Causes of Death in Anti-IgLON5 Disease: A Novel Case Report and Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Description
2.2. Systematic Literature Research
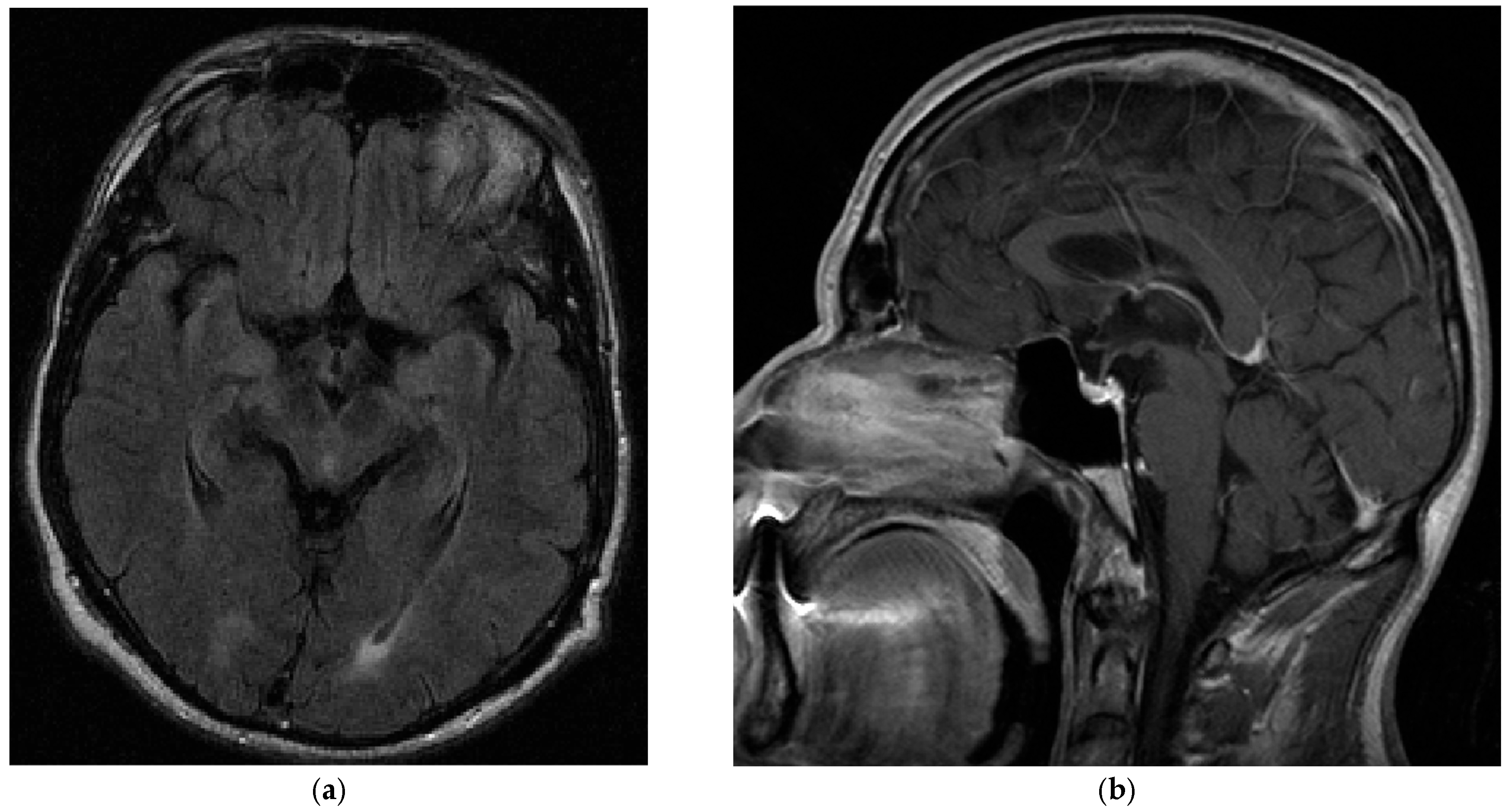
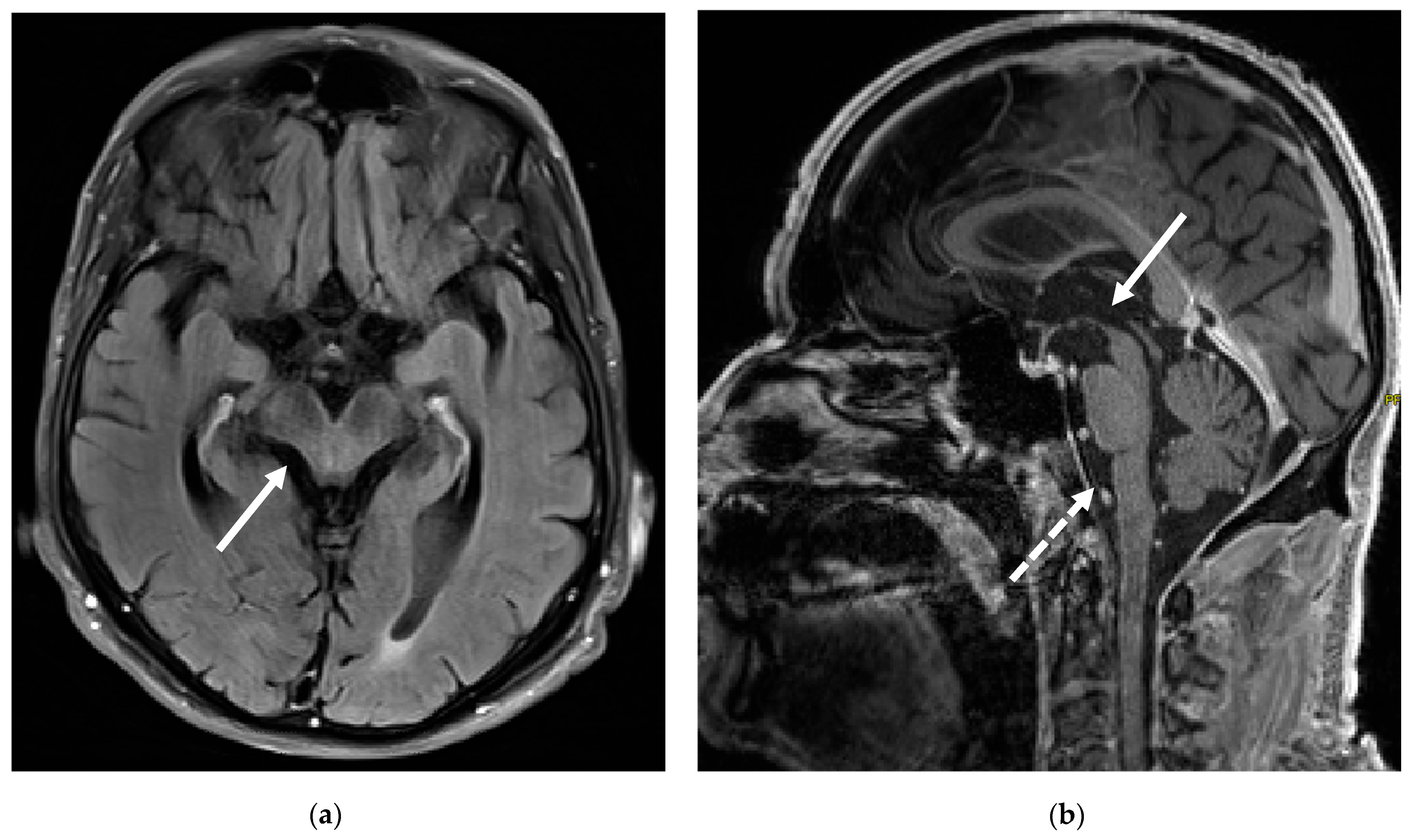
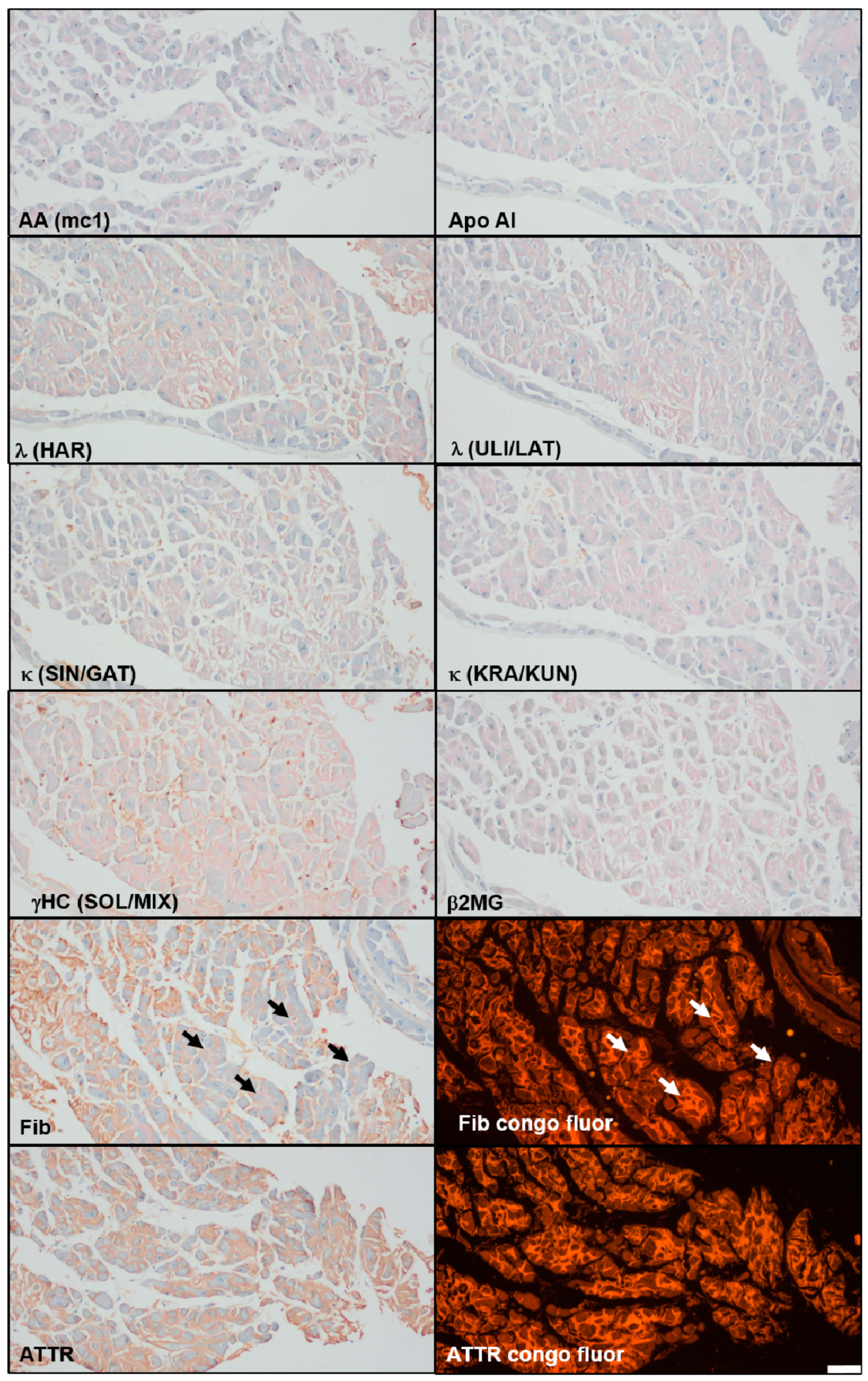
3. Case Report
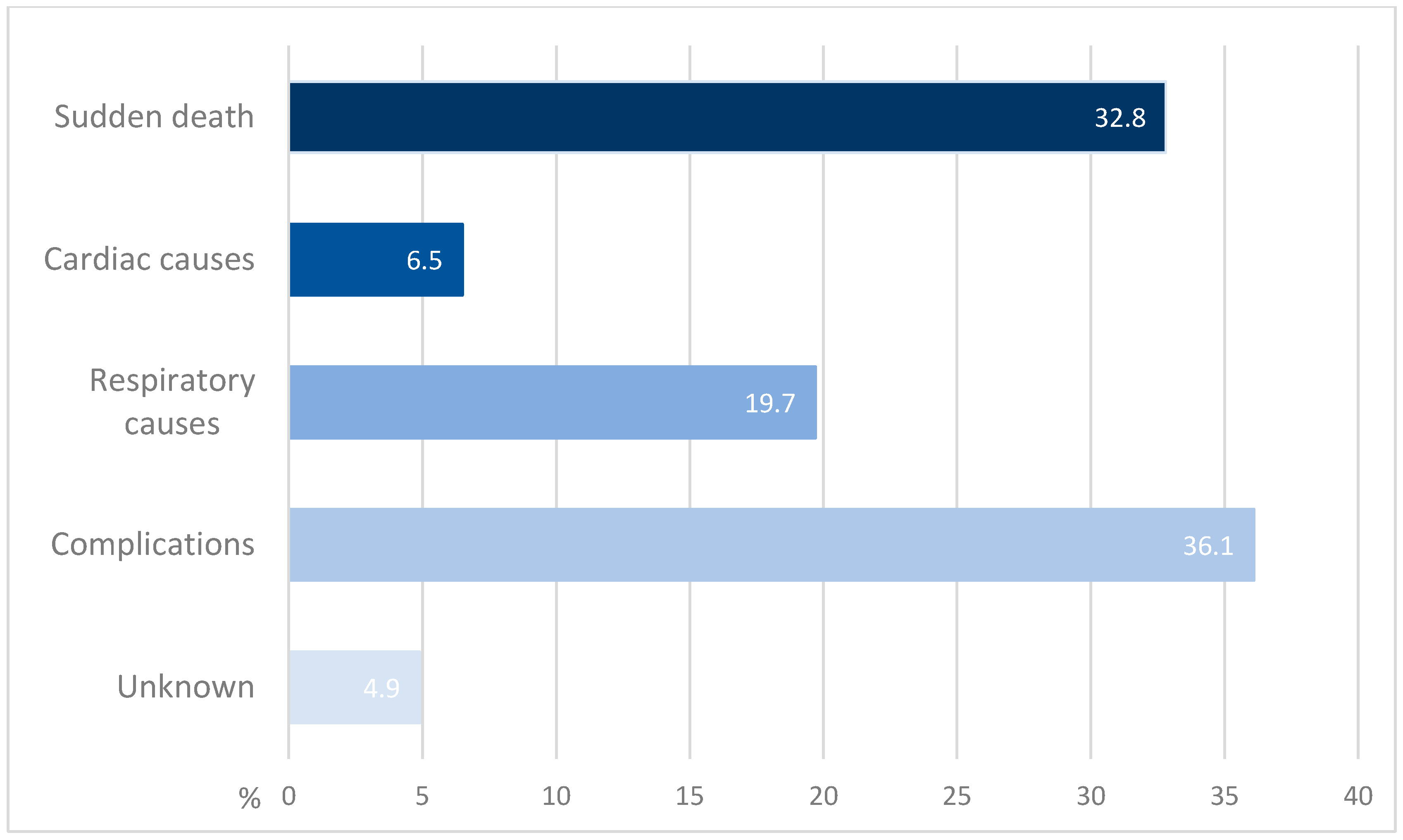
4. Results of the Literature Review
| Publication | Causes of Death | Notes |
|---|---|---|
| Gaig et al. [2] | Sudden death (n = 6) Aspiration pneumonia (n = 6) Hypernephroma progression (n = 1) | 22 patients in total, 13 died (59%). Of the patients who died because of sudden death, two died during wakefulness, two while sleeping, and for two, the time was unknown. Heart complications in 4 patients. |
| Erro et al. [17] | Sudden death (n = 1) | |
| Sista et al. [18] | Sudden death (n = 1) Cardiac arrest (n = 1) | Case reports of four patients, two died. |
| Grüter et al. [6] | Central hypoventilation (n = 4) Aspiration (n = 1) Complications after traumatic fall (n = 1) Cardiac arrhythmia (n = 1) Pulmonary embolism (n = 1) Unclear reason (n = 2) | 52 patients in total, 10 died. |
| Sabater et al. [1] | Sudden death (n = 5) | Study with 8 patients. Two of the five patients who died because of sudden death were awake, two were asleep. For the other sudden death, there is no more precise information available. For the other three patients there is no further information either; one died in the intensive care unit, but no reason is given. |
| Bahtz et al. [19] | Sudden death (n = 1) | |
| Högl et al. [11] | Aspiration pneumonia (n = 1) | |
| Schröder et al. [20] | Cardiac infarction (n = 1) | Reference was made to a recent study, but no exact source was given. Comorbidities were type 2 diabetes and arterial hypertension. |
| Wenninger et al. [21] | Sudden death (n = 1) | During sleep. |
