Interaction Between α-Synuclein and DJ-1 in Parkinson’s Disease
Abstract
1. Parkinson’s Disease
2. α-Synuclein
2.1. Structure
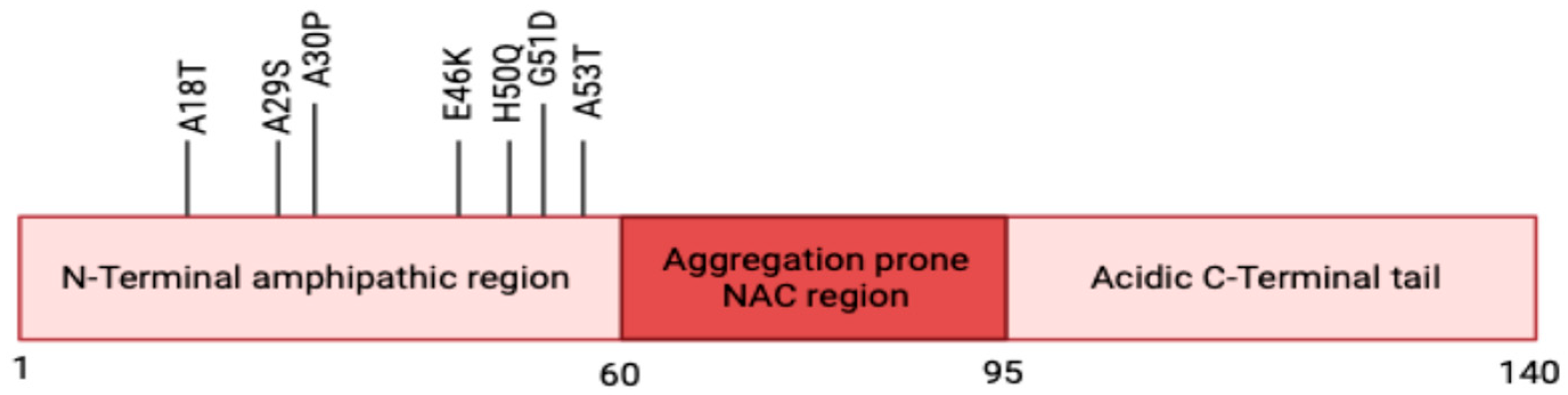
2.2. Physiological Roles of α-Syn
2.3. α-Syn in Parkinson’s Disease Pathogenesis
2.3.1. Membrane Disruption and Ion Dysregulation
2.3.2. Neuroinflammation via Microglial and Astrocytic Activation
2.3.3. Mitochondrial Dysfunction
2.3.4. ER Stress and the Unfolded Protein Response
2.3.5. Disruption of Proteostasis Pathways
2.3.6. Post-Translational Modifications of α-Synuclein
3. DJ-1
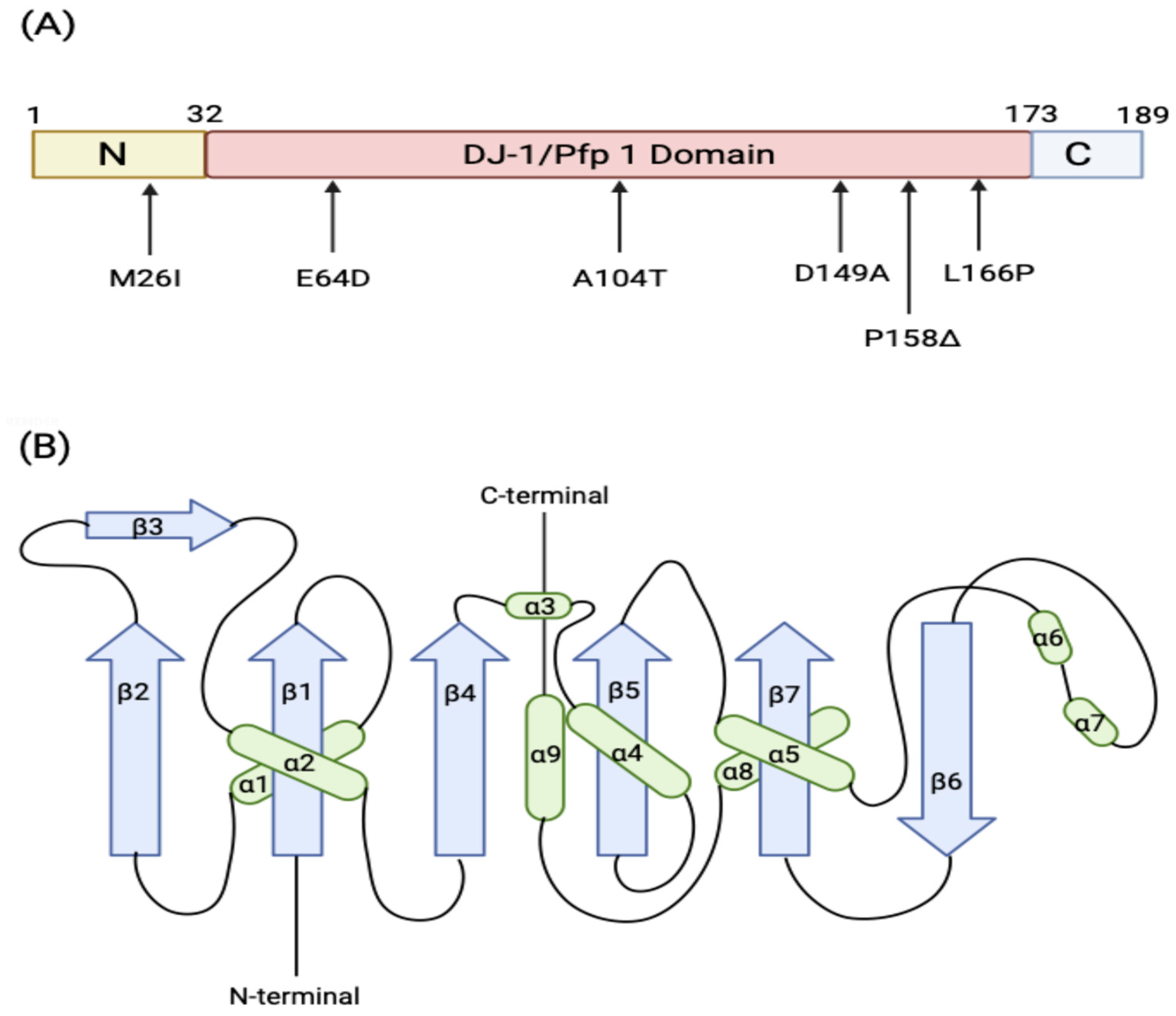
3.1. Structure
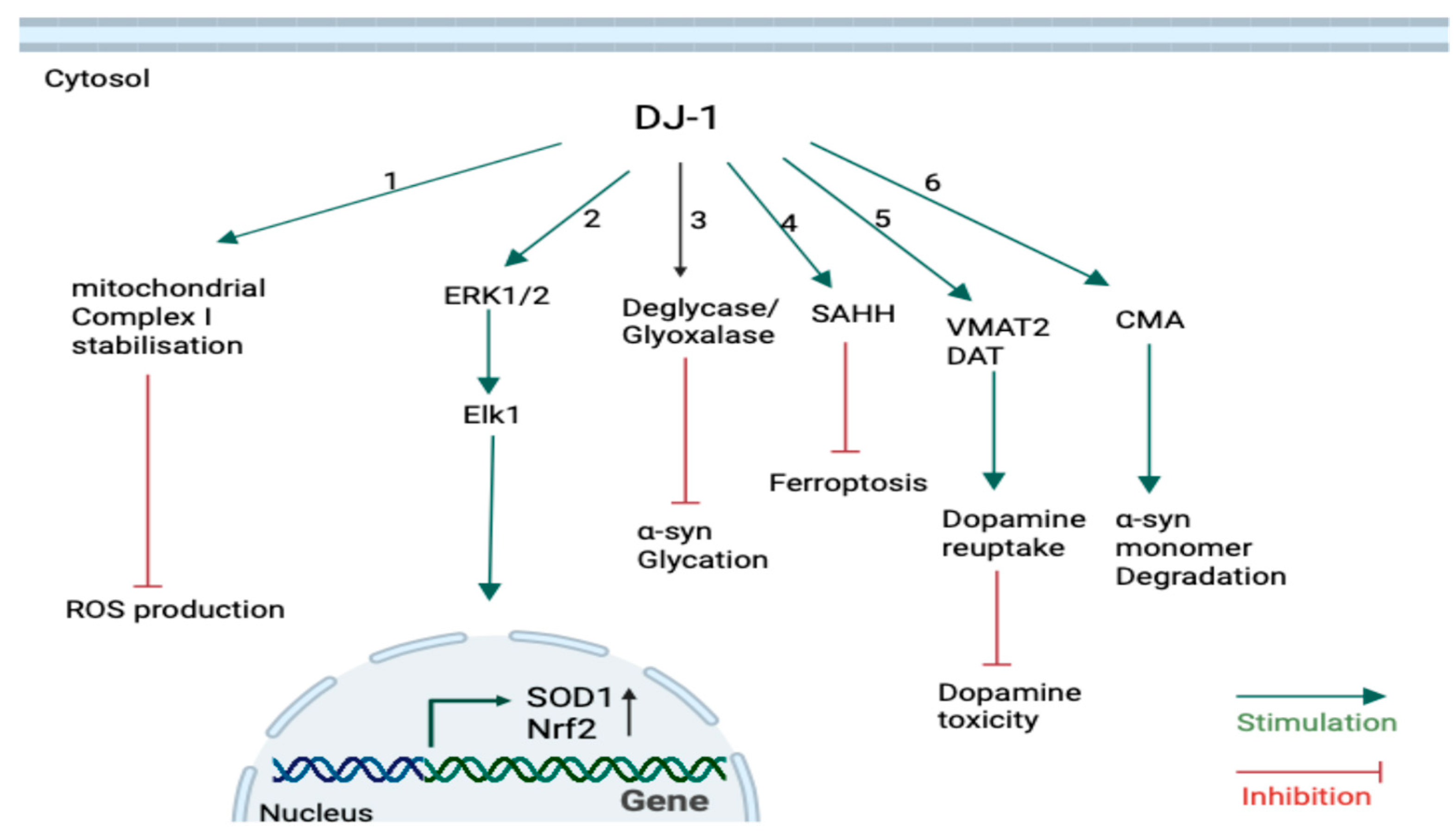
3.2. Physiological Roles of DJ-1
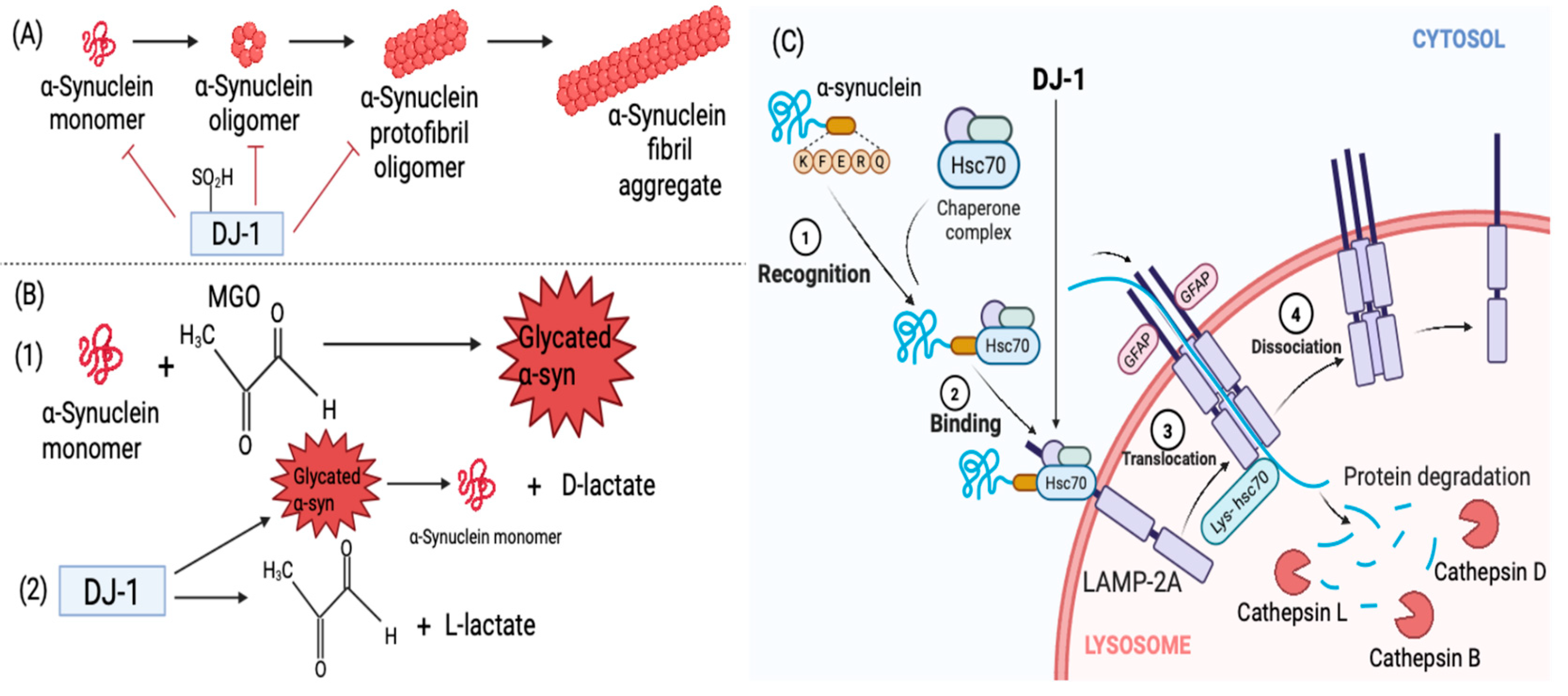
3.3. Interaction Between DJ-1 and α-Synuclein
4. Therapeutic Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| DJ-1 | Dutch juvenile -1 |
| α-Syn | α-synuclein |
| SN | Substantia Nigra |
| LRRK2 | Leucine-rich repeat kinase 2 |
| GBA | Glucocerebrosidase |
| PDI | Protein disulfide isomerase |
| SDS | Sodium Dodecyl Sulfate |
| NAC | Non-amyloid-β component |
| WT | Wild type |
| SNARE | Soluble N-ethylmaleimide-sensitive factor activating protein receptor |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| DOPAL | 3,4-Dihydroxyphenylacetaldehyde |
| TLR2/4 | Toll-like receptors 2 and 4 |
| IL-1β | Interleukin-1β |
| TNF-α | Tumour Necrosis factor-α |
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| ATP | Adenosine triphosphate |
| Ca2+ | Calcium |
| ER | Endothelium reticulum |
| UPR | Unfolded protein response |
| BiP | Binding immunoglobulin protein |
| PERK | PRKR-like ER kinase |
| IRE1α | Inositol-requiring enzyme 1α |
| ATF6 | Activating transcription factor 6 |
| ATF4 | Activating transcription factor 4 |
| CaBP1 | Calcium-binding protein 1 |
| IP3R | Inositol 1,4,5-trisphosphate receptors |
| SERCA | Sarco/endoplasmic reticulum Ca2+-ATPase |
| Rab1A | Ras-associated binding1A |
| GTPase | Guanosine triphosphatase |
| MAM | Mitochondria-associated ER membranes |
| UPS | Ubiquitin–proteasome system |
| CMA | Chaperone-mediated autophagy |
| Hsc70 | Heat-shock cognate protein of 70 kDa |
| LAMP-2A | Lysosome-associated membrane protein type 2A |
| MGO | Methylglyoxal |
| AGE | Advanced glycation end points |
| MG-H1 | Methylglyoxal-derived hydroimidazolone |
| CEL | Nε-carboxy-ethyl-lysine |
| RAGE | Receptor for advanced glycated end points |
| NFkB | Nuclear factor kappa B |
| TIQs | Tetrahydroisoquinolines |
| Ser129 | Serine 129 |
| C106 | Cysteine 106 |
| NDUFA4 | NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 4 |
| ND1 | NADH dehydrogenase subunit 1 |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| SOD1 | Superoxide dismutase 1 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| PSF | Pyrimidine tract-binding protein-associated splicing factor |
| VMAT2 | Vesicular monoamine transporter 2 |
| DAT | Dopamine active transporter |
| PLOO- | Phospholipid peroxides |
| SAHH | S-adenosyl homocysteine hydrolase |
| GSH | Glutathione |
References
- Mencke, P.; Boussaad, I.; Romano, C.D.; Kitami, T.; Linster, C.L.; Krüger, R. The Role of DJ-1 in Cellular Metabolism and Pathophysiological Implications for Parkinson’s Disease. Cells 2021, 10, 347. [Google Scholar] [CrossRef]
- Dolgacheva, L.P.; Berezhnov, A.V.; Fedotova, E.I.; Zinchenko, V.P.; Abramov, A.Y. Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J. Bioenerg. Biomembr. 2019, 51, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Adam, H.; Subash, C.B.; Gopinath Arshad, M.; Adam, T.; Parmin, N.A.; Husein, I.; Hashim, U. An update on pathogenesis and clinical scenario for Parkinson’s disease: Diagnosis and treatment. 3 Biotech 2023, 13, 142. [Google Scholar] [CrossRef]
