Application of Concomitant Transcranial Direct Current Stimulation (tDCS) and Cognitive Behavioral-Oriented Training (CBT) for Pragmatic Skills Improvement in Young Adults with Autism Spectrum Disorder (ASD): Preliminary Data from a Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
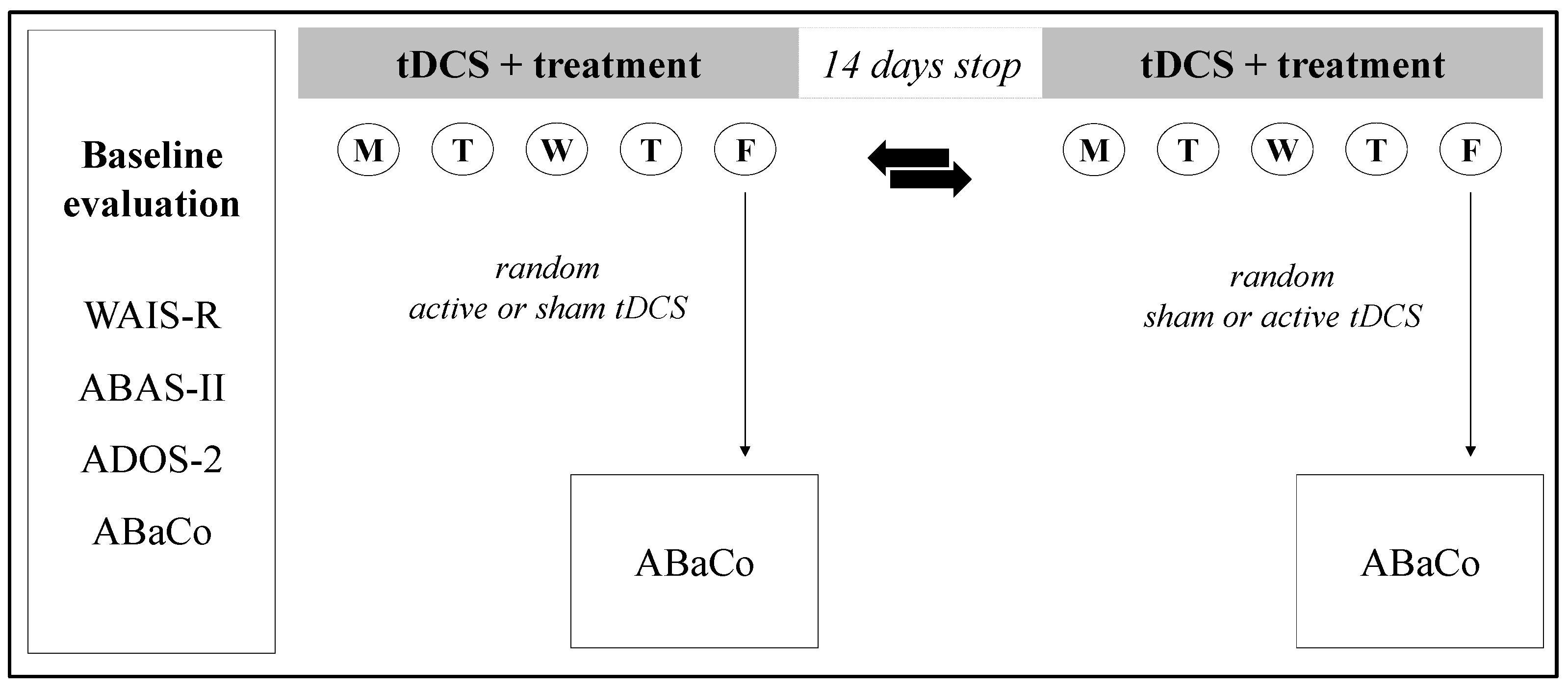
2.2. Experimental Procedure
2.3. Neuropsychiatric Assessment
2.4. Pragmatic Skills Training
2.5. Transcranial Direct Current Stimulation (tDCS)
2.6. Statistical Analyses
3. Results
3.1. Baseline Demographic and Clinical Features
3.2. Post-Treatment Changes in Pragmatic and Communicative Skills
3.3. Correlations Between Clinical Features and Pragmatic and Communicative Skills Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; text rev. American Psychiatric Publishing: Arlington, VA, USA, 2022. [Google Scholar]
- Rose, V.; Trembath, D.; Keen, D.; Paynter, J. The proportion of minimally verbal children with autism spectrum disorder in a community-based early intervention programme. J. Intellect. Disabil. Res. 2016, 60, 464–477. [Google Scholar] [CrossRef]
- Tager-Flusberg, H.; Joseph, R.M. Identifying neurocognitive phenotypes in autism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 303–314. [Google Scholar] [CrossRef]
- Noens, I.L.; van Berckelaer-Onnes, I.A. Captured by details: Sense-making, language and communication in autism. J. Commun. Disord. 2005, 38, 123–141. [Google Scholar] [CrossRef]
- Wodka, E.L.; Mathy, P.; Kalb, L. Predictors of phrase and fluent speech in children with Autism Spectrum Disorder. Pediatrics 2013, 131, e1128–e1134. [Google Scholar] [CrossRef]
- Duvall, L.; May, K.E.; Waltz, A.; Kana, R.K. The neurobiological map of theory of mind and pragmatic communication in autism. Soc. Neurosci. 2023, 18, 191–204. [Google Scholar] [CrossRef]
