Management and Outcomes of Multiple Unruptured Cerebral Aneurysms: A Descriptive Cohort Analysis
Abstract
1. Introduction
2. Methods
3. Results
3.1. Patient Demographics, Risk Factors and Symptoms
3.2. Aneurysm Characteristics
3.3. Treatment Stages and Interventions
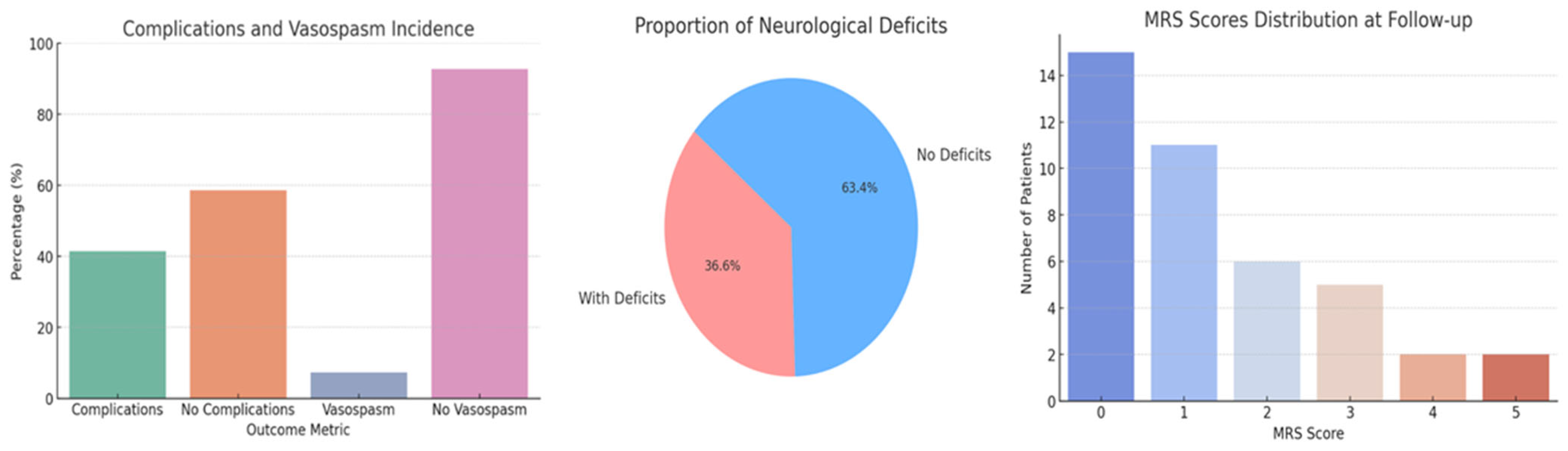
3.4. Outcome Metrics
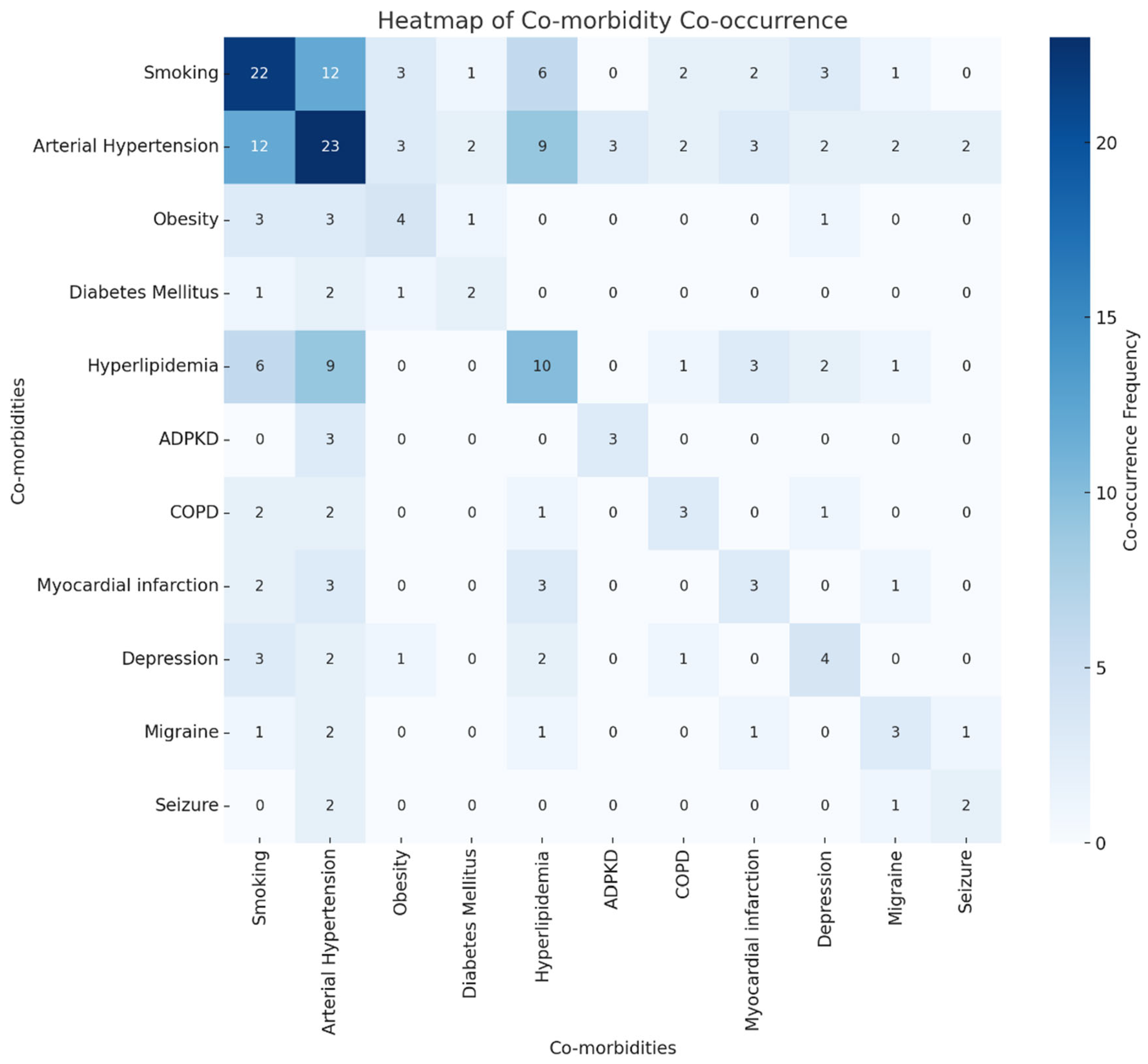
3.5. Impact of Co-Morbidity Combinations on Outcomes
4. Discussion
4.1. Patient Demographics, Risk Factors and Symptoms
4.2. Aneurysm Characteristics
4.3. Treatment Stages and Outcomes
4.4. Impact of Co-Morbidity Combinations on Outcomes
4.5. Future Perspectives
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ruigrok, Y.M. Management of Unruptured Cerebral Aneurysms and Arteriovenous Malformations. Continuum 2020, 26, 478–498. [Google Scholar] [CrossRef] [PubMed]
- Renowden, S.; Nelson, R. Management of incidental unruptured intracranial aneurysms. Pract. Neurol. 2020, 20, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lehnen, N.C.; Haase, R.; Schmeel, F.C.; Vatter, H.; Dorn, F.; Radbruch, A.; Paech, D. Automated Detection of Cerebral Aneurysms on TOF-MRA Using a Deep Learning Approach: An External Validation Study. AJNR Am. J. Neuroradiol. 2022, 43, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Laukka, D.; Kivelev, J.; Rahi, M.; Vahlberg, T.; Paturi, J.; Rinne, J.; Hirvonen, J. Detection Rates and Trends of Asymptomatic Unruptured Intracranial Aneurysms From 2005 to 2019. Neurosurgery 2024, 94, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Feng, X.; Tong, X.; Wen, Z.; Zhu, Y.; Xu, A.; Huang, M.; Ma, G.; Hu, Y.; Shi, H.; et al. Stent-to-vessel diameter ratio is associated with in-stent stenosis after flow-diversion treatment of intracranial aneurysms. J. Stroke Cerebrovasc. Dis. 2024, 33, 107833. [Google Scholar] [CrossRef] [PubMed]
- Rinkel, G.J.; Ruigrok, Y.M. Preventive screening for intracranial aneurysms. Int. J. Stroke 2022, 17, 30–36. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, Z.; Zhao, Y.; Gu, X.; Fang, J.; Yang, J.; Li, T.; Fan, B. Systematic Review of Treatment for Unruptured Intracranial Aneurysms: Clipping Versus Coiling. Turk. Neurosurg. 2024, 34, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Krzyzewski, R.M.; Kliś, K.M.; Kucala, R.; Polak, J.; Kwinta, B.M.; Starowicz-Filip, A.; Stachura, K.; Piszczek, K.; Moskała, M.; Tomaszewski, K.A. Intracranial aneurysm distribution and characteristics according to gender. Br. J. Neurosurg. 2018, 32, 541–543. [Google Scholar] [CrossRef]
- Xin, W.Q.; Sun, P.J.; Li, F.; Cheng, M.X.; Yang, S.X.; Cui, B.L.; Wang, Z.G.; Yang, X.Y. Risk factors involved in the formation of multiple intracranial aneurysms. Clin. Neurol. Neurosurg. 2020, 198, 106172. [Google Scholar] [CrossRef] [PubMed]