- Silva, S.; Almeida, A.J.; Vale, N. Importance of Nanoparticles for the Delivery of Antiparkinsonian Drugs. Pharmaceutics 2021, 13, 508. [Google Scholar] [CrossRef]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. Journal of Neurology. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Di Cagno, A.; Buonsenso, A.; Centorbi, M.; Manni, L.; Di Costanzo, A.; Casazza, G.; Parisi, A.; Guerra, G.; Calcagno, G.; Iuliano, E.; et al. Whole body-electromyostimulation effects on serum biomarkers, physical performances and fatigue in Parkinson’s patients: A randomized controlled trial. Front. Aging Neurosci. 2023, 15, 1086487. [Google Scholar] [CrossRef]
- Kahle, P.J. α-Synucleinopathy models and human neuropathology: Similarities and differences. Acta Neuropathol. 2008, 115, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bendor, J.T.; Logan, T.; Edwards, R.H. The Function of α-Synuclein. Neuron 2013, 79, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef]
- Guan, Y.; Zhao, X.; Liu, F.; Yan, S.; Wang, Y.; Du, C.; Cui, X.; Li, R.; Zhang, C.X. Pathogenic Mutations Differentially Regulate Cell-to-Cell Transmission of α-Synuclein. Front. Cell Neurosci. 2020, 14, 159. [Google Scholar] [CrossRef]
- Chartier-Harlin, M.C.; Kachergus, J.; Roumier, C.; Mouroux, V.; Douay, X.; Lincoln, S.; Levecque, C.; Larvor, L.; Andrieux, J.; Hulihan, M.; et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1167–1169. [Google Scholar] [CrossRef]
- Ibáñez, P.; Bonnet, A.M.; Débarges, B.; Lohmann, E.; Tison, F.; Agid, Y.; Dürr, A.; Brice, A.; Pollak, P. Causal relation between α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Pei, Y.; Zhang, Z.; Xu, L.; Liu, X.; Jiang, L.; Pielak, G.J.; Zhou, X.; Liu, M.; Li, C. C-terminal truncation modulates α-Synuclein’s cytotoxicity and aggregation by promoting the interactions with membrane and chaperone. Commun. Biol. 2022, 5, 798. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.; Roeters, S.J.; Kogan, V.; Woutersen, S.; Claessens, M.M.A.E.; Subramaniam, V. C-Terminal Truncated α-Synuclein Fibrils Contain Strongly Twisted β-Sheets. J. Am. Chem. Soc. 2017, 139, 15392–15400. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; West, N.; Colla, E.; Pletnikova, O.; Troncoso, J.C.; Marsh, L.; Dawson, T.M.; Jäkälä, P.; Hartmann, T.; Price, D.L.; et al. Aggregation promoting C-terminal truncation of α-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. USA 2005, 102, 2162–2167. [Google Scholar] [CrossRef]
- Anderson, J.P.; Walker, D.E.; Goldstein, J.M.; de Laat, R.; Banducci, K.; Caccavello, R.J.; Barbour, R.; Huang, J.; Kling, K.; Lee, M.; et al. Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease. J. Biol. Chem. 2006, 281, 29739–29752. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumar, A. The Involvement of His50 during Protein Disulfide Isomerase Binding Is Essential for Inhibiting α-Syn Fibril Formation. Biochemistry 2016, 55, 2677–2680. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, L.; Wang, C. Domain a’ of protein disulfide isomerase plays key role in inhibiting α-synuclein fibril formation. Cell Stress Chaperon. 2009, 15, 415–421. [Google Scholar] [CrossRef]
- Daher, J.; Ying, M.; Banerjee, R.; McDonald, R.S.; Hahn, M.; Yang, L.; Flint Beal, M.; Thomas, B.; Dawson, V.L.; Dawson, T.M.; et al. Conditional transgenic mice expressing C-terminally truncated human α-synuclein (αSyn119) exhibit reduced striatal dopamine without loss of nigrostriatal pathway dopaminergic neurons. Mol. Neurodegener. 2009, 4, 34. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Vijayaraghavan, N.; Gorion, K.M.M.; Riffe, C.J.; Strang, K.H.; Caldwell, J.; Giasson, B.I. Physiological C-terminal truncation of α-synuclein potentiates the prion-like formation of pathological inclusions. J. Biol. Chem. 2018, 293, 18914–18932. [Google Scholar] [CrossRef]
- Weinreb, P.H.; Zhen, W.; Poon, A.W.; Conway, K.A.; Lansbury, P.T. NACP, A Protein Implicated in Alzheimer’s Disease and Learning, Is Natively Unfolded†. Biochemistry 1996, 35, 13709–13715. [Google Scholar] [CrossRef]
