The Health Benefits and Challenges of Exercise Training in Persons Living with Schizophrenia: A Pilot Study
Abstract
:1. Introduction
2. Methodology
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
2.1. Physical Measures
2.1.1. Anthropometry and Body Composition
2.1.2. Resting Lipid Profile
2.1.3. Resting Blood Pressure and Heart Rate
2.1.4. Aerobic Fitness
2.1.5. Physical Activity
2.2. Exercise Intervention
2.2.1. Aerobic Exercise Intervention
| Program Stage | Week | Frequency (day/week) | Intensity | Duration (min) | |||
|---|---|---|---|---|---|---|---|
| %HRR | %HRmax | RPE | Breathing Rate | ||||
| Initial Stage | 1 | 3 | 20–39 | 50–63 | 1.5–2.5 | Slightly Increased | 15–30 |
| 2 | 3 | 40–50 | 64–70 | 3–4 | 15–30 | ||
| 3 | 3 | 40–50 | 64–70 | 3–4 | Noticeably Increased | 30 | |
| 4 | 3 | 50–60 | 70–77 | 3–5 | 30 | ||
| Improvement | 5–7 | 3 | 50–60 | 70–77 | 3–5 | Noticeably Increased | 30 |
| 8–10 | 3 | 60–70 | 77–84 | 3–6 | 30 | ||
| 11–13 | 3 | 60–70 | 77–84 | 3–6 | 30 | ||
| 14–16 | 3 | 65–75 | 80–87 | 5–7 | 30 | ||
| 17–20 | 3 | 65–75 | 80–87 | 5–7 | More Difficulty Talking while Exercising | 30 | |
| 21–24 | 3 | 70–80 | 84–90 | 5–8 | 30 | ||
| Maintenance | 25–28 | 3 | 70–80 | 84–90 | 5–8 | More Difficulty Talking while Exercising | 30–45 |
2.2.2. Resistance Training Intervention
| Program Stage | Week | Frequency (day/week) | Intensity | Sets | Muscle Groups |
|---|---|---|---|---|---|
| Familiarization | 1–4 | 1–2 | Light to Moderate 15–20 repetitions RPE = 2–3 | 1 | 8 Major Muscle Groups |
| Goal Specific | 5–24 | 2–3 | Moderate 10–15 repetitions * RPE = 3–5 | 1–2 | |
| Motor Function and Health-Related Physical Fitness Maintenance | >24 | 2–3 | Moderate 10–15 repetitions * RPE = 3–5 | 2–3 |
2.3. Statistical Analyses
3. Results
| Variable | Baseline Mean SD | |
|---|---|---|
| Age | 30.9 ± 7.2 | |
| PANSS | 99.2 ± 11.7 | |
| Gender | ||
| Male | 7 | |
| Female | 6 | |
| Ethnicity (N) | ||
| Caucasian | 9 | |
| Aboriginal | 3 | |
| Haitian | 1 | |
| Education (years) | 10.8 ± 1.4 | |
| WTAR Full Scale Predicted IQ | 94.6 ± 6.8 | |
| KBIT | 81.3 ± 15.0 | |
| ROCFT | ||
| Immediate (T score) | 20.3 ± 7.5 | |
| Delayed (T score) | 19.7 ± 7.7 | |
| HVLT-R | ||
| Immediate | 29.2 ± 11.2 | |
| Delayed | 25.1 ± 7.2 | |
| Trails A (raw score) | 43.1 ± 24.0 | |
| Trails B (raw score) | 142.3 ± 106.5 | |
| Digit Span (Standardized score) | 22.8 ± 4.6 | |
| COWA | 28.4 ± 7.5 | |
| * Antipsychotic Medications | ||
| Clozapine | 246.7 mg•day−1 | |
| Olanzapine | 11.6 mg•day−1 | |
| Aripriprazole | 15.0 mg•day−1 | |
