Clinical Management of Synthetic-Cannabinoid-Induced Psychosis: A Systematic Review of Treatment Strategies and Outcomes
Abstract
1. Introduction
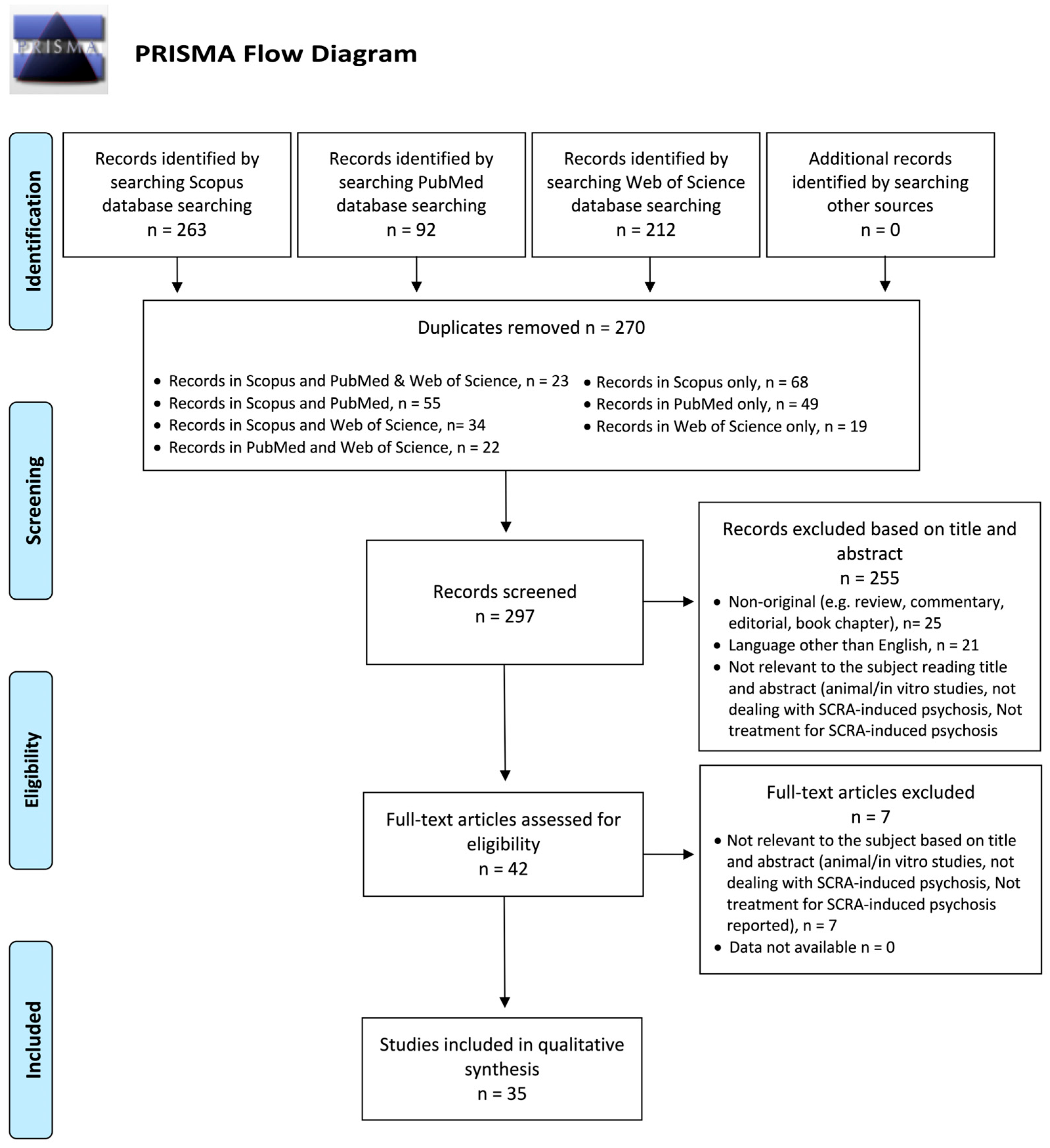
2. Materials and Methods
2.1. Systematic Review Procedures
2.2. Protocol and Registration
2.3. Eligibility Criteria (PICO Framework)
2.4. Data Synthesis Strategy
2.5. Risk of Bias and Quality of Evidence
3. Results
3.1. General Features
3.2. Risk of Bias
3.3. Clinical Presentation
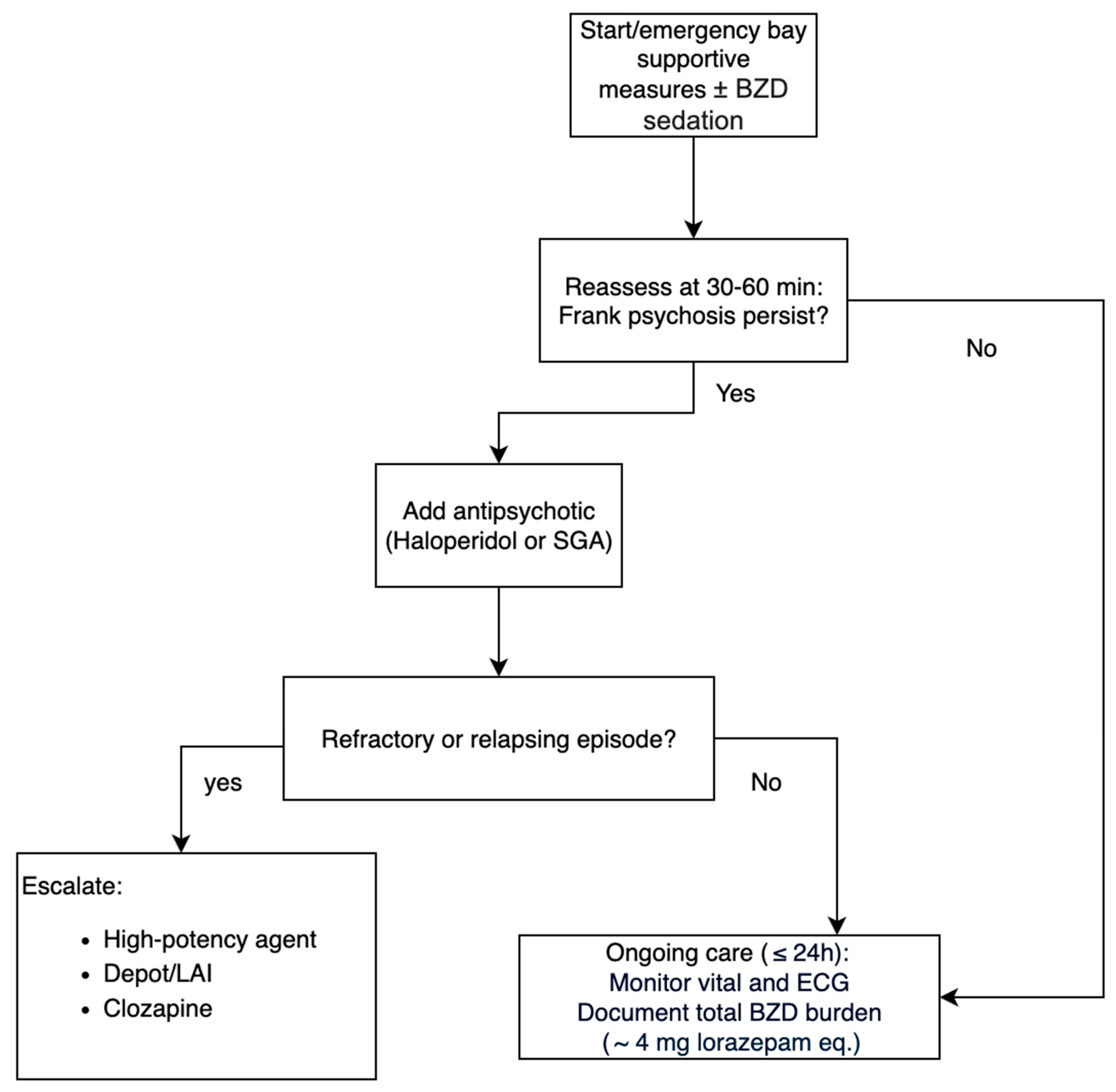
3.4. Pharmacological Management
3.5. Treatment Sequencing
3.6. Outcome and Course
3.7. Adverse Events and Safety
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Articles Excluded Based on Full Text
| Name, Year | Title | Motivation |
| Altintop, 2020 | A 4-Year Retrospective Analysis of Patients Presenting at the Emergency Department with Synthetic Cannabinoid Intoxication in Turkey | No treatment for SCRA-induced psychosis |
| Altintop et al., 2019 | Assessment of Patients Admitted to Emergency Rooms with Synthetic Cannabinoid Intoxication: A Prospective Study | No treatment for SCRA-induced psychosis |
| Benford et al., 2011 | Psychiatric Sequelae of Spice, K2, and Synthetic Cannabinoid Receptor Agonists | Data not available |
| Gilley et al., 2021 | Synthetic Cannabinoid Exposure in Adolescents Presenting for Emergency Care | Does not deal with SCRA-induced psychosis |
| O’Mahony et al., 2024 | HHC-induced psychosis: a case series of psychotic illness triggered by a widely available semisynthetic cannabinoid. | No treatment for SCRA-induced psychosis |
| Ricci et al., 2023 | First episode psychosis with and without the use of cannabis and synthetic cannabinoids: Psychopathology, global functioning and suicidal ideation and antipsychotic effectiveness | No treatment for SCRA-induced psychosis |
| van der Veer et al., 2011 | Persistent psychosis following the use of Spice | Data not available |
Appendix B. Specific SCRAs Identified
| Name, Year | Substance |
| Abouchedid et al., 2016 [26] | JWH-18 |
| Altintas et al., 2016 [27] | Unspecified SCRAs |
| Barceló et al., 2017 [28] | 5F-ADB, MMB-2201 |
| Bassir et al., 2016 [29] | Unspecified SCRAs |
| Bebarta et al., 2012 [30] | Spice |
| Berry-Cabàn et al., 2013 [31] | Spice |
| Besli et al., 2015 [32] | Unspecified SCRAs |
| Bonaccorso et al., 2018 [33] | Unspecified SCRAs |
| Celofiga et al., 2014 [34] | Unspecified SCRAs |
| Di Petta et al., 2016 [35] | Unspecified SCRAs |
| Durand et al., 2015 [36] | Unspecified SCRAs |
| Every-Palmer et al., 2011 [37] | JWH-018 |
| El Zahran et al., 2019 [38] | Cumyl-4-cyano-BINACA |
| Glue et al., 2013 [39] | K2 |
| Haro et al., 2014 [40] | JWH-081, JWH-250, JWH-203, JWH-019 |
| Helge Müller et al., 2009 [41] | Spice |
| Hermanns-Clausen et al., 2017 [42] | AB-CHMINACA, MDMB-CHMICA |
| Hoyte et al., 2012 [43] | Unspecified SCRAs |
| Hurst et al., 2011 [44] | Spice |
| Kekelidze et al., 2019 [45] | JWH, AB-PINACA, TMCP |
| Malik et al., 2021 [46] | K2 |
| Monte et al., 2017 [47] | Unspecified SCRAs |
| Oluwabusi et al., 2012 [48] | K2 |
| Ozer et al., 2016 [49] | Unspecified SCRAs |
| Peglow et al., 2012 [50] | Spice |
| Rahmani et al., 2013 [51] | Unspecified SCRAs |
| Roberto et al., 2016 [52] | Unspecified SCRAs |
| Satodiya et al., 2020 [53] | K2 |
| Simmons et al., 2011 [54] | JWH-018, JWH-073 |
| Skryabin et al., 2019 [55] | Unspecified SCRAs |
| Skryabin et al., 2018 [56] | Spice |
| Sönmez et al., 2016 [57] | Unspecified SCRAs |
| Sweet et al., 2017 [58] | Unspecified SCRAs |
| Tung et al., 2012 [59] | Spice |
| Udow et al., 2018 [60] | Unspecified SCRAs |
Appendix C. Psychiatric Comorbidity
| Name, Year | Psychiatric Comorbidity |
| Abouchedid et al., 2016 [26] | Depression |
| Altintas et al., 2016 [27] | NA |
| Barceló et al., 2017 [28] | No |
| Bassir et al., 2016 [29] | Schizophrenia, schizoaffective disorder, bipolar disorder, MDD, others |
| Bebarta et al., 2012 [30] | No |
| Berry-Cabàn et al., 2013 [31] | No |
| Besli et al., 2015 [32] | NA |
| Bonaccorso et al., 2018 [33] | Paranoid schizophrenia, schizoaffective disorder, bipolar disorder |
| Celofiga et al., 2014 [34] | Paranoid schizophrenia, undifferentiated schizophrenia |
| Di Petta et al., 2016 [35] | Previous psychosis related to substance abuse |
| Durand et al., 2015 [36] | No |
| Every-Palmer et al., 2011 [37] | Schizophrenia, schizoaffective disorder, borderline personality |
| El Zahran et al., 2019 [38] | No |
| Glue et al., 2013 [39] | Affective disorder, psychotic episodes |
| Haro et al., 2014 [40] | NA |
| Helge Müller et al., 2009 [41] | History of recurrent cannabis-induced psychotic episodes |
| Hermanns-Clausen et al., 2017 [42] | NA |
| Hoyte et al., 2012 [43] | NA |
| Hurst et al., 2011 [44] | No |
| Kekelidze et al., 2019 [45] | NA |
| Malik et al., 2021 [46] | Schizophrenia, SUD |
| Monte et al., 2017 [47] | NA |
| Oluwabusi et al., 2012 [48] | No |
| Ozer et al., 2016 [49] | No |
| Peglow et al., 2012 [50] | Post-traumatic stress disorder, SUD |
| Rahmani et al., 2013 [51] | No |
| Roberto et al., 2016 [52] | No |
| Satodiya et al., 2020 [53] | Schizophrenia |
| Simmons et al., 2011 [54] | No |
| Skryabin et al., 2019 [55] | SCRA dependence, cannabis use disorder, SCRA abuse |
| Skryabin et al., 2018 [56] | NA |
| Sönmez et al., 2016 [57] | No |
| Sweet et al., 2017 [58] | NA |
| Tung et al., 2012 [59] | No |
| Udow et al., 2018 [60] | Occasional visual hallucinations for many years with preserved insight |
Appendix D. Poly-Abuse
| Name, Year | Poly-Abuse (Substance) |
| Abouchedid et al., 2016 [26] | LSD |
| Altintas et al., 2016 [27] | Cannabis, alcohol, stimulant, opioid |
| Barceló et al., 2017 [28] | Cannabis |
| Bassir et al., 2016 [29] | Cannabis |
| Bebarta et al., 2012 [30] | Acetaminophen, dextromethorphan, doxylamine |
| Berry-Cabàn et al., 2013 [31] | Mephedrone, cannabis, alcohol |
| Besli et al., 2015 [32] | Alcohol, amphetamines |
| Bonaccorso et al., 2018 [33] | Crack, cocaine, heroin, alcohol, MDMA, cannabis, legal highs polysubstance misuse |