- Beyer, K. α-Synuclein structure, posttranslational modification and alternative splicing as aggregation enhancers. Acta Neuropathol. 2006, 112, 237–251. [Google Scholar] [CrossRef]
- Bartels, T.; Choi, J.G.; Selkoe, D.J. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 2011, 477, 107–110. [Google Scholar] [CrossRef]
- Wang, W.; Perovic, I.; Chittuluru, J.; Kaganovich, A.; Nguyen, L.T.T.; Liao, J.; Auclair, J.R.; Johnson, D.; Landeru, A.; Simorellis, A.K.; et al. A soluble α-synuclein construct forms a dynamic tetramer. Proc. Natl. Acad. Sci. USA 2011, 108, 17797–17802. [Google Scholar] [CrossRef]
- Fauvet, B.; Mbefo, M.K.; Fares, M.B.; Desobry, C.; Michael, S.; Ardah, M.T.; Tsika, E.; Coune, P.; Prudent, M.; Lion, N.; et al. α-Synuclein in Central Nervous System and from Erythrocytes, Mammalian Cells, and Escherichia coli Exists Predominantly as Disordered Monomer. J. Biol. Chem. 2012, 287, 15345–15364. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Südhof, T.C. α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc. Natl. Acad. Sci. USA 2014, 111, E4274–E4283. [Google Scholar] [CrossRef] [PubMed]
- Biorender. Available online: https://www.biorender.com (accessed on 22 July 2025).
- Abeliovich, A.; Schmitz, Y.; Fariñas, I.; Choi-Lundberg, D.; Ho, W.-H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.G.; Armanini, M.; Ryan, A.; et al. Mice Lacking α-Synuclein Display Functional Deficits in the Nigrostriatal Dopamine System. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef]
- Rosahl, T.W.; Spillane, D.; Missler, M.; Herz, J.; Selig, D.K.; Wolff, J.R.; Hammer, R.E.; Malenka, R.C.; Sudhof, T.C. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 1995, 375, 488–493. [Google Scholar] [CrossRef]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Sudhof, T.C. α-Synuclein Promotes SNARE-Complex Assembly in Vivo and in Vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.G.; Waymire, J.C.; Lin, E.; Liu, J.J.; Guo, F.; Zigmond, M.J. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J. Neurosci. 2002, 22, 3090–3099. [Google Scholar] [CrossRef] [PubMed]
- McDowall, J.S.; Ntai, I.; Honeychurch, K.C.; Hart, J.P.; Colin, P.; Schneider, B.L.; Brown, D.R. Alpha-synuclein ferrireductase activity is detectible in vivo, is altered in Parkinson’s disease and increases the neurotoxicity of DOPAL. Mol. Cell. Neurosci. 2017, 85, 1–11. [Google Scholar] [CrossRef]
- Davies, P.; Moualla, D.; Brown, D.R. Alpha-Synuclein Is a Cellular Ferrireductase. PLoS ONE 2011, 6, e15814. [Google Scholar] [CrossRef]
- McDowall, J.S.; Ntai, I.; Hake, J.; Whitley, P.R.; Mason, J.M.; Pudney, C.R.; Brown, D.R. Steady-State Kinetics of α-Synuclein Ferrireductase Activity Identifies the Catalytically Competent Species. Biochemistry 2017, 56, 2497–2505. [Google Scholar] [CrossRef]
- Calabresi, P.; Mechelli, A.; Natale, G.; Volpicelli-Daley, L.; Di Lazzaro, G.; Ghiglieri, V. Alpha-synuclein in Parkinson’s disease and other synucleinopathies: From overt neurodegeneration back to early synaptic dysfunction. Cell Death Dis. 2023, 14, 176. [Google Scholar] [CrossRef]
- Ko, L.; Ko, H.C.; Lin, W.L.; Kulathingal, J.G.; Yen, S.H.C. Aggregates Assembled From Overexpression of Wild-Type α-Synuclein are not Toxic to Human Neuronal Cells. J. Neuropathol. Exp. Neurol. 2008, 67, 1084–1096. [Google Scholar] [CrossRef]
- Guzzo, A.; Delarue, P.; Rojas, A.; Nicolaï, A.; Maisuradze, G.G.; Senet, P. Wild-Type α-Synuclein and Variants Occur in Different Disordered Dimers and Pre-Fibrillar Conformations in Early Stage of Aggregation. Front. Mol. Biosci. 2022, 9, 910104. [Google Scholar] [CrossRef] [PubMed]
- Winner, B.; Jappelli, R.; Maji, S.K.; Desplats, P.A.; Boyer, L.; Aigner, S.; Hetzer, C.; Loher, T.; Vilar, M.; Campioni, S.; et al. In vivo demonstration that α-synuclein oligomers are toxic. Proc. Natl. Acad. Sci. USA 2011, 108, 4194–4199. [Google Scholar] [CrossRef]