| Risperidone | LA 25 mg Q2w | |
| Paliperidone | 100 mg•day−1 | |
| Flupenthixol | LA inj. 100 mg | |
| Loxapine | 80 mg•day−1 | |
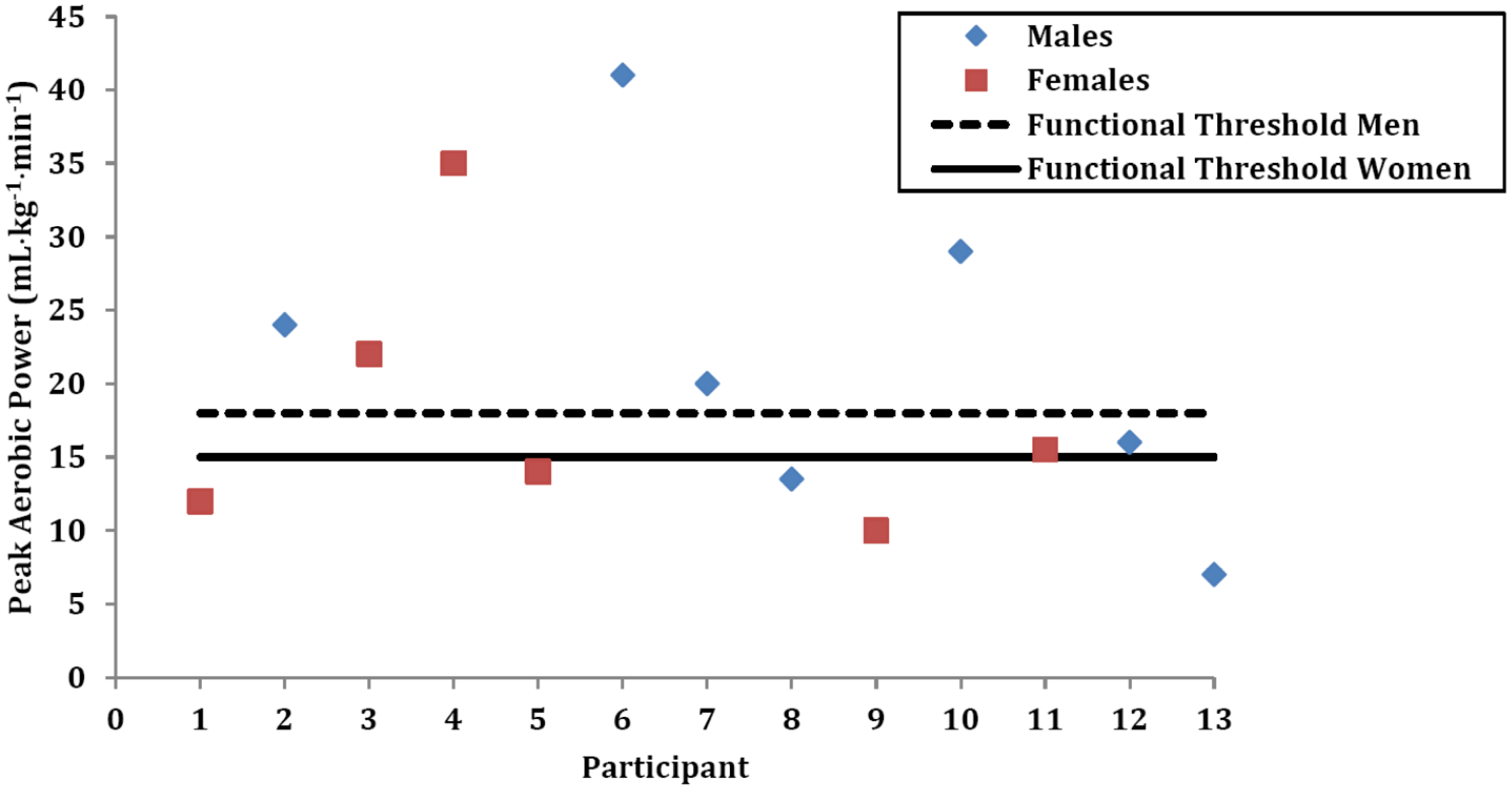
| Gender | Height (cm) | Weight (kg) | Body Mass Index (kg•m−2) | Overweight and Obesity Class | Waist Circumference (cm) | Waist Circumference Classification | Sum of Five Skinfolds (mm) | Overall Risk Category | |
|---|---|---|---|---|---|---|---|---|---|
| F | 165.0 | 85.0 | 31.2 | Obesity Class I | 113.5 | Abdominal Obesity | 175.0 | Very High | |
| F | 156.4 | 58.4 | 23.9 | Normal | 97.0 | Abdominal Obesity | 87.0 | Normal | |
| F | 165.3 | 51.0 | 18.7 | Normal | 76.0 | Normal | 64.0 | Normal | |
| F | 172.3 | 64.7 | 21.8 | Normal | 69.0 | Normal | 96.0 | Normal | |
| F | 165.7 | 91.2 | 33.2 | Obesity Class I | 120.0 | Abdominal Obesity | 147.0 | Very High | |
| F | 164.0 | 106.0 | 39.4 | Obesity Class II | 121.0 | Abdominal Obesity | 210.9 | Very High | |
| Mean | - | 164.8 * | 76.1 | 28.0 | - | 99.4 | - | 130.0 | - |
| SD | - | 5.1 | 21.3 | 7.9 | - | 22.7 | - | 56.9 | - |
| M | 185.5 | 83.0 | 24.1 | Normal | 95.0 | Normal | 68.5 | Normal | |
| M | 187.5 | 97.5 | 27.7 | Overweight | 97.5 | Normal | 90.5 | Increased Risk | |
| M | 167.7 | 71.9 | 25.6 | Overweight | 90.5 | Normal | 70.3 | Increased Risk | |
| M | 177.0 | 104.5 | 33.4 | Obesity Class I | 114.0 | Abdominal Obesity | 155.4 | Very High Risk | |
| M | 179.6 | 113.7 | 35.2 | Obesity Class II | 99.0 | Normal | 122.5 | Very High Risk | |
| M | 180.0 | 96.6 | 29.8 | Overweight | 103.5 | Abdominal Obesity | 133.0 | High Risk | |
| M | 178.5 | 106.4 | 33.4 | Obesity Class I | 112.0 | Abdominal Obesity | 180.0 | Very High Risk | |
| Mean | - | 179.4 | 96.2 | 29.9 | - | 101.6 | - | 117.2 | - |
| SD | - | 6.4 | 14.4 | 4.3 | - | 8.7 | - | 42.7 | - |
| Group Mean | - | 172.7 | 86.9 | 29.0 | - | 100.6 | - | 123.1 | - |
| SD | - | 9.4 | 20.1 | 6.0 | - | 15.9 | - | 48.1 | - |
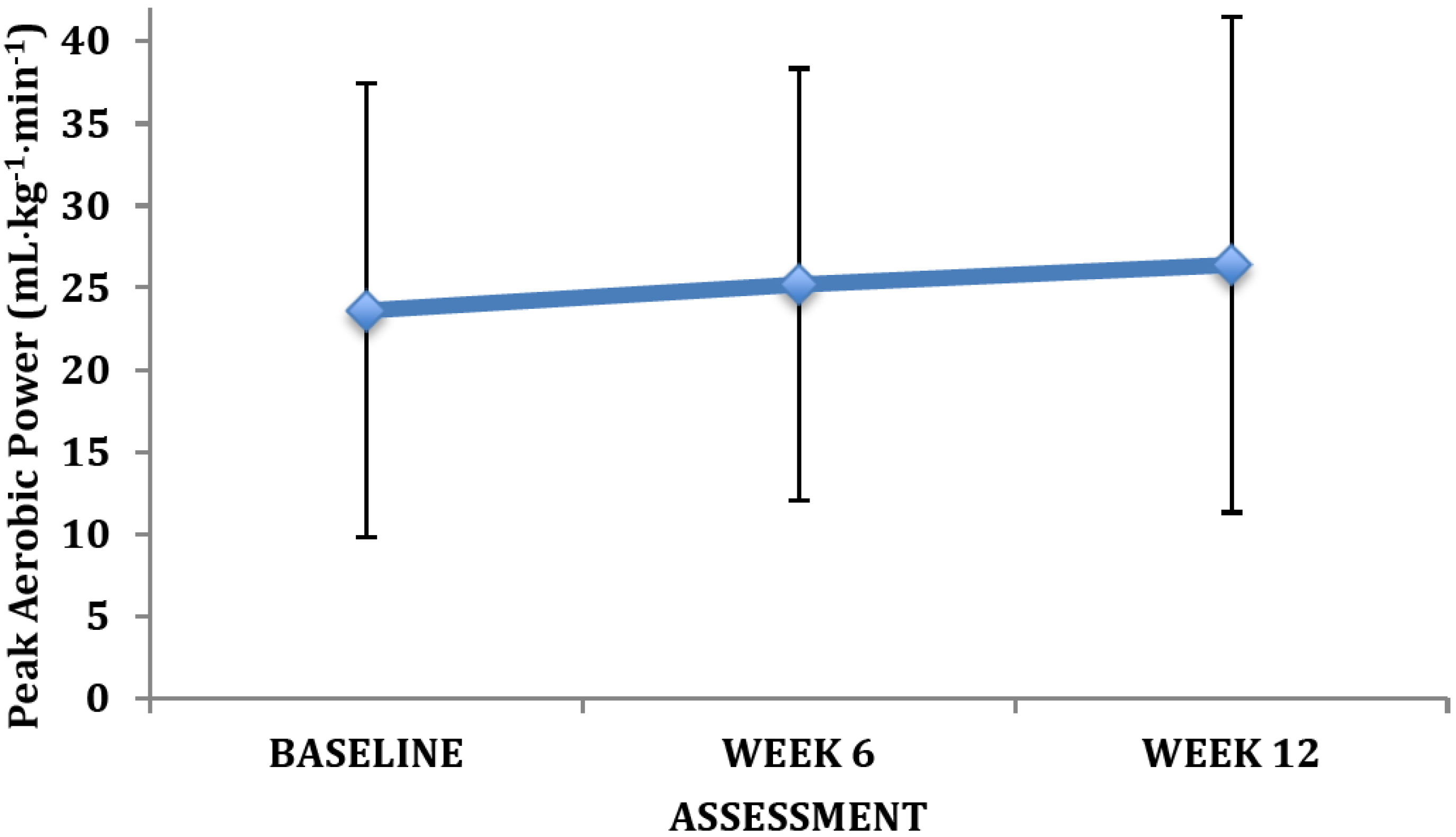
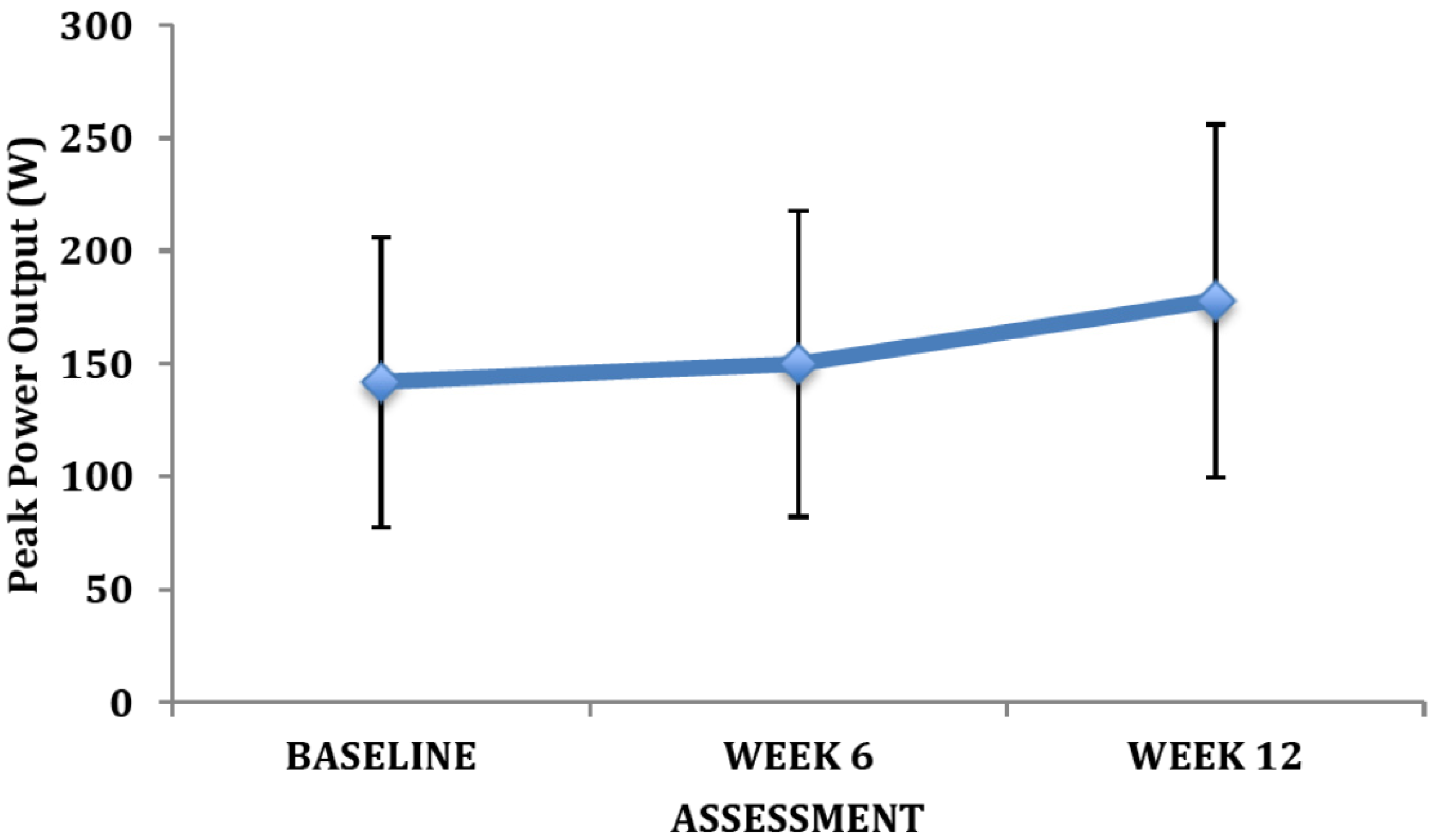
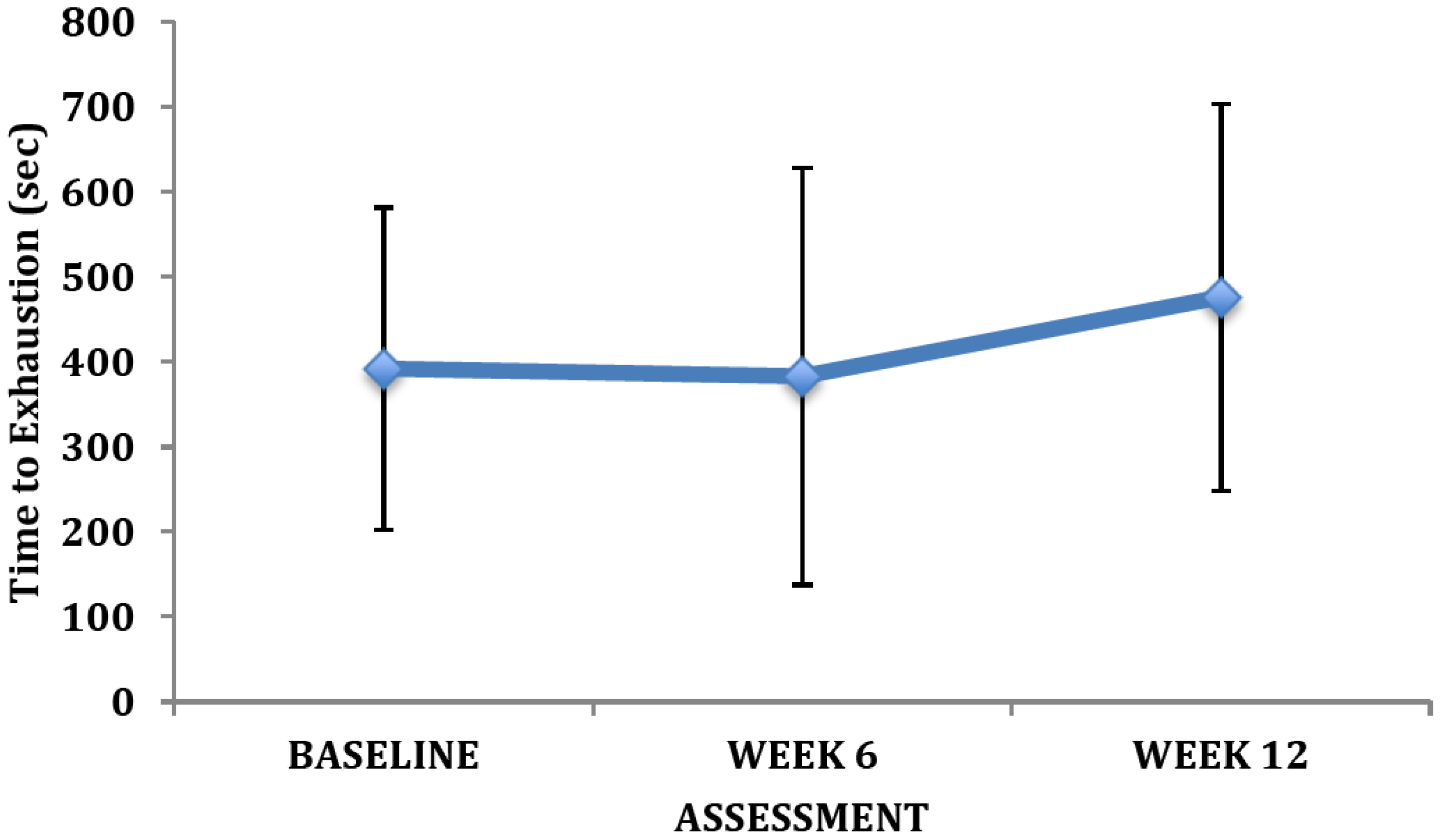
4. Discussion
5. Conclusion
Acknowledgements
References
- Hafner, H.; an der Heiden, W. Epidemiology of schizophrenia. Can. J. Psychiatry 1997, 42, 139–151. [Google Scholar]
- Public Health Agency of Canada, A Report on Mental Illnesses in Canada; Public Health Agency of Canada: Ottawa, Canada, 2002.
- Goeree, R.; Farahati, F.; Burke, N.; Blackhouse, G.; O’Reilly, D.; Pyne, J.; Tarride, J.-E. The economic burden of schizophrenia in Canada. Curr. Med. Res. Opin. 2004, 21, 2017–2028. [Google Scholar]