| Celofiga et al., 2014 [34] | NA |
| Di Petta et al., 2016 [35] | Salvia divinorum, cocaine, efedrine, ketamine, alcohol |
| Durand et al., 2015 [36] | Cannabis |
| Every-Palmer et al., 2011 [37] | No |
| El Zahran et al., 2019 [38] | No |
| Glue et al., 2013 [39] | NA |
| Haro et al., 2014 [40] | NA |
| Helge Müller et al., 2009 [41] | No |
| Hermanns-Clausen et al., 2017 [42] | Amphetamines |
| Hoyte et al., 2012 [43] | No |
| Hurst et al., 2011 [44] | Cannabis, alcohol |
| Kekelidze et al., 2019 [45] | NA |
| Malik et al., 2021 [46] | NA |
| Monte et al., 2017 [47] | NA |
| Oluwabusi et al., 2012 [48] | NA |
| Ozer et al., 2016 [49] | No |
| Peglow et al., 2012 [50] | No |
| Rahmani et al., 2013 [51] | Cannabis, LSD, psilocybin, mushrooms, Spice, bath salts, oxycodone |
| Roberto et al., 2016 [52] | No |
| Satodiya et al., 2020 [53] | NA |
| Simmons et al., 2011 [54] | No |
| Skryabin et al., 2019 [55] | NA |
| Skryabin et al., 2018 [56] | NA |
| Sönmez et al., 2016 [57] | No |
| Sweet et al., 2017 [58] | NA |
| Tung et al., 2012 [59] | Polysubstance abuse |
| Udow et al., 2018 [60] | No |
Appendix E. RoBINS-I Assessment
| Author (Year) | Study Type | Confounding | Selection of Participants | Classification of Interventions | Deviations from Intended Interventions | Missing Data | Measurement of Outcomes | Selection of Reported Result | Overall Judgment |
| Altintas et al. (2016) [27] | Single-center cross-sectional analysis | Serious—Confounding by psychiatric diagnosis and polysubstance use | Moderate—Participants were psychiatric patients, which limits representativeness | Low—Exposure classification via structured interview | Low—No deviations relevant | Moderate—Some missing records | Serious—Outcomes not blinded, limited validity | Moderate—Some selective emphasis | Serious risk of bias |
| Bassir et al. (2016) [29] | Retrospective review | Serious—Multiple confounders, no adjustment | Moderate—Hospitalized psychiatric patients only | Low—Exposure classification based on records | Low—No deviations relevant | Moderate—Some missing records | Serious—Outcomes not systematically validated | Moderate—Some selective emphasis | Serious risk of bias |
| Every-Palmer (2011) [37] | Cohort study | Serious—Multiple unmeasured confounders | Serious—Small forensic psychiatric sample, very selective | Low—Exposure classification consistent (self-report of JWH-018) | Moderate—No intervention deviations applicable | Moderate—Incomplete data from interviews | Serious—Outcomes subjective, not standardized | Serious—Results selectively described | Serious risk of bias |
| Glue et al. (2013) [39] | Retrospective observational study | Serious—Confounding by severity of illness | Moderate—All hospitalizations reviewed, small sample | Low—Exposure classification clear (SC identified) | Low—No deviations relevant | Moderate—Some missing documentation | Serious—Outcomes variable, not standardized | Moderate—Some selective reporting | Serious risk of bias |
| Hermanns-Clausen et al. (2018) [42] | Prospective observational study | Serious—Multiple confounding variables not controlled | Low—Consecutive ED patients included | Low—Exposure confirmed analytically | Low—No deviations, observational design | Moderate—Some missing clinical and lab data | Serious—Heterogeneous outcome measures across sites | Moderate—Selective reporting possible | Serious risk of bias |
| Hoyte et al. (2012) [43] | Observational study | Serious—Confounding by reporting patterns | Low—All NPDS cases included | Low—Exposure classification robust for poison center data | Low—Observational, no deviations relevant | Moderate—Some missing data in registry | Serious—Outcomes inconsistently categorized | Moderate—Registry limitations | Serious risk of bias |
| Kekelidze et al. (2019) [45] | Interventional study | Serious—No confounder adjustment | Moderate—Psychiatric hospital patients, limited representativeness | Low—Exposure classification by clinical record | Low—Observational, no deviations relevant | Moderate—Some incomplete records | Serious—Outcomes not validated | Moderate—Selective reporting likely | Serious risk of bias |
| Monte et al. (2017) [47] | Cohort study | Serious—Confounding by indication and severity, no comparator | Low—All cases from registry included consecutively | Low—Exposure classification robust (clinical registry) | Low—No deviations relevant, observational design | Moderate—Some incomplete clinical details | Serious—Outcomes heterogeneous across centers | Moderate—Registry structure limits selective reporting | Serious risk of bias |
| Skryabin et al. (2019) [55] | Observational study | Serious—No adjustment for confounding variables | Moderate—Participants were consecutive SC users but not population-based | Low—Exposure classification clear (self-report + clinical diagnosis) | Moderate—Deviations possible, treatment not standardized | Moderate—Some missing follow-up data | Serious—Outcomes not systematically validated | Moderate—Some selective reporting likely | Serious risk of bias |
| Skryabin et al. (2018) [56] | Longitudinal, observational cohort study | Serious—No confounder adjustment, polysubstance use not controlled | Moderate—Consecutive inpatients but not representative of general population | Low—Exposure classification clinical + confirmed SC use | Low—Observational, no intervention deviations | Moderate—Missing follow-up details for some patients | Serious—Psychiatric outcomes not systematically validated | Moderate—Potential selective emphasis in reporting | Serious risk of bias |
Appendix F. CARE Checklist—Case Reports
| Author (Year) | Title | Keywords | Abstract | Introduction | Patient Information | Clinical Findings | Timeline | Diagnostic Assessment | Therapeutic Intervention | Follow-Up and Outcomes | Discussion | Patient Perspective | Informed Consent |
| Abouchedid et al. (2016) [26] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Present |