- Tager-Flusberg, H. Language impairments in children with complex neurodevelopmental disorders: The case of autism. In Language Competence Across Populations: Toward a Definition of Specific Language Impairment; Levy, Y., Schaeffer, J.C., Eds.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2003; pp. 297–321. [Google Scholar]
- Liu, Y.; Tian, X.; Mao, H.; Cheng, L.; Wang, P.; Gao, Y. Research on pragmatic impairment in autistic children during the past two decades (2001–2022): Hot spots and frontiers-based on CiteSpace bibliometric analysis. Front. Psychol. 2024, 15, 1276001. [Google Scholar] [CrossRef] [PubMed]
- Adibi, A.E. Pragmatic Language Skills Underlying Social Competence of Reading Disability in Middle School. Ph.D. Thesis, University of Denver, Denver, CO, USA, 2010. [Google Scholar]
- Hage, S.V.R.; Sawasaki, L.Y.; Hyter, Y.; Fernandes, F.D.M. Social communication and pragmatic skills of children with Autism Spectrum Disorder and Developmental Language Disorder. CoDAS 2021, 34, e20210075. [Google Scholar] [CrossRef] [PubMed]
- Siracusano, M.; Calsolaro, J.; Riccioni, A.; Gialloreti, L.E.; Benvenuto, A.; Giovagnoli, G.; Curatolo, P.; Mazzone, L. TrASDition Training: An online parental training for transition-age youth with autism spectrum disorder. Psychiatry Res. 2021, 300, 113930. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Autism Spectrum Disorder in Adults: Diagnosis and Management. Clinical Guideline. Published 27 June 2012. Available online: www.nice.org.uk/guidance/cg142 (accessed on 16 January 2025).
- National Institute for Health and Care Excellence (NICE). Autism Spectrum Disorder in Under 19s: Support and Management. Clinical Guideline. Published 28 August 2013. Available online: www.nice.org.uk/guidance/cg170 (accessed on 16 January 2025).