- Penades, R.; Catalan, R.; Puig, O.; Masana, G.; Pujol, N.; Navarro, V.; Guarch, J.; Gasto, C. Executive function needs to be targeted to improve social functioning with cogitive remediation therapy (CRT) in schizophrenia. Psychiatry Res. 2010, 1771, 41–45. [Google Scholar]
- Aubin, G.; Stip, E.; Gelinas, I.; Rainville, C.; Chapparo, C. Daily activities, cognition and community functioning in persons with schizophrenia. Schizophr. Res. 2009, 107, 313–318. [Google Scholar] [CrossRef]
- Geuze, E.; Vermetten, E.; Bremner, J.D. MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatrici disorders. Mol. Psychiatry 2005, 10, 160–184. [Google Scholar]
- Tamminga, C.A.; Stan, A.D.; Wagner, A.D. The hippocampa formation in schizophrenia. Am. J. Psychiatry 2010, 167, 1178–1193. [Google Scholar] [CrossRef]
- Meda, S.A.; Stevens, M.C.; Folley, B.S.; Calhoun, V.D.; Pearlson, G.D. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: An ICA based analysis. PLoS One 2009, 4, e7911. [Google Scholar]
- Schobel, S.A.; Kelly, M.A.; Corcoran, C.M.; Van Heertum, K.; Seckinger, R.; Goetz, R.; Harkavy-Friedman, J.; Malaspina, D. Anterior hippocampal and orbitofrontal cortical structural brain abnormalities in association with cognitive deficits in schizophrenia. Schizophr. Res. 2009, 114, 110–118. [Google Scholar] [CrossRef]
- Ingenhoven, T.; Lafay, P.; Rinne, T.; Passchier, J.; Duivenvoorden, H. Effectiveness of pharmacotherapy for severe personality disorders: Meta-analyses of randomized control trials. J. Clin. Psychiatry 2010, 71, 14–25. [Google Scholar]
- Penn, D.L.; Keefe, R.S.; Davis, S.M.; Meyer, P.S.; Perkins, D.O.; Losardo, D.; Lieberman, J.A. The effects of antipsychotic medications on emotion perception in patients with chronic schizophrenia in the CATIE trial. Schizophr. Res. 2009, 115, 17–23. [Google Scholar] [CrossRef]
- De Hert, M.; Dekker, J.M.; Wood, D.; Kahl, K.G.; Holt, R.I.; Moller, H.J. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur. Psychiatry 2009, 24, 412–424. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Vancampfort, D.; Sweers, K.; van Winkel, R.; Yu, W.; de Hert, M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr. Bull. 2013, 39, 306–318. [Google Scholar] [CrossRef]
- Coodin, S. Body mass index in persons with schizophrenia. Can. J. Psychiatry 2001, 46, 549–555. [Google Scholar]
- Parks, J.; Svendsen, D.; Singer, P.; Foti, M. Morbidity and Mortality in People with Serious Mental Illness; National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council: Alexandria, VA, USA, 2006; p. 87. [Google Scholar]
