Systematic Review of the Treatment of Anosognosia for Hemiplegia in Stroke
Abstract
1. Introduction
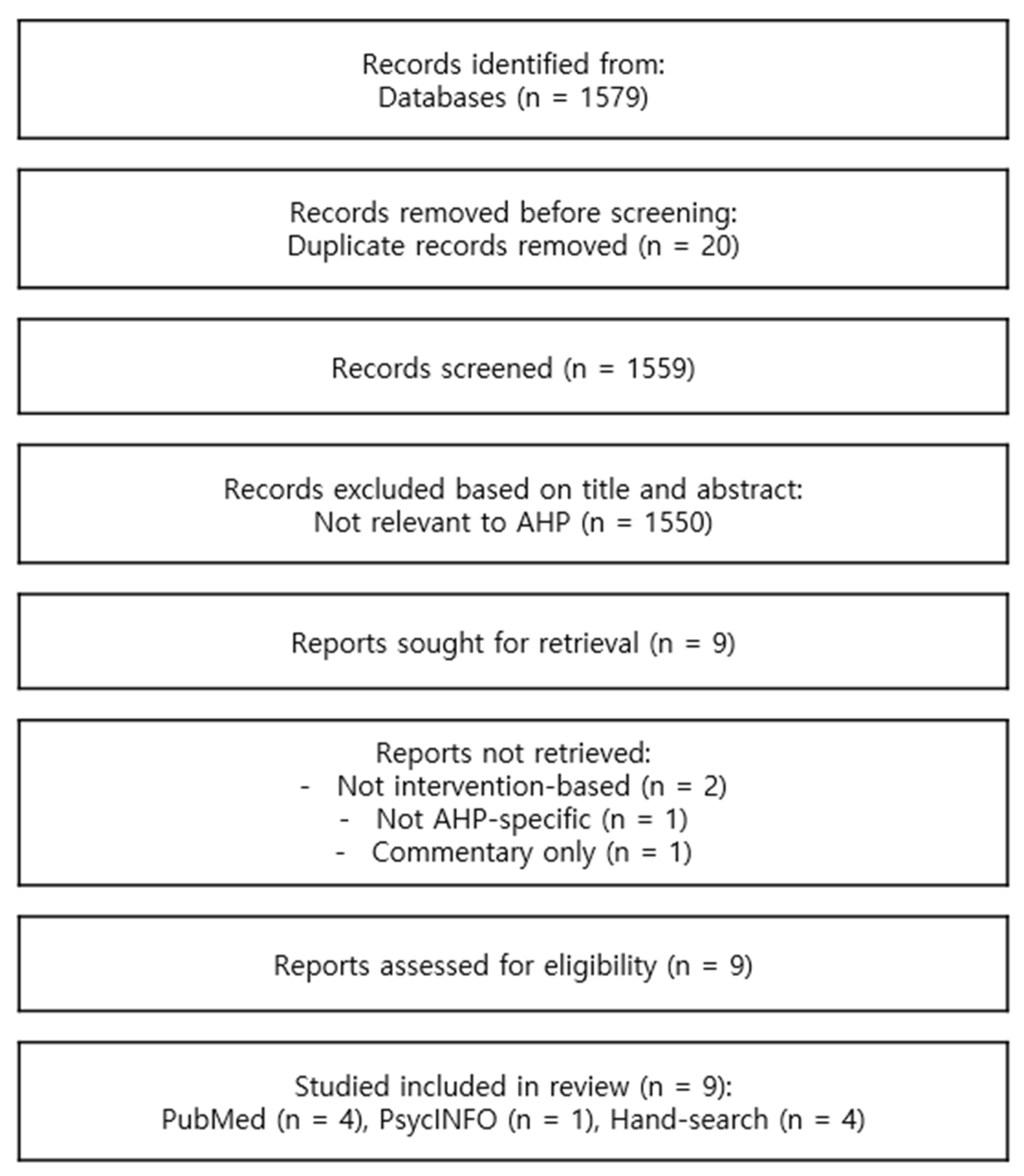
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHP | Anosognosia for HemiPlegia |
| DMN | Default Mode Network |
| JAT | Judgments of Action Test |
| MUNA | Motor Unawareness Assessment |
| tDCS | transcranial Direct Current Stimulation |
| TENS | Transcutaneous Electrical Nerve Stimulation |
| UMAS | Unawareness of Motor and somatosensory deficits After Stroke questionnaire |
| VATAm | Visual-Analogue Test for Anosognosia for motor impairments |
References
- Prigatano, G.P. Disturbances of self-awareness and rehabilitation of patients with traumatic brain injury: A 20-year perspective. J. Head Trauma Rehabil. 2005, 20, 19–29. [Google Scholar] [CrossRef]
- Orfei, M.D.; Caltagirone, C.; Spalletta, G. The evaluation of anosognosia in stroke patients. Cerebrovasc. Dis. 2009, 27, 280–289. [Google Scholar] [CrossRef]
- Mograbi, D.C.; Morris, R.G. Anosognosia. Cortex 2018, 103, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Toglia, J.; Kirk, U. Understanding awareness deficits following brain injury. NeuroRehabilitation 2000, 15, 57–70. [Google Scholar] [CrossRef]
- Jenkinson, P.M.; Preston, C.; Ellis, S.J. Unawareness after stroke: A review and practical guide to understanding, assessing, and managing anosognosia for hemiplegia. J. Clin. Exp. Neuropsychol. 2011, 33, 1079–1093. [Google Scholar] [CrossRef]
- Langer, K.G. Babinski’s anosognosia for hemiplegia in early twentieth-century French neurology. J. Hist. Neurosci. 2009, 18, 387–405. [Google Scholar] [CrossRef]
- Fotopoulou, A.; Pernigo, S.; Maeda, R.; Rudd, A.; Kopelman, M.A. Implicit awareness in anosognosia for hemiplegia: Unconscious interference without conscious re-representation. Brain 2010, 133, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Moro, V.; Scandola, M.; Bulgarelli, C.; Avesani, R.; Fotopoulou, A. Error-based training and emergent awareness in anosognosia for hemiplegia. Neuropsychol. Rehabil. 2015, 25, 593–616. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. History of Anosognosia. Front. Neurol. Neurosci. 2019, 44, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Therriault, J.; Ng, K.P.; Pascoal, T.A.; Mathotaarachchi, S.; Kang, M.S.; Struyfs, H.; Shin, M.; Benedet, A.L.; Walpola, I.C.; Nair, V.; et al. Anosognosia predicts default mode network hypometabolism and clinical progression to dementia. Neurology 2018, 90, e932–e939. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.G.; Bogousslavsky, J. The Merging Tracks of Anosognosia and Neglect. Eur. Neurol. 2020, 83, 438–446. [Google Scholar] [CrossRef]
- Barrett, A.M. Spatial Neglect and Anosognosia After Right Brain Stroke. Continuum 2021, 27, 1624–1645. [Google Scholar] [CrossRef]