- Freneau, M.; Baron-Menguy, C.; Vion, A.C.; Loirand, G. Why Are Women Predisposed to Intracranial Aneurysm? Front. Cardiovasc. Med. 2022, 9, 815668. [Google Scholar] [CrossRef]
- Ahmad, S. Clinical outcome of endovascular coil embolization for cerebral aneurysms in Asian population in relation to risk factors: A 3-year retrospective analysis. BMC Surg. 2020, 20, 104. [Google Scholar] [CrossRef]
- Karhunen, V.; Bakker, M.K.; Ruigrok, Y.M.; Gill, D.; Larsson, S.C. Modifiable Risk Factors for Intracranial Aneurysm and Aneurysmal Subarachnoid Hemorrhage: A Mendelian Randomization Study. J. Am. Heart Assoc. 2021, 10, e022277. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Wang, J.; Zhang, L.; Li, C. The Biological Effects of Smoking on the Formation and Rupture of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Front. Neurol. 2022, 13, 862916. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Can, A.; Castro, V.M.; Dligach, D.; Finan, S.; Yu, S.; Gainer, V.; Shadick, N.A.; Savova, G.; Murphy, S.; Cai, T.; et al. Lipid-Lowering Agents and High HDL (High-Density Lipoprotein) Are Inversely Associated With Intracranial Aneurysm Rupture. Stroke 2018, 49, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.K. Headache and Aneurysm. Neuroimaging Clin. N. Am. 2019, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, B.; Ma, C.; Liu, L.; Ren, L.; Fang, Y.; Dai, D.; Chen, S.; Lu, J.; Boltze, J. 3D contrast enhancement-MR angiography for imaging of unruptured cerebral aneurysms: A hospital-based prevalence study. PLoS ONE 2014, 9, e114157. [Google Scholar] [CrossRef]
- Vu, D.L.; Nguyen, V.H.; Nguyen, H.A.; Nguyen, Q.A.; Tran, A.T.; Le, H.K.; Nguyen, T.T.; Nguyen, T.T.; Tran, C.; Tran, X.B.; et al. Hemodynamic Characteristics in Ruptured and Unruptured Intracranial Aneurysms: A Prospective Cohort Study Utilizing the AneurysmFlow Tool. AJNR Am. J. Neuroradiol. 2025, 46, 75–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, L.; Wang, H.; Chen, Y.; Dai, Y.; Lin, B.; Liang, F.; Wan, J.; Yang, Y.; Zhao, B. Morphological and Hemodynamic Factors Associated with Ruptured Middle Cerebral Artery Mirror Aneurysms: A Retrospective Study. World Neurosurg. 2020, 137, e138–e143. [Google Scholar] [CrossRef] [PubMed]
- Soldozy, S.; Norat, P.; Elsarrag, M.; Chatrath, A.; Costello, J.S.; Sokolowski, J.D.; Tvrdik, P.; Kalani, M.Y.S.; Park, M.S. The biophysical role of hemodynamics in the pathogenesis of cerebral aneurysm formation and rupture. Neurosurg. Focus 2019, 47, E11. [Google Scholar] [CrossRef]