- Regenold, W.T.; Thapar, R.K.; Marano, C.; Gavirneni, S.; Kondapavuluru, P.V. Increased prevalence of type 2 diabetes mellitus among psychiatric inpatients with bipolar I affective and schizoaffective disorders independent of psychotropic drug use. J. Affect. Disord. 2002, 70, 19–26. [Google Scholar] [CrossRef]
- De Hert, M.; Mauri, M.; Shaw, K.; Wetterling, T.; Doble, A.; Giudicelli, A.; Falissard, B. The METEOR study of diabetes and other metabolic disorders in patients with schizophrenia treated with antipsychotic drugs. I. Methodology. Int. J. Methods Psychiatr. Res. 2010, 19, 195–210. [Google Scholar] [CrossRef]
- De Hert, M.; Correll, C.U.; Bobes, J.; Cetkovich-Bakmas, M.; Cohen, D.; Asai, I.; Detraux, J.; Gautam, S.; Moller, H.J.; Ndetei, D.M.; et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry 2011, 10, 52–77. [Google Scholar]
- Bushe, C.; Holt, R. Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br. J. Psychiatry Suppl. 2004, 47, S67–S71. [Google Scholar] [CrossRef]
- Laursen, T.M.; Munk-Olsen, T.; Vestergaard, M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr. Opin. Psychiatry 2012, 25, 83–88. [Google Scholar] [CrossRef]
- Protopopova, D.; Masopust, J.; Maly, R.; Valis, M.; Bazant, J. The prevalence of cardiometabolic risk factors and the ten-year risk of fatal cardiovascular events in patients with schizophrenia and related psychotic disorders. Psychiatr. Danub. 2012, 24, 307–313. [Google Scholar]
- Meunch, J.; Hamer, A.M. Adverse effects of antipsychotic medications. Am. Fam. Physician 2010, 81, 617–622. [Google Scholar]
- Maayan, L.; Vakhrusheva, J. Risperidone associated weight, leptin, and anthropometric changes in children and adolescents with psychotic disorders in early treatment. Hum. Psychopharmacol. 2010, 25, 133–138. [Google Scholar] [CrossRef]
- Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schmid, F.; Schwarz, S.; Duggan, L.; Kissling, W.; Leucht, S. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Stahl, S.M.; Mignon, L.; Meyer, J.M. Which comes first: Atypical antipsychotic treatment or cardiometabolic risk? Acta Psychiat. Scand. 2009, 120, 500–501. [Google Scholar] [CrossRef]
- Paterson, D.H.; Warburton, D.E. Physical activity and functional limitations in older adults: A systematic review related to Canada’s Physical Activity Guidelines. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 38. [Google Scholar] [CrossRef]