| Barceló et al. (2017) [28] | Present | Present | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Bebarta et al. (2012) [30] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Berry-Cabán et al. (2013) [31] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Besli et al. (2015) [32] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Bonaccorso et al. (2018) [33] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Celofiga et al. (2014) [34] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Di Petta (2016) [35] | Present | Absent | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Durand et al. (2015) [36] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| El Zahran et al. (2018) [38] | Present | Present | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Haro et al. (2014) [40] | Present | Absent | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Hurst et al. (2011) [44] | Present | Present | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Malik et al. (2020) [46] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Oluwabusi et al. (2012) [48] | Present | Absent | Absent | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Ozer et al. (2016) [49] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Present |
| Peglow et al. (2012) [50] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Rahmani et al. (2014) [51] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Roberto et al. (2016) [52] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Present |
| Satodiya & Palekar (2020) [53] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Absent | Present | Absent | Present |
| Sönmez & Köşger (2016) [57] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Sweet et al. (2017) [58] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Tung et al. (2012) [59] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
| Udow et al. (2018) [60] | Present | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Absent | Absent |
References
- Schifano, F.; Chiappini, S.; Corkery, J.M.; Scherbaum, N.; Guirguis, A. The e-psychonaut drugs’ psychopharmacology. Curr. Opin. Pharmacol. 2021, 57, 165–174. [Google Scholar] [CrossRef]
- Chiappini, S.; Vaccaro, G.; Mosca, A.; Miuli, A.; Stigliano, G.; Stefanelli, G.; Giovannetti, G.; Carullo, R.; D’aNdrea, G.; Di Carlo, F.; et al. New trends of drug abuse in custodial settings: A systematic review on the misuse of over-the-counter drugs, prescription-only-medications, and new psychoactive substances. Neurosci. Biobehav. Rev. 2024, 162, 105691. [Google Scholar] [CrossRef]
- Fiorentini, A.; Cantù, F.; Crisanti, C.; Cereda, G.; Oldani, L.; Brambilla, P. Substance-Induced Psychoses: An Updated Literature Review. Front. Psychiatry 2021, 12, 694863. [Google Scholar] [CrossRef]
- Papanti, D.; Schifano, F.; Botteon, G.; Bertossi, F.; Mannix, J.; Vidoni, D.; Impagnatiello, M.; Pascolo-Fabrici, E.; Bonavigo, T. “Spiceophrenia”: A systematic overview of “Spice”-related psychopathological issues and a case report. Hum. Psychopharmacol. 2013, 28, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Mosca, A.; Semeraro, F.; Amerio, A.; Berardelli, I.; Cremaschi, L.; Di Bernardo, I.; Pettorruso, M.; Serafini, G.; Dell’OSso, B.; et al. Navigating the challenges of substance use and psychopathology in depression, bipolar disorder, and schizophrenia. Compr. Psychiatry 2025, 142, 152616. [Google Scholar] [CrossRef]
- Martinotti, G.; De Risio, L.; Vannini, C.; Schifano, F.; Pettorruso, M.; Di Giannantonio, M. Substance-related exogenous psychosis: A postmodern syndrome. CNS Spectr. 2021, 26, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Ricci, V.; Ceci, F.; Di Carlo, F.; Lalli, A.; Ciavoni, L.; Mosca, A.; Sepede, G.; Salone, A.; Quattrone, D.; Fraticelli, S.; et al. Cannabis use disorder and dissociation: A report from a prospective first-episode psychosis study. Drug Alcohol Depend. 2021, 229, 109118. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Orsolini, L.; Corkery, J.M.; Guirguis, A.; Pettorruso, M.; Martinotti, G.; Di Giannantonio, M.; et al. New Psychoactive Substances and Suicidality: A Systematic Review of the Current Literature. Medicina 2021, 57, 580. [Google Scholar] [CrossRef]
- Chiappini, S.; Miuli, A.; Mosca, A.; Pettorruso, M.; Guirguis, A.; John, M.C.; Martinotti, G.; Di Giannantonio, M.; Schifano, F. The Benzydamine Experience: A Systematic Review of Benzydamine Abuse. Curr. Neuropharmacol. 2021, 19, 1728–1737. [Google Scholar] [CrossRef]
- Davey, Z.; Schifano, F.; Corazza, O.; Deluca, P.; Psychonaut Web Mapping Group. e-Psychonauts: Conducting research in online drug forum communities. J. Ment. Health 2012, 21, 386–394. [Google Scholar] [CrossRef]
- Bucci, S.; Schwannauer, M.; Berry, N. The digital revolution and its impact on mental health care. Psychol Psychother 2019, 92, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fu, L. Recent findings and advancements in the detection of designer benzodiazepines: A brief review. Arch. Ind. Hyg. Toxicol. 2023, 74, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J., Jr.; Raffa, R.; LeQuang, J.A.K.; Breve, F.; Varrassi, G. Old Drugs and New Challenges: A Narrative Review of Nitazenes. Cureus 2023, 15, e40736. [Google Scholar] [CrossRef]
- Lynn, R.R.; Galinkin, J. Naloxone dosage for opioid reversal: Current evidence and clinical implications. Ther. Adv. Drug Saf. 2018, 9, 63–88. [Google Scholar] [CrossRef]
- Hohmann, N.; Mikus, G.; Czock, D. Effects and Risks Associated with Novel Psychoactive Substances. Dtsch. Ärzteblatt Int. 2014, 111, 139–147. [Google Scholar] [CrossRef][Green Version]
- Schifano, F.; Napoletano, F.; Chiappini, S.; Guirguis, A.; Corkery, J.M.; Bonaccorso, S.; Ricciardi, A.; Scherbaum, N.; Vento, A. New/emerging psychoactive substances and associated psychopathological consequences. Psychol. Med. 2021, 51, 30–42. [Google Scholar] [CrossRef]
- Ricci, V.; Chiappini, S.; Martinotti, G.; Maina, G. Cannabis use and psychotic-like experiences: A systematic review of biological vulnerability, potency effects, and clinical trajectories. Psychiatry Res. 2025, 348, 116496. [Google Scholar] [CrossRef]
