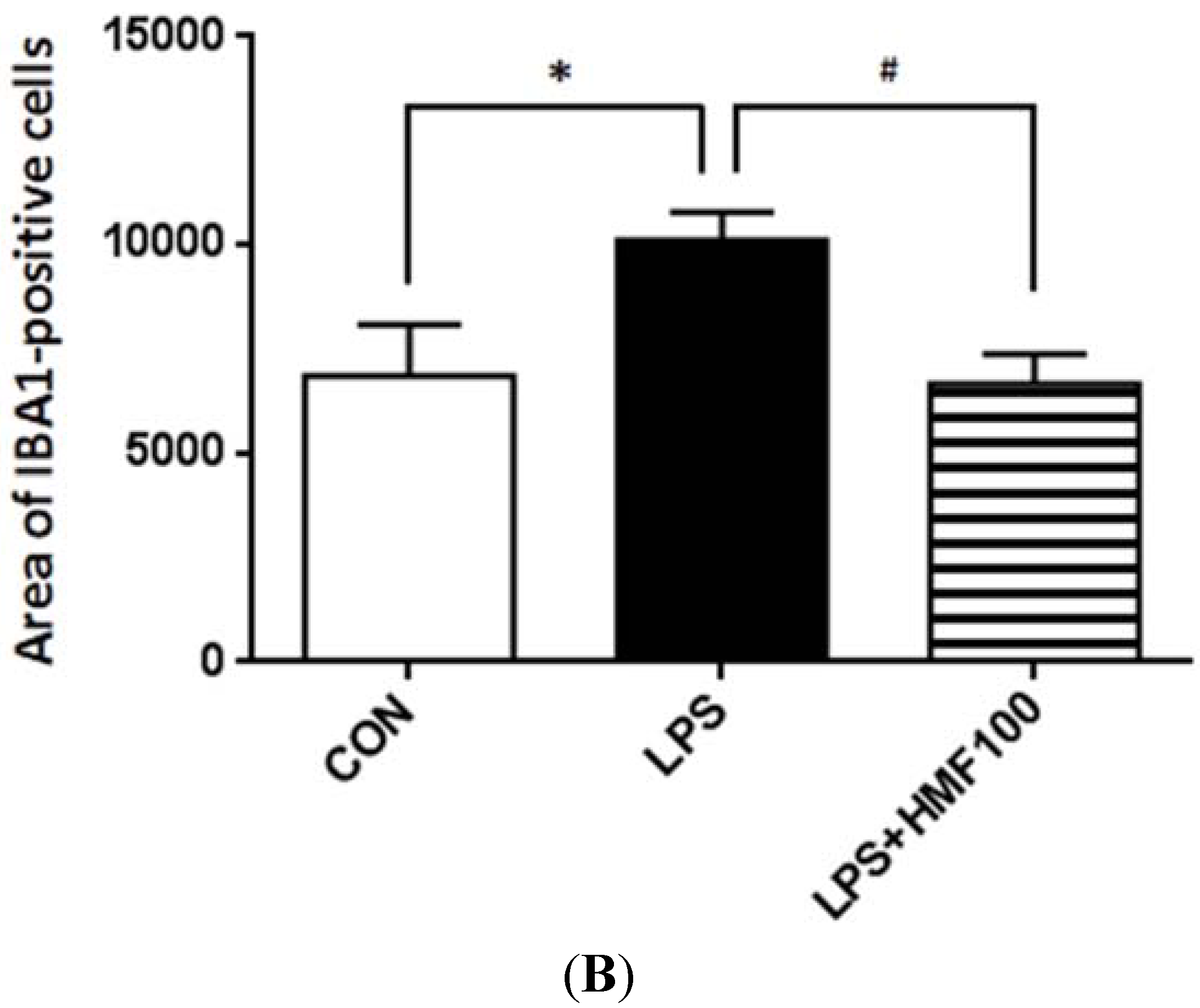
3.2. Effects of HMF on the LPS-Induced Activation of Microglia
3.3. Effect of HMF on the LPS-Induced Expression of Proinflammatory Mediator and Cytokines
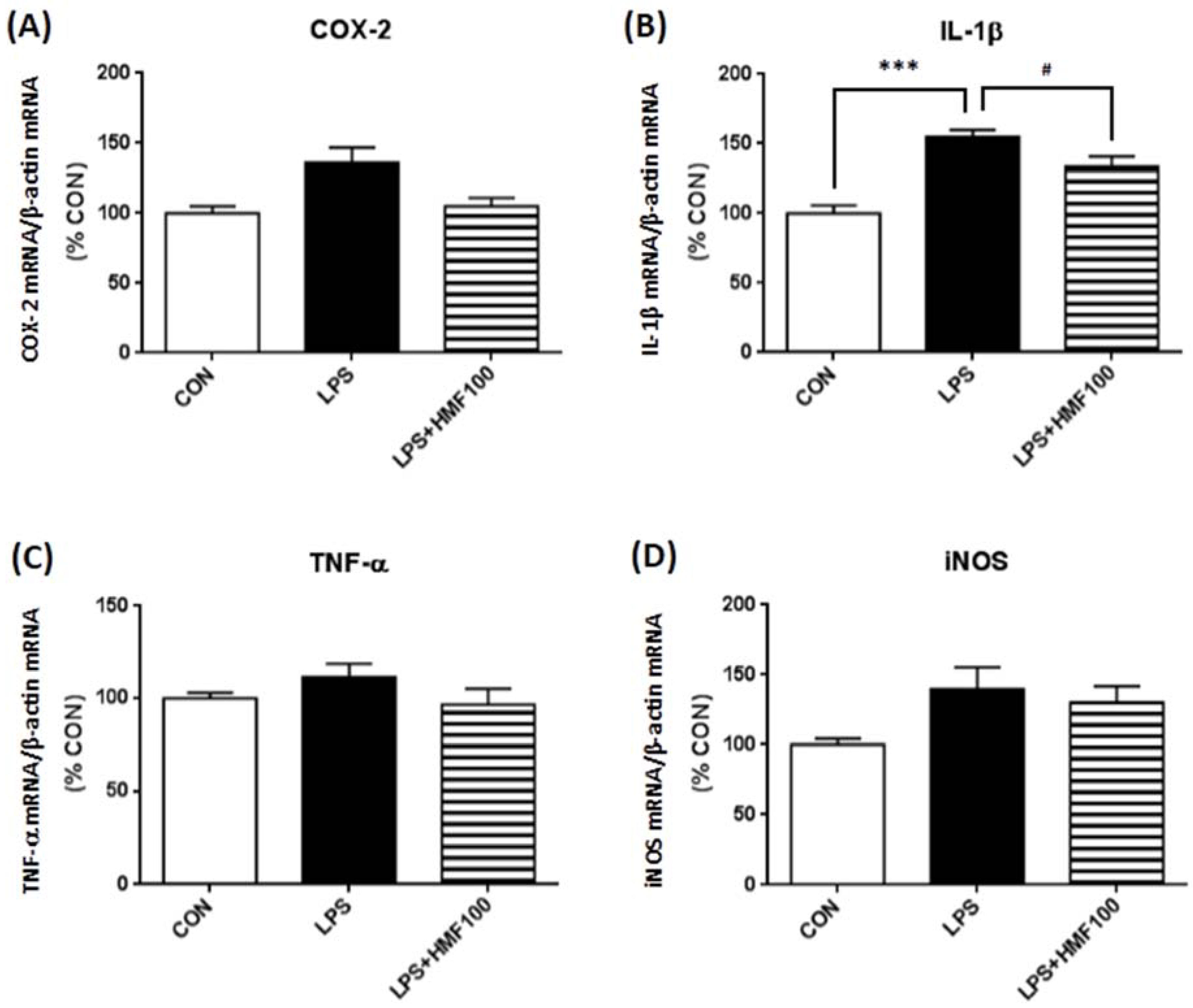
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harukuni, I.; Bhardwaj, A. Mechanisms of Brain Injury after Global Cerebral Ischemia. Neurol. Clin. 2006, 24, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Niesman, I.R.; Schilling, J.M.; Shapiro, L.A.; Kellerhals, S.E.; Bonds, J.A.; Kleschevnikov, A.M.; Cui, W.; Voong, A.; Krajewski, S.; Ali, S.S.; et al. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflammation 2014, 11. [Google Scholar] [CrossRef]
- Townsend, K.P.; Pratico, D. Novel therapeutic opportunities for Alzheimer’s disease: Focus on nonsteroidal anti-inflammatory drugs. FASEB J. 2005, 19, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Rios, M.A.; Toral-Rios, D.; Franco-Bocanegra, D.; Villeda-Hernandez, J.; Campos-Pena, V. Inflammatory process in Alzheimer’s Disease. Front. Integr. Neurosci. 2013, 7. [Google Scholar] [CrossRef]
- Cheng, Y.D.; Al-Khoury, L.; Zivin, J.A. Neuroprotection for ischemic stroke: Two decades of success and failure. NeuroRX 2004, 1, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.F.; Zhang, Z.; Wang, F.; Chen, J.G. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol. Sin. 2010, 31, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pan, M.H.; Lo, C.Y.; Tan, D.; Wang, Y.; Shahidi, F.; Ho, C.T. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. J. Funct. Foods 2009, 1, 2–12. [Google Scholar] [CrossRef]
- Manthey, J.A.; Grohmann, K.; Montanari, A.; Ash, K.; Manthey, C.L. Polymethoxylated Flavones Derived from Citrus Suppress Tumor Necrosis Factor-α Expression by Human Monocytes. J. Nat. Prod. 1999, 62, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Sato, T.; Takayama, Y.; Mimaki, Y.; Sashida, Y.; Yano, M.; Ito, A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem. Pharmacol. 2003, 65, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Li, S.; Chai, C.Y.; Lo, C.Y.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Inhibitory effect of citrus 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on 12-O-tetradecanoylphorbol 13-acetate-induced skin inflammation and tumor promotion in mice. Carcinogenesis 2007, 28, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ito, C.; Itoigawa, M.; Okada, T.; Furukawa, H. Effect of natsudaidain isolated from Citrus plants on TNF-alpha and cyclooxygenase-2 expression in RBL-2H3 cells. J. Pharm. Pharmacol. 