- Warburton, D.E.; Charlesworth, S.; Ivey, A.; Nettlefold, L.; Bredin, S.S. A systematic review of the evidence for Canada’s Physical Activity Guidelines for Adults. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 39. [Google Scholar] [CrossRef]
- Vancampfort, D.; Probst, M.; Helvik Skjaerven, L.; Catalan-Matamoros, D.; Lundvik-Gyllensten, A.; Gomez-Conesa, A.; Ijntema, R.; de Hert, M. Systematic review of the benefits of physical therapy within a multidisciplinary care approach for people with schizophrenia. Phys. Ther. 2012, 92, 11–23. [Google Scholar] [CrossRef]
- Beebe, L.H.; Tian, L.; Morris, N.; Goodwin, A.; Allen, S.S.; Kuldau, J. Effects of exercise on mental and physical health parameters of persons with schizophrenia. Issues Ment. Health Nurs. 2005, 26, 661–676. [Google Scholar] [CrossRef]
- Acil, A.A.; Dogan, S.; Dogan, O. The effects of physical exercises to mental state and quality of life in patients with schizophrenia. J. Psychiat. Ment. Health Nurs. 2008, 15, 808–815. [Google Scholar] [CrossRef]
- Gorczynski, P.; Falkner, G. Exercise therapy for schizophrenia. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Fleschner, M. Physical activity and stress resistance: Sympathetic nervous system adaptations prevent stress-induced immunosuppression. Exerc. Sport Sci. Rev. 2005, 33, 120–126. [Google Scholar] [CrossRef]
- Chaddock, L.; Erickson, K.I.; Prakash, R.S.; Kim, J.S.; Voss, M.W.; Vanpatter, M.; Pontifex, M.B.; Raine, L.B.; Konkel, A.; Hillman, C.H.; et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010, 1358, 172–183. [Google Scholar] [CrossRef]
- Laske, C.; Banschbach, S.; Stransky, E.; Bosch, S.; Straten, G.; Machann, J.; Fritsche, A.; Hipp, A.; Niess, A.; Eschweiler, G.W. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int. J. Neuropsychopharmacol. 2010, 13, 595–602. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Jamnik, V.K.; Bredin, S.S.D.; Gledhill, N. The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit. J. Can. 2011, 4, 3–23. [Google Scholar]
- Warburton, D.E.R.; Bredin, S.S.D.; Jamnik, V.; Gledhill, N. Validation of the PAR-Q+ and ePARmed-X+. Health Fit. J. Can. 2011, 4, 38–46. [Google Scholar]
- Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. National Institutes of Health. Obes. Res. 1998, 6 (Suppl. 2), 51S–209S. [CrossRef]
- Gledhill, N.; Jamnik, V. Canadian Physical Activity, Fitness and Lifestyle Approach, 3rd ed; Canadian Society for Exercise Physiology: Ottawa, Canada, 2003. [Google Scholar]
- National Institutes of Health, The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Institutes of Health: Bethesda, MD, USA, 2004.