| Honorat et al. [4] | Respiratory failure (n = 2) | The paper reports 4 deaths but only mentions the causes of two patients. |
| Berger-Sieczkowski et al. [10] | Subdural hemorrhage after a fall (n = 1) Pneumonia/sepsis (n = 1) Respiratory failure (n = 1) | The paper also reports other cases which are already mentioned above. |
| Asioli et al. [22] | Sudden death (n = 1) | During sleep. |
| Della Marca et al. [23] | Sudden death (n = 1) | During sleep. |
| Chen et al. [24] | Sudden death (n = 1) | |
| Liu et al. [12] | Cerebral hemorrhage (n = 1) | |
| Montojo et al. [13] | Aspiration pneumonia (n = 1) | |
| Klein da Costa et al. [14] | Pneumonia (n = 1) | Septic shock secondary to pneumonia. |
| Li et al. [25] | Sudden death (n = 1) | |
| Gelpi E. et al. [16] | Pneumonia (n = 4) Respiratory failure (n = 4) Respiratory insufficiency (n = 1) Unknown (n = 1) Urinary infection and sepsis (n = 1) | 22 cases in total, 10 cases were already published. From the 12 remaining cases, one was excluded because there was no antibody testing. One patient with pneumonia developed sepsis, one patient with pneumonia developed status epilepticus. The patient with the unknown cause was found dead at home. |
| Postuma R. et al. [15] | COVID-19 infection (n = 1) Sudden death (n = 1) | The patient with sudden death required intubation, became comatose, and died. |
| Cagnin et al. [26] | Bradycardia (n = 1) | The patient had recurrent hypoxic events during the disease. |
5. Discussion
5.1. Case Report
5.2. Causes of Death
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATTR | Amyloid transthyretin |
| CSF | Cerebrospinal fluid |
| FLAIR | Fluid attenuated inversion recovery |
| HLA | Human leukocyte antigen |
| MRI | Magnetic resonance imaging |
| OSAS | Obstructive sleep apnea syndrome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PSP | Progressive supranuclear palsy |