2009, 61, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, K.; Tada, K.; Harita, G.; Fujimoto, T.; Tsukayama, M.; Tsuji, A. Sudachitin, a polymethoxyflavone from Citrus sudachi, suppresses lipopolysaccharide-induced inflammatory responses in mouse macrophage-like RAW264 cells. Biosci. Biotechnol. Biochem. 2012, 76, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Okada, T.; Kozaki, S. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Makihata, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Nakajima, M.; Furukawa, Y. Oenothein B Suppresses Lipopolysaccharide (LPS)-Induced Inflammation in the Mouse Brain. Int. J. Mol. Sci. 2013, 14, 9767–9778. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Teismann, P.; Schulz, J.B. Cellular pathology of Parkinson’s disease: Astrocytes, microglia and inflammation. Cell Tissue Res. 2004, 318, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Jackson-Lewis, V.; Guegan, C.; Wu, D.C.; Teismann, P.; Choi, D.K.; Tieu, K.; Przedborski, S. The role of glial cells in Parkinson’s disease. Curr. Opin. Neurol. 2001, 14, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Ladecola, C.; Anrather, J. The immunology of stroke: From mechanisms and translation. Nat. Med. 2012, 17, 796–808. [Google Scholar] [CrossRef]
- Nakayama, M.; Uchimura, K.; Zhu, R.L.; Nagayama, T.; Rose, M.E.; Stetler, R.A.; Isakson, P.C.; Chen, J.; Graham, S.H. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc. Natl. Acad. Sci. USA 1998, 95, 10954–10959. [Google Scholar] [CrossRef] [PubMed]
- Cote, S.; Carmichael, P.H.; Verreault, R.; Lindsay, J.; Lefebvre, J.; Laurin, D. Nonsteroidal anti-inflammatory drug use and the risk of cognitive impairment and Alzheimer’s disease. Alzheimers Dement. 2012, 8, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, S.; Herkenham, M. Toll-like receptor 4 on nonhematopoietic cells sustains CNS inflammation during endotoxemia, independent of systemic cytokines. J. Neurosci. 