- Cmiel-Smorzyk, K.; Kawlewska, E.; Wolanski, W.; Hebda, A.; Ladzinski, P.; Kaspera, W. Morphometry of cerebral arterial bifurcations harbouring aneurysms: A case-control study. BMC Neurol. 2022, 22, 49. [Google Scholar] [CrossRef]
- Zakeri, M.; Atef, A.; Aziznia, M.; Jafari, A. A comprehensive investigation of morphological features responsible for cerebral aneurysm rupture using machine learning. Sci. Rep. 2024, 14, 15777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, L.; Dong, S.; Kheiri, A.A. Influence of physiological conditions on hemodynamics of internal carotid artery aneurysms. Sci. Rep. 2024, 14, 23106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toma, A.; De La Garza Ramos, R.; Altschul, D.J. Risk Factors for Headache Disorder in Patients With Unruptured Intracranial Aneurysms. Cureus 2023, 15, e38385. [Google Scholar] [CrossRef] [PubMed]
- Valenca, M.M.; Andrade-Valenca, L.P.; Martins, C.; de Aragão, M.F.V.; Batista, L.L.; Peres, M.F.P.; da Silva, W.F. Cluster headache and intracranial aneurysm. J. Headache Pain. 2007, 8, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liu, A.; Yang, X.; Li, Y.; Jiang, C.; Wu, Z. Incidence and predictors of headache relief after endovascular treatment in patients with unruptured intracranial aneurysms. Interv. Neuroradiol. 2017, 23, 18–27. [Google Scholar] [CrossRef]
- Schneider, A.M.; Moore, J.M.; Adeeb, N.; Gupta, R.; Griessenauer, C.J.; Winkler, P.A.; Sieber, S.; Alturki, A.Y.; Ogilvy, C.S.; Thomas, A.J. Self-Reported Headaches in Patients with Unruptured Intracranial Aneurysms Treated with the Pipeline Embolization Device. World Neurosurg. 2018, 113, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.L.; Lopes, D.; Miller, D.A.; Tawk, R.G.; Brasiliense, L.B.; Ringer, A.; Sauvageau, E.; Powers, C.J.; Arthur, A.; Hoit, D.; et al. The fate of cranial neuropathy after flow diversion for carotid aneurysms. J. Neurosurg. 2016, 124, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.K. Motor Aphasia Attributed to Middle Cerebral Artery Aneurysm. J. Neurol. Neurosci. 2017, 8, i232. [Google Scholar] [CrossRef]
- Fukuda, T.; Imamura, H.; Tani, S.; Adachi, H.; Fukumitsu, R.; Sunohara, T.; Omura, Y.; Funakoshi, Y.; Sasaki, N.; Matsui, Y.; et al. Treatment Strategies for Unruptured Multiple Intracranial Aneurysms. Neurol. Surg. 2019, 47, 943–947. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, R.; Wei, Y.; Chen, R.; Zhang, X.; Xue, G.; Lv, N.; Duan, G.; Wang, C.; Yu, Y.; et al. Comparison of staged-stent and stent-assisted coiling technique for ruptured saccular wide-necked intracranial aneurysms: Safety and efficacy based on a propensity score-matched cohort study. Front. Neurol. 2023, 14, 1101859. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nounaka, Y.; Shirokane, K.; Matano, F.; Koketsu, K.; Kubota, A.; Morita, A.; Murai, Y. The Second-Set Surgeries for Multiple Unruptured Aneurysms Do Not Increase Perioperative Complications. Neurosurg. Pract. 2024, 5, e00037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohammad, F.; Horiguchi, T.; Mizutani, K.; Yoshida, K. Clipping versus coiling in unruptured anterior cerebral circulation aneurysms. Surg. Neurol. Int. 2020, 11, 50. [Google Scholar] [CrossRef]
- Asilturk, M.; Abdallah, A. Clinical outcomes of multiple aneurysms microsurgical clipping: Evaluation of 90 patients. Neurol. Neurochir. Pol. 2018, 52, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Podila, S.S.; Girotra, T. Unruptured aneurysmal clipping complicated by delayed and refractory vasospasm: Case report. BMC Neurol. 2020, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Campe, C.; Neumann, J.; Sandalcioglu, I.E.; Rashidi, A.; Luchtmann, M. Vasospasm and delayed cerebral ischemia after uneventful clipping of an unruptured intracranial aneurysm—A case report. BMC Neurol. 2019, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Tsyben, A.; Paldor, I.; Laidlaw, J. Cerebral vasospasm and delayed ischaemic deficit following elective aneurysm clipping. J. Clin. Neurosci. 2016, 34, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, J. Complex interactions and composite burden of risk factors in vascular cognitive impairment. J. Neurol. Sci. 2025, 468, 123367. [Google Scholar] [CrossRef] [PubMed]
- Algra, A.M.; Lindgren, A.; Vergouwen, M.D.I.; Greving, J.P.; van der Schaaf, I.C.; van Doormaal, T.P.C.; Rinkel, G.J.E. Procedural Clinical Complications, Case-Fatality Risks, and Risk Factors in Endovascular and Neurosurgical Treatment of Unruptured Intracranial Aneurysms: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 282–293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fingerlin, T.J.; Rychen, J.; Roethlisberger, M.; Taub, E.; Mariani, L.; Guzman, R.; Zumofen, D.W. Long-term aneurysm recurrence and de novo aneurysm formation after surgical treatment of unruptured intracranial aneurysms: A cohort study and systematic review. Neurol Res. 2020, 42, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Egeto, P.; Loch Macdonald, R.; Ornstein, T.J.; Schweizer, T.A. Neuropsychological function after endovascular and neurosurgical treatment of subarachnoid hemorrhage: A systematic review and meta-analysis. J. Neurosurg. 2018, 128, 768–776. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.H.; Jeon, J.P.; Choi, H.C.; Choi, H.J. Clinical Factors Contributing to Cognitive Function in the Acute Stage after Treatment of Intracranial Aneurysms: A Cross-Sectional Study. J. Clin. Med. 2022, 11, 5053. [Google Scholar] [CrossRef]