| REM | Rapid-eye-movement |
| SPECT | Single-photon emission computed tomography |
References
- Sabater, L.; Gaig, C.; Gelpi, E.; Bataller, L.; Lewerenz, J.; Torres-Vega, E.; Contreras, A.; Giometto, B.; Compta, Y.; Embid, C.; et al. A novel non-rapid-eye movement and rapid-eye-movement parasomnia with sleep breathing disorder associated with antibodies to IgLON5: A case series, characterisation of the antigen, and post-mortem study. Lancet Neurol. 2014, 13, 575–586. [Google Scholar] [CrossRef]
- Gaig, C.; Graus, F.; Compta, Y.; Högl, B.; Bataller, L.; Brüggemann, N.; Giordana, C.; Heidbreder, A.; Kotschet, K.; Lewerenz, J.; et al. Clinical manifestations of the anti-IgLON5 disease. Neurology 2017, 88, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.S.; Blaabjerg, M. Anti-IgLON5 Disease: A Case With 11-Year Clinical Course and Review of the Literature. Front. Neurol. 2019, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- Honorat, J.A.; Komorowski, L.; Josephs, K.A.; Fechner, K.; St Louis, E.K.; Hinson, S.R.; Lederer, S.; Kumar, N.; Gadoth, A.; Lennon, V.A.; et al. IgLON5 antibody: Neurological accompaniments and outcomes in 20 patients. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e385. [Google Scholar] [CrossRef] [PubMed]
- Heidbreder, A.; Philipp, K. Anti-IgLON 5 Disease. Curr. Treat. Options Neurol. 2018, 20, 29. [Google Scholar] [CrossRef]
- Grüter, T.; Möllers, F.E.; Tietz, A.; Dargvainiene, J.; Melzer, N.; Heidbreder, A.; Strippel, C.; Kraft, A.; Höftberger, R.; Schöberl, F.; et al. Clinical, serological and genetic predictors of response to immunotherapy in anti-IgLON5 disease. Brain 2023, 146, 600–611. [Google Scholar] [CrossRef]