- Cooney, M.T.; Vartiainen, E.; Laatikainen, T.; Juolevi, A.; Dudina, A.; Graham, I.M. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2010, 159, 612–619. [Google Scholar] [CrossRef]
- Jones, N.L. Clinical Exercise Testing, 3rd ed; Saunders: Philadelphia, PA, USA, 1988. [Google Scholar]
- Godin, G.; Shephard, R.J. A simple method to assess exercise behavior in the community. Can. J. Appl. Sport Sci. 1985, 10, 141–146. [Google Scholar]
- Godin, G.; Jobin, J.; Bouillon, J. Assessment of leisure time exercise behavior by self-report: A concurrent validity study. Can. J. Public Health 1986, 77, 359–362. [Google Scholar]
- Trinh, L.; Plotnikoff, R.C.; Rhodes, R.E.; North, S.; Courneya, K.S. Correlates of physical activity in a population-based sample of kidney cancer survivors: An application of the theory of planned behavior. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 96. [Google Scholar] [CrossRef]
- Godin, A. The Godin—Shephard Leisure—Time Physical Activity Questionnaire. Health Fit. J. Can. 2011, 4, 18–22. [Google Scholar]
- Tremblay, M.S.; Warburton, D.E.; Janssen, I.; Paterson, D.H.; Latimer, A.E.; Rhodes, R.E.; Kho, M.E.; Hicks, A.; Leblanc, A.G.; Zehr, L.; et al. New canadian physical activity guidelines. Appl. Physiol. Nutr. Metab. 2011, 36, 36–46. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.; Bredin, S.S. Prescribing exercise as preventive therapy. Can. Med. Assoc. J. 2006, 174, 961–974. [Google Scholar] [CrossRef]
- Warburton, D.E.; Nicol, C.; Bredin, S.S. Health benefits of physical activity: The evidence. Can. Med. Assoc. J. 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D.; Giacomantonio, N. Clinical Exercise Prescription for Atrial Fibrillation; International Collaboration on Clinical Exercise Prescription: Vancouver, Canada, 2012. [Google Scholar]
- Stone, J.A. Canadian Guidelines for Cardiac Rehabilitation and Cardiovascular Disease Prevention: Translating Knowledge into Action, 3rd ed; Canadian Association of Cardiac Rehabilitation: Winnipeg, Cannda, 2009. [Google Scholar]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef]
- Verweij, L.M.; Terwee, C.B.; Proper, K.I.; Hulshof, C.T.; van Mechelen, W. Measurement error of waist circumference: Gaps in knowledge. Public Health Nutr. 2013, 16, 281–288. [Google Scholar] [CrossRef]
- National Institutes of Health, The Practical Guide: The Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Institutes of Health: Bethesda, MD, USA, 2000.
- Alberti, K.G.; Zimmet, P.; Shaw, J. The metabolic syndrome—a new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Bond, D.J.; Lang, D.J.; Noronha, M.M.; Kunz, M.; Torres, I.J.; Su, W.; Honer, W.G.; Lam, R.W.; Yatham, L.N. The association of elevated body mass index with reduced brain volumes in first-episode mania. Biol. Psychiatry 2011, 70, 381–387. [Google Scholar] [CrossRef]
- Ward, M.A.; Carlsson, C.M.; Trivedi, M.A.; Sager, M.A.; Johnson, S.C. The effect of body mass index on global brain volume in middle-aged adults: A cross sectional study. BMC Neurol. 2005, 5, 23. [Google Scholar] [CrossRef]
- Taki, Y.; Kinomura, S.; Sato, K.; Inoue, K.; Goto, R.; Okada, K.; Uchida, S.; Kawashima, R.; Fukuda, H. Relationship between body mass index and gray matter volume in 1428 healthy individuals. Obesity (Silver Spring) 2008, 16, 119–124. [Google Scholar] [CrossRef]
- Gunstad, J.; Paul, R.H.; Cohen, R.A.; Tate, D.F.; Spitznagel, M.B.; Grieve, S.; Gordon, E. Relationship between body mass index and brain volume in healthy adults. Int. J. Neurosci. 2008, 118, 1582–1593. [Google Scholar] [CrossRef]
- Soreca, I.; Rosano, C.; Jennings, J.R.; Sheu, L.K.; Kuller, L.H.; Matthews, K.A.; Aizenstein, H.J.; Gianaros, P.J. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom. Med. 2009, 71, 485–490. [Google Scholar] [CrossRef]
- Pedrini, S.; Thomas, C.; Brautigam, H.; Schmeidler, J.; Ho, L.; Fraser, P.; Westaway, D.; Hyslop, P.S.; Martins, R.N.; Buxbaum, J.D.; et al. Dietary composition modulates brain mass and solubilizable Abeta levels in a mouse model of aggressive Alzheimer’s amyloid pathology. Mol. Neurodegener. 2009, 4, 40. [Google Scholar] [CrossRef]
- Wang, P.W.; Sachs, G.S.; Zarate, C.A.; Marangell, L.B.; Calabrese, J.R.; Goldberg, J.F.; Sagduyu, K.; Miyahara, S.; Ketter, T.A. Overweight and obesity in bipolar disorders. J. Psychiatr. Res. 2006, 40, 762–764. [Google Scholar] [CrossRef]
- Fagiolini, A.; Frank, E.; Scott, J.A.; Turkin, S.; Kupfer, D.J. Metabolic syndrome in bipolar disorder: Findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005, 7, 424–430. [Google Scholar] [CrossRef]
- Fagiolini, A.; Frank, E.; Houck, P.R.; Mallinger, A.G.; Swartz, H.A.; Buysse, D.J.; Ombao, H.; Kupfer, D.J. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J. Clin. Psychiatry 2002, 63, 528–533. [Google Scholar] [CrossRef]
- Fagiolini, A.; Kupfer, D.J.; Rucci, P.; Scott, J.A.; Novick, D.M.; Frank, E. Suicide attempts and ideation in patients with bipolar I disorder. J. Clin. Psychiatry 2004, 65, 509–514. [Google Scholar] [CrossRef]
- Fagiolini, A.; Kupfer, D.J.; Houck, P.R.; Novick, D.M.; Frank, E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am. J. Psychiatry 2003, 160, 112–117. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef]
- Katzmarzyk, P.T.; Craig, C.L.; Bouchard, C. Original article underweight, overweight and obesity: Relationships with mortality in the 13-year follow-up of the Canada Fitness Survey. J. Clin. Epidemiol. 2001, 54, 916–920. [Google Scholar] [CrossRef]
- Statistics Canada Health Indicators. Available online: http://www.statcan.gc.ca/ (accessed on 1 December 2006).