- Fletcher-Watson, S.; Happé, F. Autism: A New Introduction to Psychological Theory and Current Debate; Routledge/Taylor & Francis Group: London, UK, 2019. [Google Scholar] [CrossRef]
- Salehinejad, M.A.; Ghanavati, E.; Glinski, B.; Hallajian, A.-H.; Azarkolah, A. A systematic review of randomized controlled trials on efficacy and safety of transcranial direct current stimulation in major neurodevelopmental disorders: ADHD, autism, and dyslexia. Brain Behav. 2022, 12, e2724. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Moffa, A.H.; Fregni, F.; Palm, U.; Padberg, F.; Blumberger, D.M.; Daskalakis, Z.J.; Bennabi, D.; Haffen, E.; Alonzo, A.; et al. Transcranial Direct Current Stimulation as a Monotherapy for Major Depressive Disorder: A Systematic Review and Meta-analysis of Randomized Controlled Trials. JAMA Psychiatry 2016, 208, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Brunoni, A.R.; Valiengo, L.; Baccaro, A.; Zanão, T.A.; de Oliveira, J.F.; Goulart, A.; Boggio, P.S.; Lotufo, P.A.; Benseñor, I.M.; Fregni, F. The sertraline vs. electrical current therapy for treating depression clinical study. JAMA Psychiatry 2013, 70, 383–391. [Google Scholar] [CrossRef]
- Carvhalo, S.; Gonçalves, Ó.F.; Brunoni, A.R.; Fernandes-Gonçalves, A.; Fregni, F.; Leite, J. Transcranial Direct Current Stimulation as an Add-on Treatment to Cognitive-Behavior Therapy in First Episode Drug-Naïve Major Depression Patients: The ESAP Study Protocol. Front. Psychiatry 2020, 11, 563058. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Pantazatos, S.P.; Schneider, H.; Hirsch, J. Neural systems for speech and song in autism. Brain 2012, 135, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Cherkassky, V.L.; Mason, R.A.; Keller, T.A.; Minshew, N.J.; Just, M.A. Brain function differences in language processing in children and adults with autism. Autism Res. 2013, 6, 288–302. [Google Scholar] [CrossRef]
- Cheng, L.; Zhan, L.; Huang, L.; Zhang, H.; Sun, J.; Huang, G.; Wang, Y.; Li, M.; Li, H.; Gao, Y.; et al. The atypical functional connectivity of Broca’s area at multiple frequency bands in autism spectrum disorder. Brain Imaging Behav. 2022, 16, 2627–2636. [Google Scholar] [CrossRef]
- Marangolo, P.; Fiori, V.; Calpagnano, M.A.; Campana, S.; Razzano, C.; Caltagirone, C.; Marini, A. tDCS over the left inferior frontal cortex improves speech production in aphasia. Front. Hum. Neurosci. 2013, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Urgesi, C. Please get to the point! A cortical correlate of linguistic informativeness. J. Cogn. Neurosci. 2012, 24, 2211–2222. [Google Scholar] [CrossRef]
- Arvidsson, C.; Torubarova, E.; Pereira, A.; Uddén, J. Conversational production and comprehension: fMRI-evidence reminiscent of but deviant from the classical Broca–Wernicke model. Cereb. Cortex 2024, 34, bhae073. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Arlington, VA, USA, 2013. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-R Manual: Wechsler Adult Intelligence Scale-Revised; Psychological Corporation: New York, NY, USA, 1981. [Google Scholar]
- Harrison, P.L.; Oakland, T. Adaptive Behavior Assessment System, 2nd ed.; (ABAS-II); The Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S.L. Autism Diagnostic Observation Schedule, 2nd ed.; (ADOS-2) Manual; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Hus, V.; Lord, C. The Autism Diagnostic Observation Schedule, Module 4: Revised algorithm and standardized severity scores. J. Autism Dev. Disord. 2014, 44, 1996–2012. [Google Scholar] [CrossRef]
- Sacco, K.; Bara, B.; Colle, L.; Mate, D.; Angeleri, R.; Bosco, F. Assessment Battery for Communication—ABaCo: A new instrument for the evaluation of pragmatic abilities. J. Commun. Sci. 2008, 9, 111–157. [Google Scholar] [CrossRef]
- Stallard, P.; Good, T. Feel Good: A Cognitive Behavioural Therapy Workbook for Children and Young People, 2nd ed.; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- You, X.R.; Gong, X.R.; Guo, M.R.; Ma, B.X. Cognitive behavioural therapy to improve social skills in children and adolescents with autism spectrum disorder: A meta-analysis of randomised controlled trials. J. Affect. Disord. 2024, 344, 8–16. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef]
- Fertonani, A.; Rosini, S.; Cotelli, M.; Rossini, P.M.; Miniussi, C. Naming facilitation induced by transcranial direct current stimulation. Behav. Brain Res. 2010, 208, 311–318. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, Version 22.0; IBM Corp: Armonk, NY, USA, 2013.