- Koneczny, I.; Macher, S.; Hutterer, M.; Seifert-Held, T.; Berger-Sieczkowski, E.; Blaabjerg, M.; Breu, M.; Dreyhaupt, J.; Dutra, L.A.; Erdler, M.; et al. HLA dependency and possible clinical relevance of intrathecally synthesized anti-IgLON5 IgG4 in anti-IgLON5 disease. Front. Immunol. 2024, 15, 1376456. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Linke, R.P. On typing amyloidosis using immunohistochemistry. Detailled illustrations, review and a note on mass spectrometry. Prog. Histochem. Cytochem. 2012, 47, 61–132. [Google Scholar] [CrossRef]
- Berger-Sieczkowski, E.; Endmayr, V.; Haider, C.; Ricken, G.; Jauk, P.; Macher, S.; Pirker, W.; Högl, B.; Heidbreder, A.; Schnider, P.; et al. Analysis of inflammatory markers and tau deposits in an autopsy series of nine patients with anti-IgLON5 disease. Acta Neuropathol. 2023, 146, 631–645. [Google Scholar] [CrossRef]
- Högl, B.; Heidbreder, A.; Santamaria, J.; Graus, F.; Poewe, W. IgLON5 autoimmunity and abnormal behaviours during sleep. Lancet 2015, 385, 1590. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Z.; Chen, X.; Zhang, Y.; He, F.; Ma, X.; Ke, Q. Case report: A longitudinal study of an unusual rapidly progressive dementia case. Front. Neurol. 2023, 14, 1151130. [Google Scholar] [CrossRef]
- Montojo, T.; Piren, V.; Benkhadra, F.; Codreanu, A.; Diederich, N.J. Gaze Palsy, Sleep and Gait Disorder, as Well as Tako-Tsubo Syndrome in a Patient with IgLON5 Antibodies. Mov. Disord. Clin. Pract. 2017, 4, 441–443. [Google Scholar] [CrossRef]
- Klein da Costa, B.; de Oliveira Pinto, P.; Staub, L.; Hansel, G.; Vanik Pinto, G.; Porcello Schilling, L.; Rodrigues Dos Passos, G.; Alves Martins, W.; Becker, J.; Machado Castilhos, R.; et al. Neurological syndromes and potential triggers associated with antibodies to neuronal surface antigens. Mult. Scler. Relat. Disord. 2023, 80, 105022. [Google Scholar] [CrossRef]
- Postuma, R.; Vorasoot, N.; St Louis, E.K.; Pelletier, A.; Lim, M.M.; Elliott, J.; Gagnon, J.F.; Gan-Or, Z.; Forsberg, L.K.; Fields, J.A.; et al. IGLON5 Frequency in Idiopathic REM Sleep Behavior Disorder: A Multicenter Study. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200311. [Google Scholar] [CrossRef]