- Mosca, A.; Chiappini, S.; Miuli, A.; Mancusi, G.; Santovito, M.C.; Di Carlo, F.; Pettorruso, M.; Corkery, J.M.; Canessa, C.; Martinotti, G.; et al. Ibogaine/Noribogaine in the Treatment of Substance Use Disorders: ASystematic Review of the Current Literature. Curr. Neuropharmacol. 2023, 21, 2178–2194. [Google Scholar] [CrossRef]
- Cavallotto, C.; Chiappini, S.; Mosca, A.; D’andrea, G.; Di Carlo, F.; Piro, T.; Susini, O.; Stefanelli, G.; Di Cesare, A.; Ricci, V.; et al. Examining Lurasidone Efficacy in Patients with Schizophrenia Spectrum Illness and Concurrent Alcohol and Substance Use Disorder: A Prospective, Multicentric, Real-World Investigation. J. Clin. Med. 2024, 13, 2206. [Google Scholar] [CrossRef]
- Chiappini, S.; Cavallotto, C.; Mosca, A.; Di Carlo, F.; Piro, T.; Giovannetti, G.; Pasino, A.; Vicinelli, M.; Lorenzini, C.; Di Paolo, M.; et al. Investigating the Effectiveness of Brexpiprazole in Subjects with Schizophrenia Spectrum Illness and Co-Occurring Substance Use Disorder: A Prospective, Multicentric, Real-World Study. Pharmaceuticals 2024, 17, 535. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE Guidelines: Consensus-Based Clinical Case Reporting Guideline Development. Headache 2013, 53, 1541–1547. [Google Scholar] [CrossRef]
- Abouchedid, R.; Ho, J.H.; Hudson, S.; Dines, A.; Archer, J.R.H.; Wood, D.M.; Dargan, P.I. Acute Toxicity Associated with Use of 5F-Derivations of Synthetic Cannabinoid Receptor Agonists with Analytical Confirmation. J. Med. Toxicol. 2016, 12, 396–401. [Google Scholar] [CrossRef]
- Altintas, M.; Inanc, L.; Oruc, G.A.; Arpacioglu, S.; Gulec, H. Clinical characteristics of synthetic cannabinoid-induced psychosis in relation to schizophrenia: A single-center cross-sectional analysis of concurrently hospitalized patients. Neuropsychiatr. Dis. Treat 2016, 12, 1893–1900. [Google Scholar] [CrossRef]
- Barceló, B.; Pichini, S.; López-Corominas, V.; Gomila, I.; Yates, C.; Busardò, F.P.; Pellegrini, M. Acute intoxication caused by synthetic cannabinoids 5F-ADB and MMB-2201: A case series. Forensic Sci. Int. 2017, 273, e10–e14. [Google Scholar] [CrossRef]
- Nia, A.B.; Medrano, B.; Perkel, C.; Galynker, I.; Hurd, Y.L. Psychiatric comorbidity associated with synthetic cannabinoid use compared to cannabis. J. Psychopharmacol. 2016, 30, 1321–1330. [Google Scholar] [CrossRef]
- Bebarta, V.S.; Ramirez, S.; Varney, S.M. Spice: A New “Legal” Herbal Mixture Abused by Young Active Duty Military Personnel. Subst. Abus. 2012, 33, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Berry-Cabán, C.S.; Ee, J.; Ingram, V.; Berry, C.E.; Kim, E.H. Synthetic Cannabinoid Overdose in a 20-Year-Old Male US Soldier. Subst. Abus. 2013, 34, 70–72. [Google Scholar] [CrossRef]
- Besli, G.E.; Ikiz, M.A.; Yildirim, S.; Saltik, S. Synthetic Cannabinoid Abuse in Adolescents: A Case Series. J. Emerg. Med. 2015, 49, 644–650. [Google Scholar] [CrossRef]
- Bonaccorso, S.; Metastasio, A.; Ricciardi, A.; Stewart, N.; Jamal, L.; Rujully, N.-U.; Theleritis, C.; Ferracuti, S.; Ducci, G.; Schifano, F. Synthetic Cannabinoid use in a Case Series of Patients with Psychosis Presenting to Acute Psychiatric Settings: Clinical Presentation and Management Issues. Brain Sci. 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Celofiga, A.; Koprivsek, J.; Klavz, J. Use of Synthetic Cannabinoids in Patients with Psychotic Disorders: Case Series. J. Dual Diagn. 2014, 10, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Di Petta, G. “Synthetic Psychosis” by Novel Psychoactive Substances: A Psychopathological Understanding of a Clinical Case. In An Experiential Approach to Psychopathology; Stanghellini, G., Aragona, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–188. [Google Scholar] [CrossRef]
- Durand, D.; Delgado, L.L.; Parra-Pellot, D.M.D.L.; Nichols-Vinueza, D. Psychosis and Severe Rhabdomyolysis Associated with Synthetic Cannabinoid Use: A Case Report. Clin. Schizophr. Relat. Psychoses 2015, 8, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Every-Palmer, S. Synthetic cannabinoid JWH-018 and psychosis: An explorative study. Drug Alcohol Depend. 2011, 117, 152–157. [Google Scholar] [CrossRef]
- El Zahran, T.; Gerona, R.; Morgan, B.W.; Pomerleau, A.C. A novel synthetic cannabinoid (Cumyl-4-cyano-BINACA) resulting in hyperthermia, rhabdomyolysis, and renal failure in a 29-year-old patient: It’s not meningitis. Clin. Toxicol. 2019, 57, 421–422. [Google Scholar] [CrossRef]
- Glue, P.; Courts, J.; Gray, A.; Patterson, T. Influence of law changes affecting synthetic cannabinoid availability and frequency of hospital presentations: 4-year national survey. N. Z. Med. J. 2016, 129, 37–40. [Google Scholar]
- Haro, G.; Ripoll, C.; Ibáñez, M.; Orengo, T.; Liaño, V.M.; Meneu, E.; Hernández, F.; Traver, F. Could Spice Drugs Induce Psychosis with Abnormal Movements Similar to Catatonia? Psychiatry Interpers. Biol. Process. 2014, 77, 206–208. [Google Scholar] [CrossRef]
- Müller, H.; Sperling, W.; Köhrmann, M.; Huttner, H.B.; Kornhuber, J.; Maler, J.-M. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr. Res. 2010, 118, 309–310. [Google Scholar] [CrossRef]
- Hermanns-Clausen, M.; Müller, D.; Kithinji, J.; Angerer, V.; Franz, F.; Eyer, F.; Neurath, H.; Liebetrau, G.; Auwärter, V. Acute side effects after consumption of the new synthetic cannabinoids AB-CHMINACA and MDMB-CHMICA. Clin. Toxicol. 2018, 56, 404–411. [Google Scholar] [CrossRef]
- Hoyte, C.O.; Jacob, J.; Monte, A.A.; Al-Jumaan, M.; Bronstein, A.C.; Heard, K.J. A Characterization of Synthetic Cannabinoid Exposures Reported to the National Poison Data System in 2010. Ann. Emerg. Med. 2012, 60, 435–438. [Google Scholar] [CrossRef]
- Hurst, D.; Loeffler, G.; McLay, R. Psychosis Associated with Synthetic Cannabinoid Agonists: A Case Series. AJP 2011, 168, 1119. [Google Scholar] [CrossRef]
- Kekelidze, Z.I.; Klimenko, T.V.; Kozlov, A.A.; Shakhova, S.M. Differential approaches to the treatment of acute psychosis due to the use of synthetic cannabinoids. Z. Nevrol. Psikhiatr. Im. S. S. Korsakova 2017, 117, 21. [Google Scholar] [CrossRef]