- Losh, M.; Capps, L. Narrative ability in high-functioning children with autism or Asperger’s syndrome. J. Autism Dev. Disord. 2003, 33, 239–251. [Google Scholar] [CrossRef]
- Schneider, S.; Rapp, A.M.; Haeußinger, F.B.; Ernst, L.H.; Hamm, F.; Fallgatter, A.J.; Ehlis, A.C. Beyond the N400: Complementary access to early neural correlates of novel metaphor comprehension using combined electrophysiological and haemodynamic measurements. Cortex 2014, 53, 25–59. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.B.; Engberg-Pedersen, E.; Wallentin, M. Context predicts word order processing in Broca’s region. J. Cogn. Neurosci. 2014, 26, 2762–2777. [Google Scholar] [CrossRef]
- Li, Q.; Fu, Y.; Liu, C.; Meng, Z. Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front. Behav. Neurosci. 2022, 16, 893955. [Google Scholar] [CrossRef] [PubMed]
- Catani, M.; Bambini, V. A model for Social Communication and Language Evolution and Development (SCALED). Curr. Opin. Neurobiol. 2014, 28, 165–171. [Google Scholar] [CrossRef]
- Ouerchefani, R.; Ouerchefani, N.; Rejeb, M.R.B.; Le Gall, D. Pragmatic language comprehension: Role of theory of mind, executive functions, and the prefrontal cortex. Neuropsychologia 2024, 194, 108756. [Google Scholar] [CrossRef]
- McConathey, E.M.; White, N.C.; Gervits, F.; Ash, S.; Coslett, H.B.; Grossman, M.; Hamilton, R.H. Baseline performance predicts tDCS-mediated improvements in language symptoms in primary progressive aphasia. Front. Hum. Neurosci. 2017, 11, 347. [Google Scholar] [CrossRef]
- Looi, C.Y.; Duta, M.; Brem, A.K.; Huber, S.; Nuerk, H.C.; Cohen Kadosh, R. Combining brain stimulation and video game to promote long-term transfer of learning and cognitive enhancement. Sci. Rep. 2016, 6, 22003. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Charman, T.; Havdahl, A.; Carbone, P.; Anagnostou, E.; Boyd, B.; Carr, T.; de Vries, P.J.; Dissanayake, C.; Divan, G.; et al. The Lancet Commission on the future of care and clinical research in autism. Lancet 2022, 399, 271–334. [Google Scholar] [CrossRef] [PubMed]
- Frith, U. Autism: Explaining the Enigma, 2nd ed.; Blackwell Publishing: Oxford, UK, 2003. [Google Scholar]
- Hill, E.L. Executive dysfunction in autism. Trends Cogn. Sci. 2004, 8, 26–32. [Google Scholar] [CrossRef]
- Ozonoff, S.; Pennington, B.F.; Rogers, S.J. Executive function deficits in high-functioning autistic individuals: Relationship to theory of mind. J. Child Psychol. Psychiatry 1991, 32, 1081–1105. [Google Scholar] [CrossRef]
- Thompson-Schill, S.L.; D’Esposito, M.; Aguirre, G.K.; Farah, M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A re-evaluation. Proc. Natl. Acad. Sci. USA 1997, 94, 14792–14797. [Google Scholar] [CrossRef]
- Fedorenko, E.; Duncan, J.; Kanwisher, N. Language-selective and domain-general regions lie side by side within Broca’s area. Curr. Biol. 2012, 22, 2059–2062. [Google Scholar] [CrossRef]
- Zhan, L.; Gao, Y.; Huang, L.; Zhang, H.; Huang, G.; Wang, Y.; Sun, J.; Xie, Z.; Li, M.; Jia, X.; et al. Brain functional connectivity alterations of Wernicke’s area in individuals with autism spectrum conditions in multi-frequency bands: A mega-analysis. Heliyon 2024, 10, e26198. [Google Scholar] [CrossRef]