- Gelpi, E.; Reinecke, R.; Gaig, C.; Iranzo, A.; Sabater, L.; Molina-Porcel, L.; Aldecoa, I.; Endmayr, V.; Högl, B.; Schmutzhard, E.; et al. Neuropathological spectrum of anti-IgLON5 disease and stages of brainstem tau pathology: Updated neuropathological research criteria of the disease-related tauopathy. Acta Neuropathol. 2024, 148, 53. [Google Scholar] [CrossRef] [PubMed]
- Erro, M.E.; Sabater, L.; Martínez, L.; Herrera, M.; Ostolaza, A.; García de Gurtubay, I.; Tuñón, T.; Graus, F.; Gelpi, E. Anti-IGLON5 disease: A new case without neuropathologic evidence of brainstem tauopathy. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e651. [Google Scholar] [CrossRef]
- Sista, S.R.; Crum, B.; Aboseif, A.; Devine, M.F.; Zekeridou, A.; Hammami, M.B.; Rezk, M.M.; Truffert, A.; Lalive, P.H.; Kunchok, A.; et al. Motor-neuron-disease-like phenotype associated with IgLON5 disease. J. Neurol. 2022, 269, 6139–6144. [Google Scholar] [CrossRef]
- Bahtz, R.; Teegen, B.; Borowski, K.; Probst, C.; Blöcker, I.; Fechner, K.; Parigger, S.; Daniel, G.; Brücke, T.; Lauenstein, A.; et al. Autoantibodies against IgLON5: Two new cases. J. Neuroimmunol. 2014, 275, 8. [Google Scholar] [CrossRef]
- Schröder, J.B.; Melzer, N.; Ruck, T.; Heidbreder, A.; Kleffner, I.; Dittrich, R.; Muhle, P.; Warnecke, T.; Dziewas, R. Isolated dysphagia as initial sign of anti-IgLON5 syndrome. Neurol. Neuroimmunol. Neuroinflamm. 2017, 4, e302. [Google Scholar] [CrossRef]
- Wenninger, S. Expanding the Clinical Spectrum of IgLON5-Syndrome. J. Neuromuscul. Dis. 2017, 4, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Asioli, G.M.; Calandra-Buonaura, G.; Mastrangelo, V.; Pierangeli, G.; Gaig, C.; Santamaria, J.; Cortelli, P.; Provini, F. Persistence of Facio-Skeletal Myorhythmia During Sleep in anti-IgLON5 Disease. Mov. Disord. Clin. Pract. 