2005, 25, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Hauss-Wegrzyniak, B.; Dobrzanski, P.; Stoehr, J.D.; Wenk, G.L. Chronic neuroinflammation in rats reproduces components of the neurobiology of Alzheimer’s disease. Brain Res. 1998, 780, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. S1), 232–240. [Google Scholar]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Iinuma, Y.; Kodani, R.; Oka, J. Neuromedin U inhibits inflammation-mediated memory impairment and neuronal cell-death in rodents. Neurosci. Res. 2008, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Dantzer, R.; Kelley, K.W.; McCusker, R.H. Central administration of insulin-like growth factor-I decreases depressive-like behavior and brain cytokine expression in mice. J. Neuroinflammation 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ide, M.; Shibutani, T.; Ohtaki, H.; Numazawa, S.; Shioda, S.; Yoshida, T. Lipopolysaccharide-induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J. Neurosci. Res. 2006, 83, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.; Tracey, K.J.; Moldawer, L.L.; Hesse, D.G.; Manogue, K.B.; Kenney, J.S.; Lee, A.T.; Kuo, G.C.; Allison, A.C.; Lowry, S.F.; et al. Antibodies to cachectin/tumor necrosis factor reduce interleukin 1 beta and interleukin 6 appearance during lethal bacteremia. J. Exp. Med. 1989, 170, 1627–1633. [Google Scholar] [CrossRef] [PubMed]
- Perlstein, R.S.; Whitnall, M.H.; Abrams, J.S.; Mougey, E.H.; Neta, R. Synergistic roles of interleukin-6, interleukin-1, and tumor necrosis factor in the adrenocorticotropin response to bacterial lipopolysaccharide in vivo. Endocrinology 1993, 132, 946–952. [Google Scholar] [PubMed]
- Gatti, S.; Bartfai, T. Induction of tumor necrosis factor-α mRNA in the brain after peripheral endotoxin treatment: Comparison with interleukin-1 family and interleukin-6. Brain Res. 1993, 624, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Laye, S.; Parnet, P.; Goujon, E.; Dantzer, R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Mol. Brain Res. 1994, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, J.; Jung, S.C.; Park, D.B.; Maeng, Y.H.; Hong, J.Y.; Kim, S.J.; Lee, S.R.; Eun, S.Y. Anti-neuroinflammatory activity of nobiletin on suppression of microglial activation. Biol. Pharm. Bull. 2010, 33, 1814–1821. [Google Scholar] [CrossRef] [PubMed]