- Fregni, F.; Nitsche, M.A.; Loo, C.K.; Brunoni, A.R.; Marangolo, P.; Leite, J.; Carvalho, S.; Bolognini, N.; Caumo, W.; Paik, N.J.; et al. Regulatory considerations for the clinical and research use of transcranial direct current stimulation (tDCS): Review and recommendations from an expert panel. Clin. Res. Regul. Aff. 2015, 32, 22–35. [Google Scholar] [CrossRef]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, G.K.; Chun, J.; Kuo, H.-J.; Curtiss, S.L.; Okyere, C. Perspectives of autistic emerging adults, parents, and practitioners on the transition to adulthood. J. Child Fam. Stud. 2023, 32, 938–950. [Google Scholar] [CrossRef]

| Sessions | Domain | Tools and Techniques | Main Topic |
|---|---|---|---|
| day 1, 5, 6, and 10 | Conversational | Free conversation |
|
| |||
| |||
| |||
| day 2 and 7 | Extralinguistic | Comic strip conversation, social stories, role playing |
|
| |||
| day 3 and 8 | Paralinguistic | Social stories, role playing, video |
|
| |||
| day 4 and 9 | Linguistics/ Context | Social stories, role playing |
|
| |||
|
| Age | 19.4 ± 1.83 |
| Sex ratio (male/female) | 10/0 |
| Full IQ | 89.0 ± 20.5 |
| Verbal IQ | 90.8 ± 24.0 |
| Performance IQ | 95.1 ± 26.4 |
| ABAS-II | |
| ABAS-II GAC | 85.1 ± 13.8 |
| ABAS-II CAD | 89.3 ± 13.9 |
| ABAS-II SAD | 81.5 ± 9.6 |
| ABAS-II PAD | 85.1 ± 16.8 |
| ADOS-2 | |
| ADOS-2 SA | 9.4 ± 3.8 |
| ADOS-2 RRB | 2 ± 1.4 |
| ADOS-2 CSS | 6 ± 2.1 |
| ABaCo GLO | 65.5 ± 11.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arturi, L.; Scoppola, C.; Riccioni, A.; Siracusano, M.; Iasevoli, L.; Civetta, G.; Spalletta, G.; Fiori, V.; Mazzone, L. Application of Concomitant Transcranial Direct Current Stimulation (tDCS) and Cognitive Behavioral-Oriented Training (CBT) for Pragmatic Skills Improvement in Young Adults with Autism Spectrum Disorder (ASD): Preliminary Data from a Pilot Study. Brain Sci. 2025, 15, 970. https://doi.org/10.3390/brainsci15090970
Arturi L, Scoppola C, Riccioni A, Siracusano M, Iasevoli L, Civetta G, Spalletta G, Fiori V, Mazzone L. Application of Concomitant Transcranial Direct Current Stimulation (tDCS) and Cognitive Behavioral-Oriented Training (CBT) for Pragmatic Skills Improvement in Young Adults with Autism Spectrum Disorder (ASD): Preliminary Data from a Pilot Study. Brain Sciences. 2025; 15(9):970. https://doi.org/10.3390/brainsci15090970
Chicago/Turabian StyleArturi, Lucrezia, Chiara Scoppola, Assia Riccioni, Martina Siracusano, Luigi Iasevoli, Giulia Civetta, Gianfranco Spalletta, Valentina Fiori, and Luigi Mazzone. 2025. "Application of Concomitant Transcranial Direct Current Stimulation (tDCS) and Cognitive Behavioral-Oriented Training (CBT) for Pragmatic Skills Improvement in Young Adults with Autism Spectrum Disorder (ASD): Preliminary Data from a Pilot Study" Brain Sciences 15, no. 9: 970. https://doi.org/10.3390/brainsci15090970
APA StyleArturi, L., Scoppola, C., Riccioni, A., Siracusano, M., Iasevoli, L., Civetta, G., Spalletta, G., Fiori, V., & Mazzone, L. (2025). Application of Concomitant Transcranial Direct Current Stimulation (tDCS) and Cognitive Behavioral-Oriented Training (CBT) for Pragmatic Skills Improvement in Young Adults with Autism Spectrum Disorder (ASD): Preliminary Data from a Pilot Study. Brain Sciences, 15(9), 970. https://doi.org/10.3390/brainsci15090970