2021, 8, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Della Marca, G.; Iorio, R.; Losurdo, A.; Mirabella, M.; Frisullo, G. Sleep disorder associated with antibodies to IgLON5: Parasomnia or agrypnia? Lancet Neurol. 2014, 13, 864. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, J.; Irani, S.R. Distinctive Magnetic Resonance Imaging Findings in IgLON5 Antibody Disease. JAMA Neurol. 2020, 77, 125–126. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Zhang, L.; Zhang, W.; Li, B.; Baizabal-Carvallo, J.F.; Song, X. IgLON5 autoimmunity in a patient with Creutzfeldt-Jakob disease: Case report and review of literature. Front. Neurol. 2024, 15, 1367361. [Google Scholar] [CrossRef]
- Cagnin, A.; Mariotto, S.; Fiorini, M.; Gaule, M.; Bonetto, N.; Tagliapietra, M.; Buratti, E.; Zanusso, G.; Ferrari, S.; Monaco, S. Microglial and Neuronal TDP-43 Pathology in Anti-IgLON5-Related Tauopathy. J. Alzheimers Dis. 2017, 59, 13–20. [Google Scholar] [CrossRef]
- Yilmaz, A.; Bauersachs, J.; Kindermann, I.; Klingel, K.; Knebel, F.; Meder, B.; Morbach, C.; Nagel, E.; Schulze-Bahr, E.; aus dem Siepen, F.; et al. Diagnostik und Therapie der kardialen Amyloidose. Kardiologie 2019, 13, 264–291. [Google Scholar] [CrossRef]
- Macher, S.; Milenkovic, I.; Zrzavy, T.; Höftberger, R.; Seidel, S.; Berger-Sieczkowski, E.; Berger, T.; Rommer, P.S.; Wiest, G. Ocular Motor Abnormalities in Anti-IgLON5 Disease. Front. Immunol. 2021, 12, 753856. [Google Scholar] [CrossRef]
- Gaig, C.; Ercilla, G.; Daura, X.; Ezquerra, M.; Fernández-Santiago, R.; Palou, E.; Sabater, L.; Höftberger, R.; Heidbreder, A.; Högl, B.; et al. HLA and microtubule-associated protein tau H1 haplotype associations in anti-IgLON5 disease. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e605. [Google Scholar] [CrossRef]