- Roberts, H.; Brown, D.R. Seeking a Mechanism for the Toxicity of Oligomeric α-Synuclein. Biomolecules 2015, 5, 282–305. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Petre, B.M.; Wall, J.; Simon, M.; Nowak, R.J.; Walz, T.; Lansbury, P.T. α-Synuclein, Especially the Parkinson’s Disease-associated Mutants, Forms Pore-like Annular and Tubular Protofibrils. J. Mol. Biol. 2002, 322, 1089–1102. [Google Scholar] [CrossRef]
- Tsigelny, I.F.; Sharikov, Y.; Wrasidlo, W.; Gonzalez, T.; Desplats, P.A.; Crews, L.; Spencer, B.; Masliah, E. Role of α-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J. 2012, 279, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, N.; Nielsen, S.B.; Buell, A.K.; Kaspersen, J.D.; Arosio, P.; Vad, B.S.; Paslawski, W.; Christiansen, G.; Valnickova-Hansen, Z.; Andreasen, M.; et al. The Role of Stable α-Synuclein Oligomers in the Molecular Events Underlying Amyloid Formation. J. Am. Chem. Soc. 2014, 136, 3859–3868. [Google Scholar] [CrossRef]
- Rooijen, B.; van Maria, M.; Subramaniam, V. Lipid bilayer disruption by oligomeric α-synuclein depends on bilayer charge and accessibility of the hydrophobic core. BBA-Biomembr. 2009, 1788, 1271–1278. [Google Scholar] [CrossRef]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric α-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for α-synuclein dependent activation of microglia and astroglia. Glia 2012, 61, 349–360. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef]
- Ludtmann, M.H.R.; Angelova, P.R.; Horrocks, M.H.; Choi, M.L.; Rodrigues, M.; Baev, A.Y.; Berezhnov, A.V.; Yao, Z.; Little, D.; Banushi, B.; et al. α-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat. Commun. 2018, 9, 2293. [Google Scholar] [CrossRef]
- Mosharov, E.V.; Staal, R.G.W.; Bové, J.; Prou, D.; Hanani, M.; Goldstein, D.S.; Greene, L.A.; Sulzer, D. α-Synuclein overexpression increases cytosolic catecholamine concentration. J. Neurosci. 2006, 26, 9304–9311. [Google Scholar] [CrossRef]
- Angelova, P.R.; Horrocks, M.H.; Klenerman, D.; Gandhi, S.; Abramov, A.Y.; Shchepinov, M.S. Lipid peroxidation is essential for α-synuclein-induced cell death. J. Neurochem. 2015, 133, 582–589. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; van Haastert, E.S.; Eikelenboom, P.; de Vos, R.A.I.; Rozemuller, J.M.; Scheper, W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007, 354, 707–711. [Google Scholar] [CrossRef]
- Kovaleva, V.; Saarma, M. Endoplasmic Reticulum Stress Regulators: New Drug Targets for Parkinson’s Disease. J. Parkinson Dis. 2021, 11, S219–S228. [Google Scholar] [CrossRef]
- Gorbatyuk, M.S.; Shabashvili, A.; Chen, W.; Meyers, C.; Sullivan, L.F.; Salganik, M.; Lin, J.H.; Lewin, A.S.; Muzyczka, N.; Gorbatyuk, O.S. Glucose Regulated Protein 78 Diminishes α-Synuclein Neurotoxicity in a Rat Model of Parkinson Disease. Mol. Ther. 2012, 20, 1327–1337. [Google Scholar] [CrossRef]
- Yamamoto, K.; Izumi, Y.; Arifuku, M.; Kume, T.; Sawada, H. α-Synuclein oligomers mediate the aberrant form of spike-induced calcium release from IP3 receptor. Sci. Rep. 2019, 9, 15977. [Google Scholar] [CrossRef] [PubMed]
- Betzer, C.; Lassen, L.B.; Olsen, A.J.; Kofoed, R.H.; Reimer, L.; Gregersen, E.; Zheng, J.; Calì, T.; Gai, W.; Chen, T.; et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018, 19, e44617. [Google Scholar] [CrossRef]
- Bravo, R.; Parra, V.; Gatica, D.; Rodriguez, A.E.; Torrealba, N.; Paredes, F.; Wang, Z.V.; Zorzano, A.; Hill, J.A.; Jaimovich, E.; et al. Endoplasmic Reticulum and the Unfolded Protein Response. Int. Rev. Cell Mol. Biol. 2013, 301, 215–290. [Google Scholar] [PubMed]
- Coune, P.G.; Bensadoun, J.C.; Aebischer, P.; Schneider, B.L. Rab1A Over-Expression Prevents Golgi Apparatus Fragmentation and Partially Corrects Motor Deficits in an Alpha-Synuclein Based Rat Model of Parkinson’s Disease. J. Parkinson Dis. 2011, 1, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Vicario, M.; Cieri, D.; Brini, M.; Calì, T. The Close Encounter Between Alpha-Synuclein and Mitochondria. Front. Neurosci. 2018, 12, 388. [Google Scholar] [CrossRef]