- Steward, K.A.; Kretzmer, T. Anosognosia in moderate-to-severe traumatic brain injury: A review of prevalence, clinical correlates, and diversity considerations. Clin. Neuropsychol. 2022, 36, 2021–2040. [Google Scholar] [CrossRef]
- Monai, E.; Pini, L.; Palacino, F.; Bisio, M.; Bernocchi, F.; Salvalaggio, A.; Corbetta, M. Convergence of Visual and Motor Awareness in Human Parietal Cortex. Ann. Neurol. 2023, 95, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Pacella, V.; Foulon, C.; Jenkinson, P.M.; Scandola, M.; Bertagnoli, S.; Avesani, R.; Fotopoulou, A.; Moro, V.; de Schotten, M.T. Anosognosia for hemiplegia as a tripartite disconnection syndrome. eLife 2019, 8, e46075. [Google Scholar] [CrossRef]
- Kortte, K.B.; Hillis, A.E. Recent trends in rehabilitation interventions for visual neglect and anosognosia for hemiplegia following right hemisphere stroke. Future Neurol. 2011, 6, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Byrd, E.M.; Jablonski, R.J.; Vance, D.E. Understanding Anosognosia for Hemiplegia After Stroke. Rehabil. Nurs. 2020, 45, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, A.; Rudd, A.; Holmes, P.; Kopelman, M. Self-observation reinstates motor awareness in anosognosia for hemiplegia. Neuropsychologia 2009, 47, 1256–1260. [Google Scholar] [CrossRef]
- Beschin, N.; Cocchini, G.; Allen, R.; Della Sala, S. Anosognosia and neglect respond differently to the same treatments. Neuropsychol. Rehabil. 2012, 22, 550–562. [Google Scholar] [CrossRef]
- Ronchi, R.; Rode, G.; Cotton, F.; Farnè, A.; Rossetti, Y.; Jacquin-Courtois, S. Remission of anosognosia for right hemiplegia and neglect after caloric vestibular stimulation. Restor. Neurol. Neurosci. 2013, 31, 19–24. [Google Scholar] [CrossRef]
- Besharati, S.; Forkel, S.J.; Kopelman, M.; Solms, M.; Jenkinson, P.M.; Fotopoulou, A. The affective modulation of motor awareness in anosognosia for hemiplegia: Behavioural and lesion evidence. Cortex 2014, 61, 127–140. [Google Scholar] [CrossRef]
- Gandola, M.; Sedda, A.; Manera, M.; Pingue, V.; Salvato, G.; Spitoni, G.F.; Pistarini, C.; Giorgi, I.; Pizzamiglio, L.; Bottini, G. Selective improvement of anosognosia for hemiplegia during transcranial direct current stimulation: A case report. Cortex 2014, 61, 107–119. [Google Scholar] [CrossRef]
- Besharati, S.; Kopelman, M.; Avesani, R.; Moro, V.; Fotopoulou, A.K. Another perspective on anosognosia: Self-observation in video replay improves motor awareness. Neuropsychol. Rehabil. 2015, 25, 319–352. [Google Scholar] [CrossRef]
- Facchin, A.; Beschin, N. Different impact of prism adaptation rehabilitation in spatial neglect and anosognosia for hemiplegia. Ann. Phys. Rehabil. Med. 2018, 61, 113–114. [Google Scholar] [CrossRef]