- Barhouse, P.; Young, M.; Taussky, P.; Pacheco-Barrios, N.; Ogilvy, C.S. Anterior choroidal artery aneurysms: A systematic review and meta-analysis of outcomes and ischemic complications following surgical and endovascular treatment. J. Neurosurg. 2024, 142, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.S.; Priola, S.M.; Katsanos, A.H.; Scalia, G.; Alves, A.C.; Srivastava, A.; Hawkes, C. The Management of Intracranial Aneurysms: Current Trends and Future Directions. Neurol. Int. 2024, 16, 74–94. [Google Scholar] [CrossRef]
- Hurford, R.; Taveira, I.; Kuker, W.; Rothwell, P.M.; Oxford Vascular Study Phenotyped Cohort. Prevalence, predictors and prognosis of incidental intracranial aneurysms in patients with suspected TIA and minor stroke: A population-based study and systematic review. J. Neurol. Neurosurg. Psychiatry 2021, 92, 542–548. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Davies, L.; Raki, C.; Lai, L.T. Natural history of small incidental intracranial aneurysms: A systematic review and pooled analysis on the influence of follow-up duration and aneurysm location on rupture risk reporting. J. Clin. Neurosci. 2025, 136, 111241. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, A.; Umman, V.; Oral, G.; Erginoz, E.; Carkman, M.S. Assessing the effectiveness of ACS surgical risk calculator versus P-POSSUM in predicting mortality and morbidity for major hepatobiliary surgery: An observational study. Medicine 2024, 103, e38973. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]



| Category | Metric | Value |
|---|---|---|
| Patient Demographics | Female | 82.93% (95% CI: 68.7–91.5) |
| Male | 17.07% (95% CI: 8.5–31.3) | |
| Age (Mean) | 56.8 ± 10.9 years | |
| Age (Median) | 58 years | |
| Risk Factors/Comorbidities | Yes | 92.7% |
| No | 7.3% | |
| Hypertension | 56.1% (95% CI: 41.0–70.1) | |
| Smoking | 53.7% (95% CI: 38.7–67.9) | |
| Hyperlipidemia | 24.4% (95% CI: 13.8–39.3) | |
| Depression | 9.8% (95% CI: 3.9–22.5) | |
| Obesity | 9.8% (95% CI: 3.9–22.5) | |
| Symptoms | Total Symptomatic | 82.9% |
| Headache | 48.8% (95% CI: 34.3–63.5) | |
| Vertigo | 17.1% (95% CI: 8.5–31.3) | |
| Visual Disturbances | 14.6% (95% CI: 6.9–28.4) | |
| Asymptomatic Rate | 17.1% (95% CI: 17.1–31.3) | |
| Family History | Yes | 12.2% (95% CI: 12.2–25.5) |
| No | 87.8% (95% CI: 74.5–94.7) | |
| Risk Factor | Prevalence by Family History | Fisher p-value |
| Hypertension | 40.0% with FH (n = 5), 58.3% without FH (n = 36) | p = 0.64 |
| Smoking | 60.0% with FH (n = 5), 52.8% without FH (n = 36) | p = 1.00 |