- Snyder, H.; Mensah, K.; Theisler, C.; Lee, J.; Matouschek, A.; Wolozin, B. Aggregated and monomeric alpha-synuclein bind to the S6’ proteasomal protein and inhibit proteasomal function. J. Biol. Chem. 2003, 278, 11753–11759. [Google Scholar] [CrossRef]
- Lindersson, E.; Beedholm, R.; Højrup, P.; Moos, T.; Gai, W.; Hendil, K.B.; Jensen, P.H. Proteasomal inhibition by alpha-synuclein filaments and oligomers. J. Biol. Chem. 2004, 279, 12924–12934. [Google Scholar] [CrossRef]
- Cuervo, A.M.; Stefanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired Degradation of Mutant Alpha-Synuclein by Chaperone-Mediated Autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Rodriguez-Oroz, M.C.; Cooper, J.M.; Caballero, C.; Ferrer, I.; Obeso, J.A.; Schapira, A.H.V. Chaperone-Mediated Autophagy Markers in Parkinson Disease Brains. Arch. Neurol. 2010, 67, 1464–1472. [Google Scholar] [CrossRef]
- Yao, R.; Shen, J. Chaperone-mediated autophagy: Molecular mechanisms, biological functions, and diseases. MedComm 2023, 4, e347. [Google Scholar] [CrossRef]
- Hodara, R.; Norris, E.H.; Giasson, B.I.; Mishizen-Eberz, A.J.; Lynch, D.R.; Lee, V.M.-Y.; Ischiropoulos, H. Functional Consequences of α-Synuclein Tyrosine Nitration. J. Biol. Chem. 2004, 279, 47746–47753. [Google Scholar] [CrossRef]
- Martinez-Vicente, M.; Talloczy, Z.; Kaushik, S.; Massey, A.C.; Mazzulli, J.; Mosharov, E.V.; Hodara, R.; Fredenburg, R.; Wu, D.-C.; Follenzi, A.; et al. Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J. Clin. Investig. 2008, 118, 777–788. [Google Scholar] [CrossRef]
- Skou, L.D.; Johansen, S.K.; Okarmus, J.; Meyer, M. Pathogenesis of DJ-1/PARK7-Mediated Parkinson’s Disease. Cells 2024, 13, 296. [Google Scholar] [CrossRef]
- Miranda, V.H.; Szego, É.M.; Oliveira, L.M.A.; Breda, C.; Darendelioglu, E.; de Oliveira, R.M.; Ferreira, D.G.; Gomes, M.A.; Rott, R.; Oliveira, M.; et al. Glycation potentiates α-synuclein-associated neurodegeneration in synucleinopathies. Brain J. Nerol. 2017, 140, 1399–1419. [Google Scholar] [CrossRef]
- Farzadfard, A.; König, A.; Petersen, S.; Nielsen, J.; Vasili, E.; Dominguez- Meijide, A.; Buell, A.K.; Tiago Fleming Outeiro Otzen, D.E. Glycation modulates alpha-synuclein fibrillization kinetics: A sweet spot for inhibition. J. Biol. Chem. 2022, 298, 101848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Niu, M.; Zhang, X.; Wang, J.; Zhou, C.; Xie, A. RAGE Silencing Ameliorates Neuroinflammation by Inhibition of p38-NF-κB Signaling Pathway in Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Poncet, G.; Herskovits, T.; Alves, R.; Corre, L.L.; Al-Azzani, M.; Koenig, A.; Birman, S.; Tiago Fleming Outeiro Mansuy, D.; Dairou, J. Inhibition of the Parkinson’s Disease-Related Protein DJ-1 by Endogenous Neurotoxins of the 1,2,3,4-Tetrahydroisoquinoline Family. ACS Chem. Neurosci. 2025, 16, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Oueslati, A.; Fournier, M.; Lashuel, H.A. Role of post-translational modifications in modulating the structure, function and toxicity of α-synuclein. Prog. Brain Res. 2010, 183, 115–145. [Google Scholar]
- Zhang, L.; Wang, J.; Wang, J.; Yang, B.; He, Q.; Weng, Q. Role of DJ-1 in Immune and Inflammatory Diseases. Front. Immunol. 2020, 11, 994. [Google Scholar] [CrossRef]
- Dash, B.K.; Urano, Y.; Saito, Y.; Noguchi, N. Redox-sensitive DJ-1 protein: An insight into physiological roles, secretion, and therapeutic target. Redox Exp. Med. 2022, 2022, R96–R115. [Google Scholar] [CrossRef]
- Bonifati, V.; Rizzu, P.; Van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.J.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 Gene Associated with Autosomal Recessive Early-Onset Parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef]