- Semmler, J.; Wachtel, H.; Endres, S. The specific type IV phosphodiesterase inhibitor rolipram suppresses tumor necrosis factor-alpha production by human mononuclear cells. Int. J. Immunopharmacol. 1993, 15, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Bendele, P. Anti-inflammatory activity of an orange peel polymethoxylated flavone, 3′,4′,3,5,6,7,8-heptamethoxyflavone, in the rat carrageenan/paw edema and mouse lipopolysaccharide-challenge assays. J. Agric. Food Chem. 2008, 56, 9399–9403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ke, H.; Colman, R.W. Identification of interaction sites of cyclic nucleotide phosphodiesterase type 3A with milrinone and cilostazol using molecular modeling and site-directed mutagenesis. Mol. Pharmacol. 2002, 62, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Bluthe, R.M.; Laye, S.; Michaud, B.; Combe, C.; Dantzer, R.; Parnet, P. Role of interleukin-1β and tumour necrosis factor-α in lipopolysaccharide-induced sickness behaviour: A study with interleukin-1 type I receptor-deficient mice. Eur. J. Neurosci. 2000, 12, 4447–4456. [Google Scholar] [PubMed]
- Takeda, H.; Muto, S.; Hattori, T.; Sadakane, C.; Tsuchiya, K.; Katsurada, T.; Ohkawara, T.; Oridate, N.; Asaka, M. Rikkunshito ameliorates the aging-associated decrease in ghrelin receptor reactivity via phosphodiesterase III inhibition. Endocrinology 2010, 151, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Sadakane, C.; Hattori, T.; Katsurada, T.; Ohkawara, T.; Nagai, K.; Asaka, M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 2008, 134, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, S.; Miyoshi, K.; Tsumura, Y.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus. Brain Sci. 2015, 5, 118-129. https://doi.org/10.3390/brainsci5020118
Okuyama S, Miyoshi K, Tsumura Y, Amakura Y, Yoshimura M, Yoshida T, Nakajima M, Furukawa Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus. Brain Sciences. 2015; 5(2):118-129. https://doi.org/10.3390/brainsci5020118
Chicago/Turabian StyleOkuyama, Satoshi, Kazuhiro Miyoshi, Yuichi Tsumura, Yoshiaki Amakura, Morio Yoshimura, Takashi Yoshida, Mitsunari Nakajima, and Yoshiko Furukawa. 2015. "3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus" Brain Sciences 5, no. 2: 118-129. https://doi.org/10.3390/brainsci5020118
APA StyleOkuyama, S., Miyoshi, K., Tsumura, Y., Amakura, Y., Yoshimura, M., Yoshida, T., Nakajima, M., & Furukawa, Y. (2015). 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammation in the Mouse Hippocampus. Brain Sciences, 5(2), 118-129. https://doi.org/10.3390/brainsci5020118