- Allum, J.; Whittaker, M.; Green, H. Knowing and not knowing: Practical reflections on video based feedback as part of neuro-rehabilitation in a case of persistent anosognosia for hemiplegia. Neurocase 2024, 31, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Berti, A.; Ladavas, E.; Della Corte, M. Anosognosia for hemiplegia, neglect dyslexia, and drawing neglect: Clinical findings and theoretical considerations. J. Int. Neuropsychol. Soc. 1996, 2, 426–440. [Google Scholar] [CrossRef]
- Feinberg, T.E.; Roane, D.M.; Ali, J. Illusory limb movements in anosognosia for hemiplegia. J. Neurol. Neurosurg. Psychiatry 2000, 68, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, S.; Cocchini, G.; Beschin, N.; Cameron, A. VATA-m: Visual-Analogue Test assessing Anosognosia for motor impairment. Clin. Neuropsychol. 2009, 23, 406–427. [Google Scholar] [CrossRef] [PubMed]
- Bisiach, E.; Vallar, G.; Perani, D.; Papagno, C.; Berti, A. Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia 1986, 24, 471–482. [Google Scholar] [CrossRef]
- Nimmo-Smith, I.; Marcel, A.J.; Tegnér, R. A diagnostic test of unawareness of bilateral motor task abilities in anosognosia for hemiplegia. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1167–1169. [Google Scholar] [CrossRef]
- Moro, V.; Pernigo, S.; Zapparoli, P.; Cordioli, Z.; Aglioti, S.M. Phenomenology and neural correlates of implicit and emergent motor awareness in patients with anosognosia for hemiplegia. Behav. Brain Res. 2011, 225, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Moro, V.; Besharati, S.; Scandola, M.; Bertagnoli, S.; Gobbetto, V.; Ponzo, S.; Bulgarelli, C.; Fotopoulou, A.; Jenkinson, P.M. The Motor Unawareness Assessment (MUNA): A new tool for the assessment of anosognosia for hemiplegia. J. Clin. Exp. Neuropsychol. 2021, 43, 91–104. [Google Scholar] [CrossRef] [PubMed]
| Author | Country | Study Design | Number of Cases | Brain Lesion | Handedness | AHP Assessment | Intervention | Outcome | Rationale |
|---|---|---|---|---|---|---|---|---|---|
| Fotopoulou et al., 2009 [18] | UK | Single case study | 1 | Right | Right-handed | Berti awareness interview [26], Feinberg awareness scale [27] | Self-observation in video replay | Observed dramatic reinstatement, instantly and permanently | judgements relying on 3rd-person and off-line self-observation |
| Beschin et al., 2012 [19] | Italy, UK | Comparative case study | 5 | Right 2; Left 3 | Not reported | VATAm [28] | Optokinetic stimulation, prism adaptation, TENS | Treatment response bias, temporarily | Testing neglect-based intervention for AHP |
| Ronchi et al., 2013 [20] | France | Single case study | 1 | Left | Ambidextrous | Anosognosia score [29] | Caloric vestibular stimulation | Temporary remission of neglect, and remission of anosognosia | Vestibular input may restore awareness |