| Hyperlipidemia | 0.0% with FH (n = 5), 27.8% without FH (n = 36) | p = 0.31 |
| Characteristic | Detail | Count |
|---|---|---|
| Aneurysm Size | Small (<5 mm) | 40 |
| Medium (5–10 mm) | 48 | |
| Large (>10 mm) | 13 | |
| Top Locations | MCAB Left | 15 |
| MCAB Right | 12 | |
| Acom | 11 | |
| MCA Left | 9 | |
| ACI C6 Left | 8 | |
| ACI C6 Right | 7 | |
| ACI C7 Left | 7 | |
| ACI C7 Right | 6 | |
| MCA Right | 5 | |
| Basilar | 4 | |
| Location Combination | ACI C7 right, MCAB right, MCA left | 2 |
| MCAB right, MCA right, ACI C6 right | 2 | |
| MCAB left, MCA left, ACI C6 left, Acom | 2 | |
| MCAB left, ACI C6 left, Pericallosal left | 2 | |
| MCAB left, ACI C6 right | 2 | |
| MCAB right, MCA left, MCA right, ACI C6 left, Acom | 2 | |
| Number of Aneurysms | 2 Aneurysms | 28 |
| 3 Aneurysms | 8 | |
| 4 Aneurysms | 4 | |
| 5 Aneurysms | 1 |
| Outcome Metrics | Details—Confidence Intervals (Wilson 95%) | |||
|---|---|---|---|---|
| Post-operative Complications * | 41.4% (95% CI: 27.6–56.6) with complications | |||
| Vasospasm Incidence | 7.3% (95% CI: 2.5–19.6) with vasospasm | |||
| Length of 1st Hospital Stay | Mean: 16.8 ± 10.8 days, Range: 7–70 days | |||
| Length of 2nd Hospital Stay | Mean: 10.6 ± 4.0 days, Range: 5–20 days | |||
| Neurological Deficits | 36.6% (95% CI: 23.5–52.0) with deficits | |||
| MRS Scores at Follow-up | Distribution: 0 (15), 1 (11), 2 (6), 3 (5), 4 (2), 5 (2) | |||
| Exploratory analyses of comorbidities and outcomes | ||||
| Comparison | Group A | Group B | Test | p-value |
| Hypertension vs. Post-op Complications | With HTN: 12/23 (52%) | No HTN: 5/18 (28%) | Fisher’s Exact | 0.20 |
| Hospital stay vs. Neurological Deficits | No deficit: median 14 days (n = 26) | With deficit: median 15 days (n = 15) | Mann–Whitney U | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atallah, O.; Alrefaie, K.; Badary, A. Management and Outcomes of Multiple Unruptured Cerebral Aneurysms: A Descriptive Cohort Analysis. Brain Sci. 2025, 15, 973. https://doi.org/10.3390/brainsci15090973
Atallah O, Alrefaie K, Badary A. Management and Outcomes of Multiple Unruptured Cerebral Aneurysms: A Descriptive Cohort Analysis. Brain Sciences. 2025; 15(9):973. https://doi.org/10.3390/brainsci15090973
Chicago/Turabian StyleAtallah, Oday, Khadeja Alrefaie, and Amr Badary. 2025. "Management and Outcomes of Multiple Unruptured Cerebral Aneurysms: A Descriptive Cohort Analysis" Brain Sciences 15, no. 9: 973. https://doi.org/10.3390/brainsci15090973
APA StyleAtallah, O., Alrefaie, K., & Badary, A. (2025). Management and Outcomes of Multiple Unruptured Cerebral Aneurysms: A Descriptive Cohort Analysis. Brain Sciences, 15(9), 973. https://doi.org/10.3390/brainsci15090973