- Abou-Sleiman, P.M.; Healy, D.G.; Quinn, N.; Lees, A.J.; Wood, N.W. The role of pathogenic DJ-1 mutations in Parkinson’s disease. Ann. Neurol. 2003, 54, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Hague, S.; Rogaeva, E.; Hernandez, D.; Gulick, C.; Singleton, A.; Hanson, M.; Johnson, J.; Weiser, R.; Gallardo, M.; Ravina, B.; et al. Early-onset Parkinson’s disease caused by a compound heterozygous DJ-1 mutation. Ann. Neurol. 2003, 54, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Hering, R.; Strauss, K.M.; Tao, X.; Bauer, A.; Woitalla, D.; Mietz, E.-M.; Petrovic, S.; Bauer, P.; Schaible, W.; Müller, T.; et al. Novel homozygous p.E64D mutation in DJ1 in early onset Parkinson disease (PARK7). Hum. Mutat. 2004, 24, 321–329. [Google Scholar] [CrossRef]
- Rannikko, E.H.; Vesterager, L.B.; Shaik, J.H.A.; Weber, S.S.; Cornejo Castro, E.M.; Fog, K.; Jensen, P.H.; Kahle, P.J. Loss of DJ-1 protein stability and cytoprotective function by Parkinson’s disease-associated proline-158 deletion. J. Neurochem. 2013, 125, 314–327. [Google Scholar] [CrossRef]
- Repici, M.; Straatman, K.R.; Balduccio, N.; Enguita, F.J.; Outeiro, T.F.; Giorgini, F. Parkinson’s disease-associated mutations in DJ-1 modulate its dimerization in living cells. J. Mol. Med. 2013, 91, 599–611. [Google Scholar] [CrossRef]
- Honbou, K.; Suzuki, N.N.; Horiuchi, M.; Niki, T.; Taira, T.; Ariga, H.; Inagaki, F. The Crystal Structure of DJ-1, a Protein Related to Male Fertility and Parkinson’s Disease. J. Biol. Chem. 2003, 278, 31380–31384. [Google Scholar] [CrossRef]
- Wilson, M.A.; Collins, J.L.; Hod, Y.; Ringe, D.; Petsko, G.A. The 1.1-A resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early onset Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 9256–9261. [Google Scholar] [CrossRef]
- Choi, J.; Levey, A.I.; Weintraub, S.T.; Rees, H.D.; Gearing, M.; Chin, L.S.; Li, L. Oxidative Modifications and Down-regulation of Ubiquitin Carboxyl-terminal Hydrolase L1 Associated with Idiopathic Parkinson’s and Alzheimer’s Diseases. J. Biol. Chem. 2004, 279, 13256–13264. [Google Scholar] [CrossRef]
- Ito, G.; Ariga, H.; Nakagawa, Y.; Iwatsubo, T. Roles of distinct cysteine residues in S-nitrosylation and dimerization of DJ-1. Biochem. Biophys. Res. Commun. 2006, 339, 667–672. [Google Scholar] [CrossRef]
- Shinbo, Y.; Niki, T.; Taira, T.; Ooe, H.; Takahashi-Niki, K.; Maita, C.; Seino, C.; Iguchi-Ariga, S.M.M.; Ariga, H. Proper SUMO-1 conjugation is essential to DJ-1 to exert its full activities. Cell Death Differ. 2006, 13, 96–108. [Google Scholar] [CrossRef]
- Hayashi, T.; Ishimori, C.; Takahashi-Niki, K.; Taira, T.; Kim, Y.; Maita, H.; Maita, C.; Ariga, H.; Iguchi-Ariga, S.M.M. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 2009, 390, 667–672. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Chen, S.; Wang, Y.; Cao, L.; Zhang, Y.; Kang, W.; Li, H.; Gui, Y.; Chen, S.; et al. DJ-1 modulates the expression of Cu/Zn- superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann. Neurol. 2011, 70, 591–599. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Jiang, L.; Bin, L.; Meng, Y.; Zhu, D.; Zhu, H.; He, Q.; Yang, B.; Ying, M. DJ-1 suppresses ferroptosis through preserving the activity of S-adenosyl homocysteine hydrolase. Nat. Commun. 2020, 11, 1251. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Cooper, J.M.; Dexter, D.; Clark, J.B.; Jenner, P.; Marsden, C.D. Mitochondrial Complex I Deficiency in Parkinson’s Disease. J. Neurochem. 1990, 54, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, C.; Giguère, N.; Bourque, M.J.; Lévesque, M.; Slack, R.S.; Trudeau, L.É. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr. Biol. 2015, 25, 2349–2360. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P.Y. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Kim, C.Y.; Rizzu, P.; Geula, C.; Porter, D.R.; Pothos, E.N.; Squitieri, F.; Heutink, P.; Xu, J. DJ-1 Transcriptionally Up-regulates the Human Tyrosine Hydroxylase by Inhibiting the Sumoylation of Pyrimidine Tract-binding Protein-associated Splicing Factor. J. Biol. Chem. 2006, 281, 20940–20948. [Google Scholar] [CrossRef]