| Besharati et al., 2014 [21] | UK | Case–control study | 8 | Right | Not reported | Berti awareness interview [26], Feinberg awareness scale [27] | Negative emotional induction | Negative emotional induction improved awareness | Experimental designed study rather than a clinical study |
| Gandola et al., 2014 [22] | Italy | Single case study | 1 | Bilateral lesion on involving predominantly Right | Right-handed | Thumb-finger opposition task, Bisiach scale [29], UMAS [30] | tDCS: sham or anodal stimulation on premotor cortex | Temporary remission of AHP in the online condition with visual feedback (eyes open) | The stimulation of the premotor cortex by tDCS activates motor comparator system |
| Moro et al., 2015 [8] | Italy, UK | Pilot case series | 4 | Right: chronic stroke | Right-handed | VATAm [28], Bisiach scale [29], Moro’s interview [31], JAT [31] | Error-based training | Improved awareness | Unsuccessful action attempts with concomitant error analysis facilitate the emergent awareness |
| Besharati et al., 2015 [23] | UK/Italy | Experimental study | 2 | Right: one acute, one chronic | Right-handed | Acute: Berti awareness interview [26], Feinberg awareness scale [27] Chronic: VATAm [28], Bisiach scale [29], modified Marcel-Moro’s interview [31] | Self-observation in video replay | Improved motor awareness after video feedback | video-based, self-observation can reinstate motor awareness by providing third person and off-line feedback |
| Facchin et al., 2018 [24] | Italy | Single case study | 1 | Right | Not reported | VATAm [28], UMAS [30] | Prism adaptation | Improved neglect; AHP unchanged | Divergent improvement of neglect and anosognosia |
| Allum et al., 2024 [25] | UK | Qualitative case study | 1 | Right | Not reported | MUNA [32] | Video-based feedback during neurorehabilitation | Dramatic effects were observed, but with a subsequent recurrence | Emotion |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.C.; Park, J.; Kim, M.W. Systematic Review of the Treatment of Anosognosia for Hemiplegia in Stroke. Brain Sci. 2025, 15, 906. https://doi.org/10.3390/brainsci15090906
Kim DC, Park J, Kim MW. Systematic Review of the Treatment of Anosognosia for Hemiplegia in Stroke. Brain Sciences. 2025; 15(9):906. https://doi.org/10.3390/brainsci15090906
Chicago/Turabian StyleKim, Dong Chan, Junghyeon Park, and Min Wook Kim. 2025. "Systematic Review of the Treatment of Anosognosia for Hemiplegia in Stroke" Brain Sciences 15, no. 9: 906. https://doi.org/10.3390/brainsci15090906
APA StyleKim, D. C., Park, J., & Kim, M. W. (2025). Systematic Review of the Treatment of Anosognosia for Hemiplegia in Stroke. Brain Sciences, 15(9), 906. https://doi.org/10.3390/brainsci15090906