- Malik, K.; Kommana, S.; Paul, J.; Krakauer, M. Synthetic cannabinoid induced ocular self-injury. Orbit 2021, 40, 326–328. [Google Scholar] [CrossRef] [PubMed]
- Monte, A.A.; Calello, D.P.; Gerona, R.R.; Hamad, E.; Campleman, S.L.; Brent, J.; Wax, P.; Carlson, R.G. Characteristics and Treatment of Patients with Clinical Illness Due to Synthetic Cannabinoid Inhalation Reported by Medical Toxicologists: A ToxIC Database Study. J. Med. Toxicol. 2017, 13, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Oluwabusi, O.O.; Lobach, L.; Akhtar, U.; Youngman, B.; Ambrosini, P.J. Synthetic Cannabinoid-Induced Psychosis: Two Adolescent Cases. J. Child Adolesc. Psychopharmacol. 2012, 22, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Urun, O.; Ceri, V.; Evren, C. Capgras syndrome after use of synthetic cannabinoids: An adolescent case. Dusunen Adam J. Psychiatry Neurol. Sci. 2016, 29, 374–378. [Google Scholar]
- Peglow, S.; Buchner, J.; Briscoe, G. Synthetic Cannabinoid Induced Psychosis in a Previously Nonpsychotic Patient. Am. J. Addict. 2012, 21, 287–288. [Google Scholar] [CrossRef]
- Rahmani, M.; Paul, S.; Nguyen, M.L. Treatment of refractory substance-induced psychosis in adolescent males with a genetic predisposition to mental illness. Int. J. Adolesc. Med. Health 2014, 26, 297–301. [Google Scholar] [CrossRef]
- Roberto, A.J.; Lorenzo, A.; Li, K.J.; Young, J.; Mohan, A.; Pinnaka, S.; Lapidus, K.A.B. First-Episode of Synthetic Cannabinoid-Induced Psychosis in a Young Adult, Successfully Managed with Hospitalization and Risperidone. Case Rep. Psychiatry 2016, 2016, 7257489. [Google Scholar] [CrossRef]
- Satodiya, R.; Palekar, N. Synthetic Cannabinoids and Its Association with Persistent Negative Symptoms of Schizophrenia. Cureus 2020, 12, e10329. [Google Scholar] [CrossRef]
- Simmons, J.; Cookman, L.; Kang, C.; Skinner, C. Three cases of “spice” exposure. Clin. Toxicol. 2011, 49, 431–433. [Google Scholar] [CrossRef]
- Skryabin, V.Y.; Vinnikova, M.A. Psychotic Disorders in Patients Who Use Synthetic Cannabinoids. J. Psychiatr. Pract. 2019, 25, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, V.Y.; Vinnikova, M.A. Clinical characteristics of synthetic cannabinoid-induced psychotic disorders: A single-center analysis of hospitalized patients. J. Addict. Dis. 2018, 37, 135–141. [Google Scholar] [CrossRef]
- Sönmez, İ.; Köşger, F. Synthetic Cannabinoid Receptor Agonist-Associated Psychotic Disorder: A Case Report. Turk Psikiyatr. Derg. 2016, 27, 63–66. [Google Scholar]
- Sweet, G.; Kim, S.; Martin, S.; Washington, N.B.; Brahm, N. Psychiatric symptoms and synthetic cannabinoid use: Information for clinicians. Ment. Health Clin. 2017, 7, 156–159. [Google Scholar] [CrossRef]
- Tung, C.K.; Chiang, T.P.; Lam, M. Acute mental disturbance caused by synthetic cannabinoid: A potential emerging substance of abuse in Hong Kong. East Asian Arch. Psychiatry 2012, 22, 31–33. [Google Scholar] [PubMed]
- Udow, S.J.; Freitas, M.E.; Fox, S.H.; Lang, A.E. Exacerbation of psychosis triggered by a synthetic cannabinoid in a 70-year-old woman with Parkinson disease. Can. Med Assoc. J. 2018, 190, E50–E52. [Google Scholar] [CrossRef]
- Malaca, S.; Busardò, F.P.; Nittari, G.; Sirignano, A.; Ricci, G. Fourth Generation of Synthetic Cannabinoid Receptor Agonists: A Review on the Latest Insights. Curr. Pharm. Des. 2022, 28, 2603–2617. [Google Scholar] [CrossRef]
- Bach, P.; Hayes, S.C. The use of acceptance and commitment therapy to prevent the rehospitalization of psychotic patients: A randomized controlled trial. J. Consult. Clin. Psychol. 2002, 70, 1129–1139. [Google Scholar] [CrossRef]
- De Berardis, D.; Serroni, N.; Campanella, D.; Olivieri, L.; Ferri, F.; Carano, A.; Cavuto, M.; Martinotti, G.; Cicconetti, A.; Piersanti, M.; et al. Update on the Adverse Effects of Clozapine: Focus on Myocarditis. Curr. Drug Saf. 2012, 7, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zaman, H.; Sampson, S.; Beck, A.; Sharma, T.; Clay, F.; Spyridi, S.; Zhao, S.; Gillies, D. Benzodiazepines for Psychosis-Induced Aggression or Agitation. Schizophr. Bull. 2018, 44, 966–969. [Google Scholar] [CrossRef]
- Baranchik, S.; Stryjer, R.; Weizman, A.; Shelef, A. Add-on benzodiazepines for psychosis-induced aggression. Int. Clin. Psychopharmacol. 2019, 34, 119–123. [Google Scholar] [CrossRef]
- Martinotti, G.; Chiappini, S.; Mosca, A.; Miuli, A.; Santovito, M.C.; Pettorruso, M.; Skryabin, V.; Sensi, S.L.; Di Giannantonio, M. Atypical Antipsychotic Drugs in Dual Disorders: Current Evidence for ClinicalPractice. Curr. Pharm. Des. 2022, 28, 2241–2259. [Google Scholar] [CrossRef]
- Gillies, D.; Beck, A.; McCloud, A.; Rathbone, J. Benzodiazepines for psychosis-induced aggression or agitation. In Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar] [CrossRef]
- Doyle, R.; Behan, C.; O’KEeffe, D.; Masterson, S.; Kinsella, A.; Kelly, A.; Sheridan, A.; Keating, D.; Hynes, C.; Madigan, K.; et al. Clozapine Use in a Cohort of First-Episode Psychosis. J. Clin. Psychopharmacol. 2017, 37, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, E.L.; Reckweg, J.T.; Hutten, N.R.P.W.; Kuypers, K.P.C.; Toennes, S.W.; Neukamm, M.A.; Halter, S.; Ramaekers, J.G. Psychotomimetic symptoms after a moderate dose of a synthetic cannabinoid (JWH-018): Implications for psychosis. Psychopharmacology 2022, 239, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, S.; Di Carlo, F.; Mosca, A.; D’ANdrea, G.; Di Paolo, M.; Lorenzini, C.; Lupica, M.G.; Sampogna, G.; Pettorruso, M.; Fiorillo, A.; et al. Efficacy of Psychosocial and Psychological Interventions in Addition to Drug Therapy to Improve Global Functioning of Inpatients with Schizophrenia Spectrum and Mood Disorders: A Real-World Observational Study. Neuropsychiatr. Dis. Treat. 2023, 19, 1887–1897. [Google Scholar] [CrossRef]
- Rocca, P.; Rucci, P.; Montemagni, C.; Rossi, A.; Bertolino, A.; Aguglia, E.; Altamura, C.A.; Amore, M.; Andriola, I.; Bellomo, A.; et al. Does social cognition change? Evidence after 4 years from the Italian Network for Research on Psychoses. Eur. Psychiatry 2023, 66, e10. [Google Scholar] [CrossRef]