- Yogeshwar, S.M.; Muñiz-Castrillo, S.; Sabater, L.; Peris-Sempere, V.; Mallajosyula, V.; Luo, G.; Yan, H.; Yu, E.; Zhang, J.; Lin, L.; et al. HLA-DQB1*05 subtypes and not DRB1*10:01 mediates risk in anti-IgLON5 disease. Brain 2024, 147, 2579–2592. [Google Scholar] [CrossRef]
- Shambrook, P.; Hesters, A.; Marois, C.; Zemba, D.; Servan, J.; Gaymard, B.; Pico, F.; Delorme, C.; Lubetzki, C.; Arnulf, I.; et al. Delayed Benefit From Aggressive Immunotherapy in Waxing and Waning Anti-IgLON5 Disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1009. [Google Scholar] [CrossRef]
- Haitao, R.; Yingmai, Y.; Yan, H.; Fei, H.; Xia, L.; Honglin, H.; Chaiyan, L.; Stöcker, W.; Liying, C.; Hongzhi, G. Chorea and parkinsonism associated with autoantibodies to IgLON5 and responsive to immunotherapy. J. Neuroimmunol. 2016, 300, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Furlepa, M.; Astin, R.; Fishman, J.; Saifee, T. Ventilation and tracheostomy insertion in anti-IgLON5 disease: A systematic review of cases. J. Neurol. Sci. 2025, 472, 123463. [Google Scholar] [CrossRef] [PubMed]

| Cause of Death | Age at Disease Onset | Disease Duration Until Death |
|---|---|---|
| Sudden death [1] | 65 | 2 months |
| Sudden death [1] | 76 | 6 months |
| Sudden death [19] | 71 | 7 months |
| Sudden death [21] | 58 | 24 months |
| Sudden death [22] | 68 | 24 months |
| Sudden death [1] | 69 | 24 months |
| Sudden death [17] | 71 | 24 months |
| Sudden death [1] | 53 | 72 months |
| Sudden death [23] | 55 | 120 months |
| Sudden death [1] | 59 | 144 months |
| Pneumonia/sepsis [16] | 81 | 6 months |
| Pneumonia/sepsis [10] | 82 | 6 months |
| Pneumonia [16] | 77 | 9 months |
| Urinary infection/sepsis [16] | 85 | 9 months |
| Infection [16] | 66 | 60 months |
| Pneumonia [16] | 61 | 108 months |
| Aspiration pneumonia [11] | 54 | 144 months |
| Pneumonia [16] | 62 | 156 months |