- Ishikawa, S.; Tanaka, Y.; Takahashi-Niki, K.; Niki, T.; Ariga, H.; Iguchi-Ariga, S.M.M. Stimulation of vesicular monoamine transporter 2 activity by DJ-1 in SH-SY5Y cells. Biochem. Biophys. Res. Commun. 2012, 421, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Luk, B.; Mohammed, M.; Liu, F.; Lee, F.J.S. A Physical Interaction between the Dopamine Transporter and DJ-1 Facilitates Increased Dopamine Reuptake. PLoS ONE 2015, 10, e0136641. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Ding, X.; Gao, L.; Han, Z.; Eleuteri, S.; Shi, W.; Shen, Y.; Song, Z.; Su, M.; Yang, Q.; Qu, Y.; et al. Ferroptosis in Parkinson’s disease: Molecular mechanisms and therapeutic potential. Ageing Res. Rev. 2023, 91, 102077. [Google Scholar] [CrossRef]
- Sharma, N.; Rao, S.P.; Kalivendi, S.V. The deglycase activity of DJ-1 mitigates α-synuclein glycation and aggregation in dopaminergic cells: Role of oxidative stress mediated downregulation of DJ-1 in Parkinson’s disease. Free Radic. Biol. Med. 2019, 135, 28–37. [Google Scholar] [CrossRef]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004, 2, e362. [Google Scholar] [CrossRef]
- Meulener, M.C.; Graves, C.L.; Sampathu, D.M.; Armstrong-Gold, C.E.; Bonini, N.M.; Giasson, B.I. DJ-1 is present in a large molecular complex in human brain tissue and interacts with α-synuclein. J. Neurochem. 2005, 93, 1524–1532. [Google Scholar] [CrossRef]
- Zhou, W.; Freed, C.R. DJ-1 Up-regulates Glutathione Synthesis during Oxidative Stress and Inhibits A53T α-Synuclein Toxicity. J. Biol. Chem. 2005, 280, 43150–43158. [Google Scholar] [CrossRef]
- Zhou, W.; Hurlbert, M.S.; Schaack, J.; Prasad, K.N.; Freed, C.R. Overexpression of human α-synuclein causes dopamine neuron death in rat primary culture and immortalized mesencephalon-derived cells. Brain Res. 2000, 866, 33–43. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, M.; Wilson, M.A.; Petsko, G.A.; Fink, A.L. The oxidation state of DJ-1 regulates its chaperone activity toward alpha-synuclein. J. Mol. Biol. 2006, 356, 1036–1048. [Google Scholar] [CrossRef]
- Zondler, L.; Miller-Fleming, L.; Repici, M.; Gonçalves, S.; Tenreiro, S.; Rosado-Ramos, R.; Betzer, C.; Straatman, K.R.; Jensen, P.H.; Giorgini, F.; et al. DJ-1 interactions with α-synuclein attenuate aggregation and cellular toxicity in models of Parkinson’s disease. Cell Death Dis. 2014, 5, e1350. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, S.; Hanpude, P.; Singh, A.K.; Johari, T.; Majumder, S.; Maiti, T.K. Partially oxidized DJ-1 inhibits α-synuclein nucleation and remodels mature α-synuclein fibrils in vitro. Commun. Biol. 2019, 2, 395. [Google Scholar] [CrossRef]
- Jin, J.; Li, G.; Davis, J.; Zhu, D.Z.; Wang, Y.; Pan, C.; Zhang, J. Identification of Novel Proteins Associated with Both α-Synuclein and DJ-1. Mol. Cell. Proteomics 2007, 6, 845–859. [Google Scholar] [CrossRef]
- Xu, C.Y.; Kang, W.Y.; Chen, Y.M.; Jiang, T.F.; Zhang, J.; Zhang, L.N.; Ding, J.Q.; Liu, J.; Chen, S.D. DJ-1 Inhibits α-Synuclein Aggregation by Regulating Chaperone-Mediated Autophagy. Front. Aging Neurosci. 2017, 9, 308. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Kwon, K.; Jang, S.; Kim, C.; Baek, K.; Kim, J.; Park, C. Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 2012, 21, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, A.; Bekkhozhin, Z.; Omertassova, N.; Baizhumanov, T.; Yeltay, G.; Akhmetali, M.; Toibazar, D.; Utepbergenov, D. The apparent deglycase activity of DJ-1 results from the conversion of free methylglyoxal present in fast equilibrium with hemithioacetals and hemiaminals. J. Biol. Chem. 2019, 294, 18863–18872. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.C.; Shuck, S.C.; Lin, J.; Moxley, M.A.; Termini, J.; Cookson, M.R.; Wilson, M.A. DJ-1 is not a deglycase and makes a modest contribution to cellular defense against methylglyoxal damage in neurons. J. Neurochem. 2022, 162, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Coukos, J.S.; Lee, C.W.; Pillai, K.S.; Shah, H.; Moellering, R.E. PARK7 Catalyzes Stereospecific Detoxification of Methylglyoxal Consistent with Glyoxalase and Not Deglycase Function. Biochemistry 2023, 62, 3126–3133. [Google Scholar] [CrossRef]