- Addington, J.; Chaves, A.; Addington, D. Diagnostic stability over one year in first-episode psychosis. Schizophr. Res. 2006, 86, 71–75. [Google Scholar] [CrossRef]
- Murrie, B.; Lappin, J.; Large, M.; Sara, G. Transition of Substance-Induced, Brief, and Atypical Psychoses to Schizophrenia: A Systematic Review and Meta-analysis. Schizophr. Bull. 2020, 46, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Rognli, E.B.; Heiberg, I.H.; Jacobsen, B.K.; Høye, A.; Bramness, J.G. Transition From Substance-Induced Psychosis to Schizophrenia Spectrum Disorder or Bipolar Disorder. Am. J. Psychiatry 2023, 180, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Myran, D.T.; Harrison, L.D.; Pugliese, M.; Solmi, M.; Anderson, K.K.; Fiedorowicz, J.G.; Perlman, C.M.; Webber, C.; Finkelstein, Y.; Tanuseputro, P. Transition to Schizophrenia Spectrum Disorder Following Emergency Department Visits Due to Substance Use with and Without Psychosis. JAMA Psychiatry 2023, 80, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
| Name, Year | Study Design | Population (N, M, F) | Mean Age ± Standard Deviation | Administration | Psychiatric Symptoms | Treatment | Outcome | Recommendation for Clinicians |
|---|---|---|---|---|---|---|---|---|
| Abouchedid et al., 2016 [26] | Case report | F = 1 | 19 | Smoked | Unspecified visual hallucinations | Single dose of midazolam (1 mg IV) stopped seizures/agitation; no further medication needed. | Acute psychosis with remission | A low dose of IV benzodiazepine is often sufficient for SCRA-induced convulsions or severe agitation. |
| Altintas et al., 2016 [27] | Single-center cross-sectional analysis | M = 50 | MA = 25.9 ± 5.5 | NA | Suicidal ideation, suicide attempt | Standard antipsychotic treatment in an acute psychiatric ward (agents not specified) | Acute psychosis with remission | Manage SCRA psychosis as primary psychosis but expect an earlier age of onset and monitor suicidality closely. |
| Barceló et al., 2017 [28] | Case series | N = 5 M = 4 F = 1 | Case 1 = 17 Case 2 = 17 Case 3 = 17 Case 4 = 14 Case 5 = 21 | Smoked | Agitation, confusion, anxiety, suicide attempt, altered language, bradypsychia, delusions of influence and grandeur |
| Acute psychosis with remission | Most mild-to-moderate SCRA intoxications settle within hours; start with calm environment and IVFs for dehydration/tachycardia. Admit only if neuro-psychiatric symptoms persist or airway risk develops. |
| Bassir et al., 2016 [29] | Retrospective review | N = 594 M = 444 F = 150 | MA = 40.6 ± 12.9 | Smoked | Agitation, suicidal ideation, mood symptoms, thought disorganization, internal preoccupation | SC-only patients required higher antipsychotic doses and longer psychiatric admissions than cannabis users. Exact drugs not specified. | NA | In SCRA users with severe psychosis, start antipsychotics at the upper end of the dosing range. Plan follow-up for sustained abstinence and psychosocial support. |
| Bebarta et al., 2012 [30] | Case series | Case 1 = M Case 2 = 2 Case 3 = M | Case 1 = 19 Case 2 = 19 Case 3 = 23 | Smoked | Aggression, agitation, panic, sedation, paranoia, visual and somatic hallucinations | Three service members: IV lorazepam (2 mg) for severe agitation (Case 1); naloxone trial in sedated patient (Case 2, no effect); IV fluids, oxygen, overnight ward observation for all. | Acute psychosis with remission | Provide airway support, hydrate generously, and give benzodiazepines for agitation. |
| Berry-Cabàn et al., 2013 [31] | Case report | M = 1 | 20 | Smoked | Thought blocking, disorganized thinking and behaviors, paranoid delusion, referential delusion, loss of ego boundaries, verbal hallucinations | ED/ward: Repeated lorazepam (≥5 mg total) for severe agitation + restraints; diphenhydramine (25 mg) + haloperidol (5 mg) IM. Later, risperidone (1 mg/night) for residual psychosis. | Onset with persistence | Manage SCRA-induced delusions with rapid benzodiazepine sedation first. Add parenteral antipsychotic if psychosis persists. Anticipate prolonged cognitive blunting and arrange close supervision. |
| Besli et al., 2015 [32] | Case series | N = 16 M = 15 F = 1 | MA = 15.4 ± 1.7 | Smoked | Agitation, anxiety, panic attack, numbness, euphoria, sympathomimetic symptoms, perceptual changes | Pediatric ED management (n = 16): IV crystalloids, benzodiazepines PRN for agitation. In total, 25% required ICU monitoring for hypotension, brady-/tachycardia. Social-work referrals for all. | Acute psychosis with remission | Treat adolescent SCRA intoxication like any unknown toxidrome: stabilize airway/BP, give benzodiazepines for neuro-behavioral control, and admit to ICU if vitals are labile. Education and early addiction follow-up are critical. |
| Bonaccorso et al., 2018 [33] | Case series | Case 1 = M Case 2 = F Case 3 = M Case 4 = M | Case 1 = 28 Case 2 = 32 Case 3 = 20 Case 4 = 39 | Smoked | Agitation, verbal and physical aggression, sexual disinhibition, disorganization, bizarre behavior, delusional mood, persecutory and grandiose delusions, auditory hallucinations | Combination regimens: Olanzapine (up to 20 mg/day), aripiprazole (9.75 mg tds), haloperidol (10 mg/day), depot zuclopenthixol (300 mg/week), clonazepam (≤8 mg/day), lithium (800 mg), sodium valproate (1200 mg). | Acute psychosis with remission/psychotic relapse | Administer first-line BDZ for agitation, then add high-dose SGA (avoid QT-prolonging FGA where possible). Monitor vitals with NEWS ≥ TDS, and tighten observation/leave until urine screens are negative. |
| Celofiga et al., 2014 [34] | Case series | M = 4 | Case 1 = 35 Case 2 = 21 Case 3 = 27 Case 4 = 29 | Smoked | Agitation, mood changes, anxiety, elevated affect, chronic paranoid and grandiose delusions, bizarre behavior, formal thought symptoms, haptic hallucinations | Escalation of existing benzodiazepines: Diazepam (up to 10 mg TID); oral lorazepam (up to 2.5 mg TID or 2 mg IM) for agitation/anxiety.