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar]
- Wilkins, K.; Campbell, N.R.; Joffres, M.R.; McAlister, F.A.; Nichol, M.; Quach, S.; Johansen, H.L.; Tremblay, M.S. Blood pressure in Canadian adults. Health Rep. 2010, 21, 37–46. [Google Scholar]
- Ciranni, M.A.; Kearney, T.E.; Olson, K.R. Comparing acute toxicity of first- and second-generation antipsychotic drugs: A 10-year, retrospective cohort study. J. Clin. Psychiatry 2009, 70, 122–129. [Google Scholar] [CrossRef]
- Institute of Medicine (U.S.), A Population-Based Policy and Systems Change Approach to Prevent and Control Hypertension; National Academies Press: Washington, DC, USA, 2010.
- Yano, K.; McGee, D.; Reed, D.M. The impact of elevated blood pressure upon 10-year mortality among Japanese men in Hawaii: The Honolulu Heart Program. J. Chronic Dis. 1983, 36, 569–579. [Google Scholar] [CrossRef]
- Wei, G.S.; Coady, S.A.; Goff, D.C., Jr.; Brancati, F.L.; Levy, D.; Selvin, E.; Vasan, R.S.; Fox, C.S. Blood Pressure and the Risk of Developing Diabetes in African Americans and Whites: ARIC, CARDIA, and the Framingham Heart Study. Diabetes Care 2011, 34, 873–879. [Google Scholar] [CrossRef]
- Jensen, M.T.; Marott, J.L.; Jensen, G.B. Elevated resting heart rate is associated with greater risk of cardiovascular and all-cause mortality in current and former smokers. Int. J. Cardiol. 2011, 151, 148–154. [Google Scholar] [CrossRef]
- Rogowski, O.; Steinvil, A.; Berliner, S.; Cohen, M.; Saar, N.; Ben-Bassat, O.K.; Shapira, I. Elevated resting heart rate is associated with the metabolic syndrome. Cardiovasc. Diabetol. 2009, 8, 55. [Google Scholar] [CrossRef]
- Casey, D.E. Dyslipidemia and atypical antipsychotic drugs. J. Clin. Psychiatry 2004, 65 (Suppl. 18), 27–35. [Google Scholar]
- Colman, R.; Walker, S. The Cost of Physical Inactivity in British Columbia; Genuine Progress Index Atlantic: Victoria, Canada, 2004; p. 31. [Google Scholar]
- Katzmarzyk, P.T.; Janssen, I. The economic costs associated with physical inactivity and obesity in Canada: An update. Can. J. Appl. Physiol. 2004, 29, 90–115. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Katzmarzyk, P.T.; Rhodes, R.E.; Shephard, R.J. Evidence-informed physical activity guidelines for Canadian adults. Appl. Physiol. Nutr. Metab. 2007, 32, S16–S68. [Google Scholar] [CrossRef]
- Ivy, J.L.; Zderic, T.W.; Fogt, D.L. Prevention and treatment of non-insulin-dependent diabetes mellitus. Exerc. Sport Sci. Rev. 1999, 27, 1–35. [Google Scholar] [CrossRef]
- Kriska, A.M.; Saremi, A.; Hanson, R.L.; Bennett, P.H.; Kobes, S.; Williams, D.E.; Knowler, W.C. Physical activity, obesity, and the incidence of type 2 diabetes in a high-risk population. Am. J. Epidemiol. 2003, 158, 669–675. [Google Scholar] [CrossRef]
- De Hert, M.; Vancampfort, D.; Correll, C.U.; Mercken, V.; Peuskens, J.; Sweers, K.; van Winkel, R.; Mitchell, A.J. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: Systematic evaluation. Br. J. Psychiatry 2011, 199, 99–105. [Google Scholar] [CrossRef]
- Faulkner, G.; Taylor, A.; Munro, S.; Selby, P.; Gee, C. The acceptability of physical activity programming within a smoking cessation service for individuals with severe mental illness. Patient Educ. Couns. 2007, 66, 123–126. [Google Scholar] [CrossRef]
- Vancampfort, D.; Probst, M.; Sweers, K.; Maurissen, K.; Knapen, J.; de Hert, M. Relationships between obesity, functional exercise capacity, physical activity participation and physical self-perception in people with schizophrenia. Acta Psychiatr. Scand. 2011, 123, 423–430. [Google Scholar] [CrossRef]
- Xiong, G.; Ziegahn, L.; Schuyler, B.; Rowlett, A.; Cassady, D. Improving dietary and physical activity practices in group homes serving residents with severe mental illness. Prog. Community Health Partnersh. 2010, 4, 279–288. [Google Scholar]
- Jerome, G.J.; Young, D.R.; Dalcin, A.; Charleston, J.; Anthony, C.; Hayes, J.; Daumit, G.L. Physical activity levels of persons with mental illness attending psychiatric rehabilitation programs. Schizophr. Res. 2009, 108, 252–257. [Google Scholar] [CrossRef]
- Daumit, G.L.; Goldberg, R.W.; Anthony, C.; Dickerson, F.; Brown, C.H.; Kreyenbuhl, J.; Wohlheiter, K.; Dixon, L.B. Physical activity patterns in adults with severe mental illness. J. Nerv. Ment. Dis. 2005, 193, 641–646. [Google Scholar] [CrossRef]
- Loitz, C.; Fraser, S.N.; Benegoechea, G.; Berry, T.R.; McGannon, K.R.; Spence, J.C. Sociodemographic patterns of leisure-time physical activity of Albertans 2000 to 2011. Health Fit. J. Can. 2012, 5, 3–15. [Google Scholar]
- Ryan, M.C.; Flanagan, S.; Kinsella, U.; Keeling, F.; Thakore, J.H. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug-naive patients with schizophrenia. Life Sci. 2004, 74, 1999–2008. [Google Scholar] [CrossRef]