- Choi, J.; Tak, S.; Jung, H.; Cha, S.; Hwang, E.; Lee, D.; Lee, J.; Ryu, K.; Park, C. Kinetic evidence in favor of glyoxalase III and against deglycase activity of DJ-1. Protein Sci. 2023, 32, e4641. [Google Scholar] [CrossRef]
- Gao, Q.; Jacob-Dolan, J.W.; Scheck, R.A. Parkinsonism- Associated Protein DJ-1 Is an Antagonist, Not an Eraser, for Protein Glycation. Biochemistry 2023, 62, 1181–1190. [Google Scholar] [CrossRef]
- Richarme, G.; Mihoub, M.; Dairou, J.; Bui, L.C.; Leger, T.; Lamouri, A. Parkinsonism-associated Protein DJ-1/Park7 Is a Major Protein Deglycase That Repairs Methylglyoxal- and Glyoxal-glycated Cysteine, Arginine, and Lysine Residues. J. Biol. Chem. 2015, 290, 1885–1897. [Google Scholar] [CrossRef] [PubMed]
- Richarme, G.; Dairou, J. Parkinsonism-associated protein DJ-1 is a bona fide deglycase. Biochem. Biophys. Res. Commun. 2017, 483, 387–391. [Google Scholar] [CrossRef]
- Mathas, N.; Braud, E.; Galardon, E.; Larigot, L.; Etheve-Quelquejeu, M.; Sari, M.-A.; Le Grand, B.; Blanc, E.B.; Coumoul, X.; Dairou, J. Park7 (DJ-1), a Parkinson-associated glyoxalase/deglycase: Characterization of enzymatic activity in biological samples using fluorescence-based liquid chromatography. Biochimie 2025, 235, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; An, C.N.; Pu, X.P. DJ-1 protein protects dopaminergic neurons against 6-OHDA/MG-132-induced neurotoxicity in rats. Brain Res. Bull. 2012, 88, 609–616. [Google Scholar] [CrossRef]
- Niki, T.; Endo, J.; Takahashi-Niki, K.; Yasuda, T.; Okamoto, A.; Saito, Y.; Ariga, H.; Iguchi-Ariga, S.M.M. DJ-1-binding compound B enhances Nrf2 activity through the PI3-kinase-Akt pathway by DJ-1-dependent inactivation of PTEN. Brain Res. 2020, 1729, 146641. [Google Scholar] [CrossRef] [PubMed]
- Inden, M.; Yanagisawa, D.; Hijioka, M.; Ariga, H.; Kitamura, Y. Therapeutic Activities of DJ-1 and Its Binding Compounds Against Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2017, 1037, 187–202. [Google Scholar]
- Jana, M.; Dasarathy, S.; Ghosh, S.; Pahan, K. Upregulation of DJ-1 in Dopaminergic Neurons by a Physically-Modified Saline: Implications for Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 4652. [Google Scholar] [CrossRef]
- Waragai, M.; Nakai, M.; Wei, J.; Fujita, M.; Mizuno, H.; Ho, G.; Masliah, E.; Akatsu, H.; Yokochi, F.; Hashimoto, M. Plasma levels of DJ-1 as a possible marker for progression of sporadic Parkinson’s disease. Neurosci. Lett. 2007, 425, 18–22. [Google Scholar] [CrossRef]
- Lin, X.; Cook, T.J.; Zabetian, C.P.; Leverenz, J.B.; Peskind, E.R.; Hu, S.-C.; Cain, K.C.; Pan, C.Q.; Edgar, J.S.; Goodlett, D.R.; et al. DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci. Rep. 2012, 2, 954. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Saigoh, K.; Saito, Y.; Ogawa, I.; Mitsui, Y.; Hamada, Y.; Samukawa, M.; Suzuki, H.; Kuwahara, M.; Hirano, M.; et al. Diagnosis of Parkinson’s disease and the level of oxidized DJ-1 protein. Neurosci. Res. 2018, 128, 58–62. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobhifar, P.; Brown, D.R. Interaction Between α-Synuclein and DJ-1 in Parkinson’s Disease. Brain Sci. 2025, 15, 899. https://doi.org/10.3390/brainsci15090899
Sobhifar P, Brown DR. Interaction Between α-Synuclein and DJ-1 in Parkinson’s Disease. Brain Sciences. 2025; 15(9):899. https://doi.org/10.3390/brainsci15090899
Chicago/Turabian StyleSobhifar, Pouya, and David R. Brown. 2025. "Interaction Between α-Synuclein and DJ-1 in Parkinson’s Disease" Brain Sciences 15, no. 9: 899. https://doi.org/10.3390/brainsci15090899
APA StyleSobhifar, P., & Brown, D. R. (2025). Interaction Between α-Synuclein and DJ-1 in Parkinson’s Disease. Brain Sciences, 15(9), 899. https://doi.org/10.3390/brainsci15090899