| Acute psychosis with remission/psychotic relapse | In stable patients with psychotic disorders, acute SCRA intoxication is usually managed by temporarily increasing benzodiazepines while maintaining the standing antipsychotic. |
| Di Petta et al., 2016 [35] | Case report | M = 1 | 28 | Smoked | Agitation, suicide attempts, irrational behavior, magical delusions, mystical ideas, bizarre delusions of greatness and persecution, Capgras syndrome, Ekbom syndrome, twilight state of consciousness, visual or auditory hallucinations, illusions | Paliperidone palmitate LAI (150 mg monthly) plus phenomenological psychotherapy | Acute psychosis with remission/onset with persistence | In chronic SCRA users with persistent delusional disorder, a long-acting injectable antipsychotic can stabilize psychosis and improve adherence. Combine this treatment with structured psychotherapy for partial functional recovery. |
| Durand et al., 2015 [36] | Case report | M = 1 | 23 | NA | Agitation, persecutory/mystical delusions |
| Acute psychosis with remission | Haloperidol (or another potent antipsychotic) plus benzodiazepines can safely control prolonged psychosis/agitation. |
| Every-Palmer et al., 2011 [37] | Cohort study | M = 15 | MA = 34 ± 7.9 | Smoked | Agitation, disorganization, paranoia, an impulse to do evil things, a sense of the end of the world | No acute drugs given in study; all 15 forensic inpatients were already on maintenance antipsychotics. | Acute psychosis with remission/psychotic relapse | |
| El Zahran et al., 2019 [38] | Case report | M = 1 | 29 | Smoked | Agitation, visual hallucinations |
| Acute psychosis with remission | Give supportive care and a benzodiazepine for behavioral control. |
| Glue et al., 2013 [39] | Retrospective observational study | N = 17 M = 10 F = 7 | MA = 26.1 ± 10 | NA | Homicidal ideation, affective changes (anxious, depressive), intense suicidal thinking/behavior, paranoia, thought disorder, disorganized behavior | Seventeen admissions (13% of total): Supportive care; those with psychosis received antipsychotics (typical or atypical) and sometimes received antidepressants. | Acute psychosis with remission/psychotic relapse | Start antipsychotics promptly, monitor suicidality, and arrange community follow-up once abstinent. |
| Haro et al., 2014 [40] | Letter to editor/case report | F = 1 | 19 | NA | Laughter forfeit, derealization, depersonalization, movement disorder similar to catatonia, soliloquy with personal hygiene deterioration, self-references, visual hallucinations | Aripiprazole (15 mg/day) + lorazepam + biperiden after drug cessation | Acute psychosis with partial remission | If SCRA use is suspected in first-episode psychosis, start atypical antipsychotic plus high-dose benzodiazepine, add anticholinergic if extrapyramidal/catatonic features appear, and insist on sustained abstinence with psychoeducation. |
| Helge Müller et al., 2009 [41] | Case report | M = 1 | 25 | Smoked | Increased anxiety, delusions of influence | Psychotic relapse | ||
| Hermanns-Clausen et al., 2017 [42] | Prospective observational study | N = 44 M = 39 F = 5 | MA = 20.5 | Oral, smoked, sniffed | Restlessness/agitation, amnesia, anxiety, acute psychosis, self-mutilating behavior |
| Acute psychosis with remission | Treat SCRA intoxication like a toxic delusion: give IV benzodiazepines early and be ready to intubate or deeply sedate for status seizures. |
| Hoyte et al., 2012 [43] | Observational study | N = 1898 M = 1005 F = 893 | MA = 22.5 ± 8.86 | Smoked | Agitation, irritability, drowsiness, lethargy, confusion, dizziness, paranoia, unspecified delusions and hallucinations | IV crystalloids ≈ 25%. Benzodiazepines ≈ 16% (for agitation/seizures). In total, >70% required no drug therapy. | Acute psychosis with remission | Most presentations resolve with supportive ED care alone. Use benzodiazepine if the patient is agitated or seizing. |
| Hurst et al., 2011 [44] | Case series | M = 10 | MA= 23 | Smoked | Insomnia, psychomotor agitation, suicidal ideation, anxiety, flat affect, alogia, paranoid delusions, thought blocking, disorganized speeches and behavior, psychomotor retardation, auditory and visual hallucinations | Antipsychotics given to 7/10 patients (agents not specified; used for active psychosis). | Acute psychosis with remission/onset with persistence | Initiate standard antipsychotic treatment, and monitor because symptoms may persist for weeks or months after intoxication. |
| Kekelidze et al., 2019 [45] | Interventional study | N = 43 M = 38 F = 5 | MA = 25 | NA | Anxiety; disorientation; dream-like clouding of consciousness; catatonic disorders; catalepsy; profound impairments to consciousness; perceptual delusions; delusional experiences; disorganization; degraded self-awareness; multiple vivid and dynamic pareidolias; visual, tactile, and auditory hallucinations; daydream-like fantastic hallucination | Standard detoxification (IV fluids + B vitamins + nootropics) for all, plus one of the following:
| Acute psychosis with remission | Choose a neuroleptic by matching it with the psychosis type and the severity of the somato-neurological signs: haloperidol shortens the psychotic phase fastest, whereas tiapride gives quicker relief of autonomic/neurological complications. Always embed antipsychotics in an early, structured detoxification regime. |
| Malik et al., 2021 [46] | Case series | Case 1 = M Case 2 = F | Case 1 = 31 Case 2 = 36 | NA | Aggressivity, bizarre behavior, a delusional self-inflicted injury to the eye | Propofol bolus/infusion to achieve deep sedation for emergency globe-repair surgery. Antipsychotic pharmacotherapy initiated post-operatively (drug not specified). | Acute psychosis with remission/psychotic relapse | In agitated SCRA-induced psychosis with self-harm, use rapid-onset IV anesthetics (propofol or ketamine) to permit life- or organ-saving procedures. Then, transfer to psychiatry for titration of antipsychotics and suicide-risk management. |
| Monte et al., 2017 [47] | Cohort study | N = 353 M = 297 F = 56 | MA = 25 | NA | Agitation, unspecified delusion | First-line benzodiazepines used in 37% of cases.