- Warburton, D.E.; Taylor, A.; Bredin, S.S.; Esch, B.T.; Scott, J.M.; Haykowsky, M.J. Central haemodynamics and peripheral muscle function during exercise in patients with chronic heart failure. Appl. Physiol. Nutr. Metab. 2007, 32, 318–331. [Google Scholar] [CrossRef]
- Ussher, M.; Stanbury, L.; Cheeseman, V.; Faulkner, G. Physical activity preferences and perceived barriers to activity among persons with severe mental illness in the United Kingdom. Psychiatr. Serv. 2007, 58, 405–408. [Google Scholar] [CrossRef]
- Sallis, J.F. Progress in behavioral research on physical activity. Ann. Behav. Med. 2001, 23, 77–78. [Google Scholar] [CrossRef]
- Warburton, D.E.; Bredin, S.S.; Horita, L.T.; Zbogar, D.; Scott, J.M.; Esch, B.T.; Rhodes, R.E. The health benefits of interactive video game exercise. Appl. Physiol. Nutr. Metab. 2007, 32, 655–663. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Bredin, S.S.D. The importance of qualified exercise professionals in Canada. Health Fit. J. Can. 2009, 2, 18–22. [Google Scholar]
- Warburton, D.E.R.; Bredin, S.S.D.; Charlesworth, S.; Foulds, H.; McKenzie, D.C.; Shephard, R.J. Evidence-based risk recommendations for best practices in the training of qualified exercise professionals working with clinical populations. Appl. Physiol. Nutr. Metab. 2011, 36, S232–S265. [Google Scholar] [CrossRef]
- Martin, J.L.; Perez, V.; Sacristan, M.; Rodriguez-Artalejo, F.; Martinez, C.; Alvarez, E. Meta-analysis of drop-out rates in randomised clinical trials, comparing typical and atypical antipsychotics in the treatment of schizophrenia. Eur. Psychiatry 2006, 21, 11–20. [Google Scholar] [CrossRef]
- Giacomantonio, N.B.; Bredin, S.S.; Foulds, H.J.; Warburton, D.E. A systematic review of the health benefits of exercise rehabilitation in persons living with atrial fibrillation. Can. J. Cardiol. 2013, 29, 483–491. [Google Scholar] [CrossRef]
- Howley, E.T. Type of activity: Resistance, aerobic and leisure versus occupational physical activity. Med. Sci. Sports Exerc. 2001, 33, S364–S369; discussion S419–S420. [Google Scholar] [CrossRef]
- Myers, J.; Kaykha, A.; George, S.; Abella, J.; Zaheer, N.; Lear, S.; Yamazaki, T.; Froelicher, V. Fitness versus physical activity patterns in predicting mortality in men. Am. J. Med. 2004, 117, 912–918. [Google Scholar] [CrossRef]
- Warren, K.R.; Ball, M.P.; Feldman, S.; Liu, F.; McMahon, R.P.; Kelly, D.L. Exercise program adherence using a 5-kilometer (5K) event as an achievable goal in people with schizophrenia. Biol. Res. Nurs. 2011, 13, 383–390. [Google Scholar] [CrossRef]
- Scheewe, T.W.; Backx, F.J.; Takken, T.; Jorg, F.; van Strater, A.C.; Kroes, A.G.; Kahn, R.S.; Cahn, W. Exercise therapy improves mental and physical health in schizophrenia: A randomised controlled trial. Acta Psychiatr. Scand. 2012. [Google Scholar] [CrossRef]
- Dodd, K.J.; Duffy, S.; Stewart, J.A.; Impey, J.; Taylor, N. A small group aerobic exercise programme that reduces body weight is feasible in adults with severe chronic schizophrenia: A pilot study. Disabil. Rehabil. 2011, 33, 1222–1229. [Google Scholar] [CrossRef]
- Pajonk, F.G.; Wobrock, T.; Gruber, O.; Scherk, H.; Berner, D.; Kaizl, I.; Kierer, A.; Muller, S.; Oest, M.; Meyer, T.; et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch. Gen. Psychiatry 2010, 67, 133–143. [Google Scholar] [CrossRef]
- Vancampfort, D.; de Hert, M.; Skjerven, L.H.; Gyllensten, A.L.; Parker, A.; Mulders, N.; Nyboe, L.; Spencer, F.; Probst, M. International Organization of Physical Therapy in Mental Health consensus on physical activity within multidisciplinary rehabilitation programmes for minimising cardio-metabolic risk in patients with schizophrenia. Disabil. Rehabil. 2012, 34, 1–12. [Google Scholar] [CrossRef]
- Bredin, S.S.D.; Warburton, D.E.R. The Physical Activity Line: Effective knowledge translation of evidence-based best practice in the real world setting. Can. Fam. Physician 2013, in press. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bredin, S.S.D.; Warburton, D.E.R.; Lang, D.J. The Health Benefits and Challenges of Exercise Training in Persons Living with Schizophrenia: A Pilot Study. Brain Sci. 2013, 3, 821-848. https://doi.org/10.3390/brainsci3020821
Bredin SSD, Warburton DER, Lang DJ. The Health Benefits and Challenges of Exercise Training in Persons Living with Schizophrenia: A Pilot Study. Brain Sciences. 2013; 3(2):821-848. https://doi.org/10.3390/brainsci3020821
Chicago/Turabian StyleBredin, Shannon S. D., Darren E. R. Warburton, and Donna J. Lang. 2013. "The Health Benefits and Challenges of Exercise Training in Persons Living with Schizophrenia: A Pilot Study" Brain Sciences 3, no. 2: 821-848. https://doi.org/10.3390/brainsci3020821
APA StyleBredin, S. S. D., Warburton, D. E. R., & Lang, D. J. (2013). The Health Benefits and Challenges of Exercise Training in Persons Living with Schizophrenia: A Pilot Study. Brain Sciences, 3(2), 821-848. https://doi.org/10.3390/brainsci3020821