| Respiratory insufficiency [16] | 81 | 11 months |
| Respiratory failure [16] | 69 | 48 months |
| Respiratory failure [16] | 70 | 72 months |
| Respiratory failure [16] | 66 | 96 months |
| Respiratory failure [10] | 76 | 108 months |
| Respiratory failure [16] | 50 | 180 months |
| Bradycardia [26] | 69 | 15 months |
| Cardiac arrest [18] | 53 | 84 months |
| Unknown [16] | 75 | 6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howischer, T.; Gattermeyer-Kell, L.; Hirschbichler, S.; Seifert-Held, T.; Bräsen, J.H.; Katschnig-Winter, P.; Kögl, M.; Franthal, S.; Enzinger, C.; Höftberger, R.; et al. Causes of Death in Anti-IgLON5 Disease: A Novel Case Report and Systematic Literature Review. Brain Sci. 2025, 15, 921. https://doi.org/10.3390/brainsci15090921
Howischer T, Gattermeyer-Kell L, Hirschbichler S, Seifert-Held T, Bräsen JH, Katschnig-Winter P, Kögl M, Franthal S, Enzinger C, Höftberger R, et al. Causes of Death in Anti-IgLON5 Disease: A Novel Case Report and Systematic Literature Review. Brain Sciences. 2025; 15(9):921. https://doi.org/10.3390/brainsci15090921
Chicago/Turabian StyleHowischer, Tina, Lukas Gattermeyer-Kell, Stephanie Hirschbichler, Thomas Seifert-Held, Jan Hinrich Bräsen, Petra Katschnig-Winter, Mariella Kögl, Sebastian Franthal, Christian Enzinger, Romana Höftberger, and et al. 2025. "Causes of Death in Anti-IgLON5 Disease: A Novel Case Report and Systematic Literature Review" Brain Sciences 15, no. 9: 921. https://doi.org/10.3390/brainsci15090921
APA StyleHowischer, T., Gattermeyer-Kell, L., Hirschbichler, S., Seifert-Held, T., Bräsen, J. H., Katschnig-Winter, P., Kögl, M., Franthal, S., Enzinger, C., Höftberger, R., & Schwingenschuh, P. (2025). Causes of Death in Anti-IgLON5 Disease: A Novel Case Report and Systematic Literature Review. Brain Sciences, 15(9), 921. https://doi.org/10.3390/brainsci15090921