| Acute psychosis with remission | Begin with benzodiazepines for agitation, seizures, or delusion. Add antipsychotics if psychosis persists. |
| Oluwabusi et al., 2012 [48] | Case series | M = 2 | Case 1 = 16 Case 2 = 17 | Smoked | Insomnia, low mood, hyperactivity, anxiety, apathy, paranoid delusions, grandiose delusions, somatic preoccupation, disorganized behavior, auditory and visual hallucinations | Case 1: Initial quetiapine, switched to aripiprazole (20 mg/day); relapse managed with olanzapine ODT titrated to 15 mg/day (symptoms cleared in 72 h). Case 2: Olanzapine (15 mg nightly); recurrence after non-adherence, which resolved again within days after restarting. | Acute psychosis with remission/psychotic relapse | In adolescents with first-episode psychosis linked to SCRAs, start an atypical antipsychotic (olanzapine or aripiprazole) and stress adherence. Screen for ongoing SCRA use and family vulnerability. Early medication plus abstinence usually restores the baseline within days. |
| Ozer et al., 2016 [49] | Case report | M = 1 | 17 | Smoked | Anxiety, agitation, irritability, confusion, insomnia, anorexia, dysphoric mood, suicidality with self-injury, Capgras syndrome, persecutory delusions | Olanzapine (10 mg/day); complete remission within 2 weeks. | Acute psychosis with remission | Atypical antipsychotics (e.g., olanzapine) are effective for SCRA-induced misidentification syndromes. |
| Peglow et al., 2012 [50] | Case report | M = 1 | 59 | Smoked | Traumatic flashbacks; disorganized, bizarre behavior; auditory and visual hallucinations | Observation only, continuing the patient’s usual outpatient regimen (aripiprazole (10 mg), gabapentin, etc.). No additional antipsychotics were required, and the symptoms cleared within 24 h each time. | Acute psychosis with remission | Rule out other drugs, and observe closely. Symptoms may remit rapidly once SCRA use stops. |
| Rahmani et al., 2013 [51] | Case series | M = 2 | Case 1 = 17 Case 2 = 17 | Smoked | Insomnia, irritability, mild agitation, delusion of influence and possession, mystical delusions, a sense of the end of the world, Capgras syndrome, bizarre and disorganized behaviors, auditory and visual hallucinations | Cases 1 and 2:
| Acute psychosis with remission/onset with persistence | If SCRAs precipitate a prolonged, antipsychotic-resistant psychosis, consider low-dose clozapine earlier than usual. A therapeutic response may occur at lower doses than in primary schizophrenia. |
| Roberto et al., 2016 [52] | Case report | M = 1 | 18 | Smoked | Confusion, amnesia, agitation, insomnia, catatonia, elevated mood, mutism, avolition, thought disorganization, paranoid delusions, persecution ideation, auditory hallucinations |
| Onset with persistence | Start a benzodiazepine promptly when catatonic features are present. Then, introduce a second-generation antipsychotic (e.g., risperidone) and monitor EPSs. The antipsychotic that worked during the index episode will usually work again after relapse if the patient resumes using SCRAs. |
| Satodiya et al., 2020 [53] | Case report | M = 1 | 32 | Smoked | Monotone speech, minimal gestures, social withdrawal, lack of spontaneity, blunted affect, avolition | Optimization of second-generation antipsychotic therapy (details not stated) | Psychotic relapse | Re-emergence or a switch to severe negative symptoms after chronic SCRA use warrants reassessment of the antipsychotic dose/choice, stimulant avoidance, and targeted psychosocial rehabilitation. |
| Simmons et al., 2011 [54] | Case series | M = 3 | Case 1 = 25 Case 2 = 21 Case 3 = 19 | Smoked | Agitation, amnesia, bizarre behavior, paranoia, unspecified delusions |
| Acute psychosis with remission | Treat agitation first with benzodiazepines. Secure airway if hypoventilating. Use haloperidol only once vital signs are stable. Most patients recover within 12–24 h. |
| Skryabin et al., 2019 [55] | Observational study | M = 60 | MA = 23.6 ± 3.5 | NA | Catatonia, anxiety, motor agitation, Kandinsky–Clerambault syndrome, delusions of influence, automatisms, telepathy, thought broadcasting and insertion, delusional ideas of interpretation, persecutory delusions, delusional ideas, cenesthopathic automatisms, tactile hallucinations, pseudo-hallucinations, acute verbal hallucinations with threatening monologues or dialogues | High-dose antipsychotics and prolonged inpatient/ICU care were frequently required. | Acute psychosis with remission | SCRA users in the referenced 60-patient cohort needed higher doses and longer hospitalizations than cannabis users. |
| Skryabin et al., 2018 [56] | Longitudinal, observational cohort study | M = 46 | MA = 23.2 ± 3.5 | NA | Psychomotor agitation, anxious–depressive symptoms, mild hypomania, negative symptoms of schizophrenia, Kandinsky–Clerambault syndrome, persecutory delusions, paranoia, auditory and visual hallucinations | Neuroleptics were introduced on day 1 with detox measures. Choice (haloperidol vs. tiapride) was tailored to clinical variant. Benzodiazepines were used for psychomotor agitation (doses not specified). | Acute psychosis with remission/onset with persistence/psychotic relapse | Begin antipsychotic treatment immediately in SCRA-related psychosis, matching the drug class with the delirious/oneiroid/amentive pattern and autonomic burden. Integrate close follow-up because ≈17% of patients later show schizophrenic-process manifestation, making long-term psychiatric supervision essential. |
| Sönmez et al., 2016 [57] | Case report | M = 1 | 31 | Smoked | Agitation, distressed mood, insomnia, ideation related to harming self and others, irritation, bursts of anger, delusions of persecution and reference, shape and content of thought altered | Inpatient olanzapine (20 mg/day × 10 days) led to complete resolution. The patient was discharged on the same dose and received cognitive-behavioral psychotherapy. | Acute psychosis with remission | Admit SCRA psychosis early, administer an adequate dose of a second-generation antipsychotic (olanzapine worked within a week), and schedule structured CBT to consolidate abstinence and reality testing. |
| Sweet et al., 2017 [58] | Case report | M = 1 | 47 | Smoked | Psychomotor agitation, paranoia |
| Acute psychosis with remission | In ED/acute-ward settings, treat SCRA-related agitation the same day: give an atypical IM antipsychotic (or haloperidol + lorazepam if unavailable), repeat q 30–60 min until calm, correct electrolytes, and watch for at least 6 h (symptoms may last up to 7 h). |
| Tung et al., 2012 [59] | Case report | M = 1 | 36 | Smoked | Agitation, insomnia, dysphoric mood, persecutory delusion, disorganized thoughts and behavior, irrelevant speech, bizarre behavior, auditory hallucination | IM midazolam for rapid tranquillization + physical restraints on arrival. No antipsychotic started. Full resolution after 3 days of drug-free observation. | Acute psychosis with remission | A single benzodiazepine dose may suffice; if symptoms settle, avoid unnecessary antipsychotics and focus on substance-use assessment and education. |
| Udow et al., 2018 [60] | Case report | F = 1 | 70 | Oral | Anxiety, persecutory delusions, bizarre visual hallucinations |
| Acute psychosis with remission/onset with persistence | Older PD patients are highly vulnerable to SCRA-induced psychosis. First withdraw the offending drug and rationalize dopaminergic therapy. Use very-low-dose clozapine (with fludrocortisone or midodrine if needed) rather than dopamine-blocking antipsychotics. |
| Therapeutic Domain | Evidence Base | Typical Dose Range Reported | Key Findings |
|---|---|---|---|
| Benzodiazepines (BZDs) | 27/35 manuscripts, >700 pts | IV/IM lorazepam (2–6 mg), diazepam (10 mg TID), midazolam (1 mg) | Universal first-line agent for agitation, convulsions, or catatonia. As a monotherapy, it achieved full clinical resolution of mild-to-moderate intoxications within 6–24 h. |
| Typical antipsychotics | 10/35 manuscripts (mainly from Eastern Europe) | Haloperidol (5–30 mg/day), IM chlorpromazine | Effective for florid psychosis but required high doses and close QT/EP symptom monitoring. |
| Second-generation antipsychotics (SGAs) | 22/35 manuscripts | Olanzapine (10–20 mg/day), risperidone (2–6 mg/day), aripiprazole (10–20 mg/day) | Favored in Western cohorts; usually started after BZD. Time to remission: 24–72 h. Adherence problems prompted two reports of LAI paliperidone. |
| Clozapine | 3 resistant cases | 50–150 mg/day (adult), 12.5–50 mg/day (older PD patient) | Robust improvement where ≥2 other antipsychotics failed. Effective at lower doses than in primary schizophrenia. |
| Anesthetic agents | 2 case series/reports | Propofol bolus/infusion | Enabled surgical airway or globe-repair procedures after extreme agitation or self-injury. |
| Detox/supportive care | Pediatric and ED cohorts | IV crystalloids, oxygen, B vitamins | In total, 70% of 1898 ED attendees required no psychotropics once hydrated and observed in a low-stimulus setting. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, A.; Chiappini, S.; Miuli, A.; Cavallotto, C.; Pettorruso, M.; Martinotti, G.; Schifano, F. Clinical Management of Synthetic-Cannabinoid-Induced Psychosis: A Systematic Review of Treatment Strategies and Outcomes. Brain Sci. 2025, 15, 1006. https://doi.org/10.3390/brainsci15091006
Mosca A, Chiappini S, Miuli A, Cavallotto C, Pettorruso M, Martinotti G, Schifano F. Clinical Management of Synthetic-Cannabinoid-Induced Psychosis: A Systematic Review of Treatment Strategies and Outcomes. Brain Sciences. 2025; 15(9):1006. https://doi.org/10.3390/brainsci15091006
Chicago/Turabian StyleMosca, Alessio, Stefania Chiappini, Andrea Miuli, Clara Cavallotto, Mauro Pettorruso, Giovanni Martinotti, and Fabrizio Schifano. 2025. "Clinical Management of Synthetic-Cannabinoid-Induced Psychosis: A Systematic Review of Treatment Strategies and Outcomes" Brain Sciences 15, no. 9: 1006. https://doi.org/10.3390/brainsci15091006
APA StyleMosca, A., Chiappini, S., Miuli, A., Cavallotto, C., Pettorruso, M., Martinotti, G., & Schifano, F. (2025). Clinical Management of Synthetic-Cannabinoid-Induced Psychosis: A Systematic Review of Treatment Strategies and Outcomes. Brain Sciences, 15(9), 1006. https://doi.org/10.3390/brainsci15091006









