Spinal Cord Injury in Real Time: Intra-Operative Ultrasound for Acute Phase Examination in Non-Human Primates
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
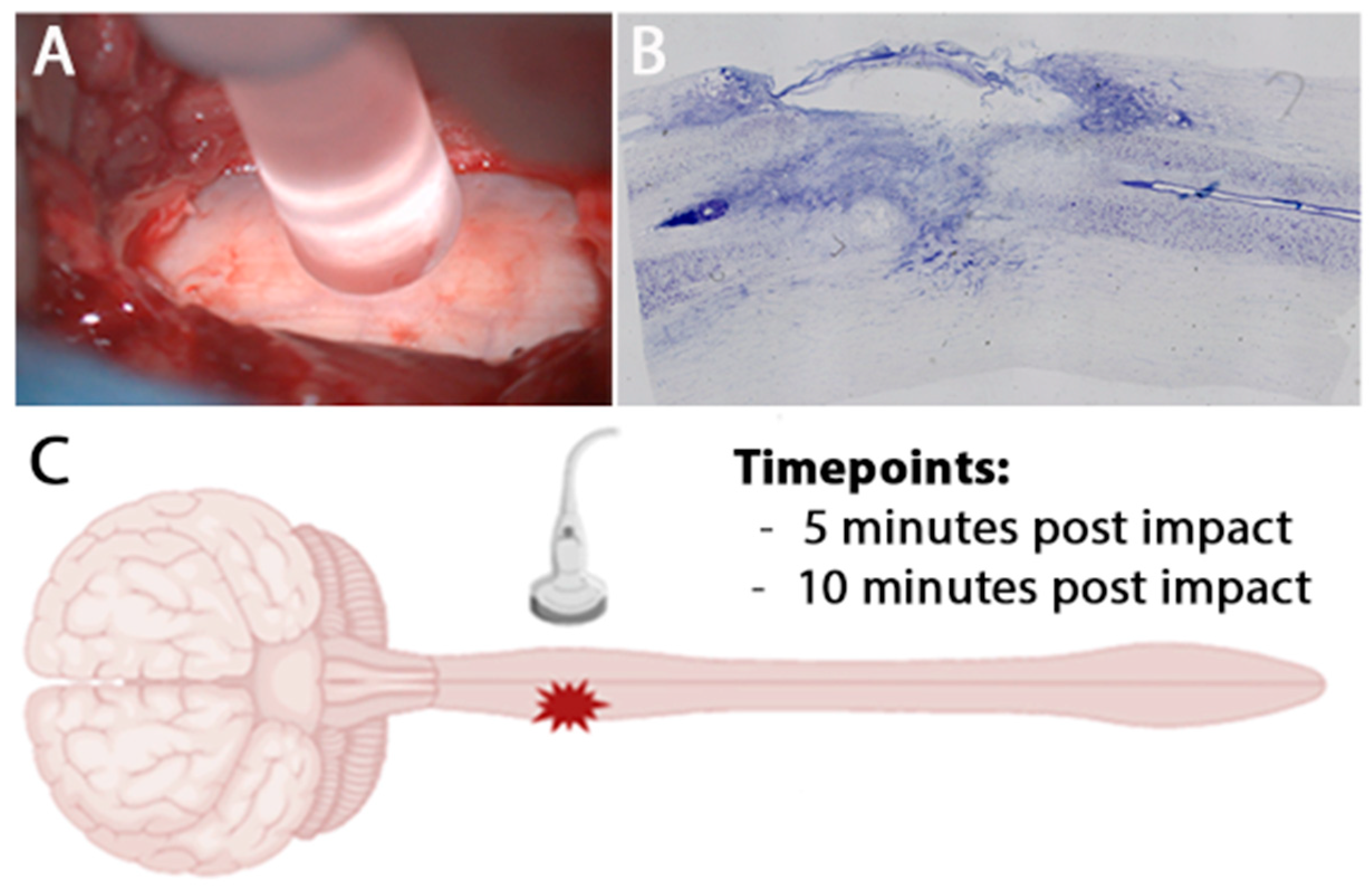
2.2. Contusion Lesion
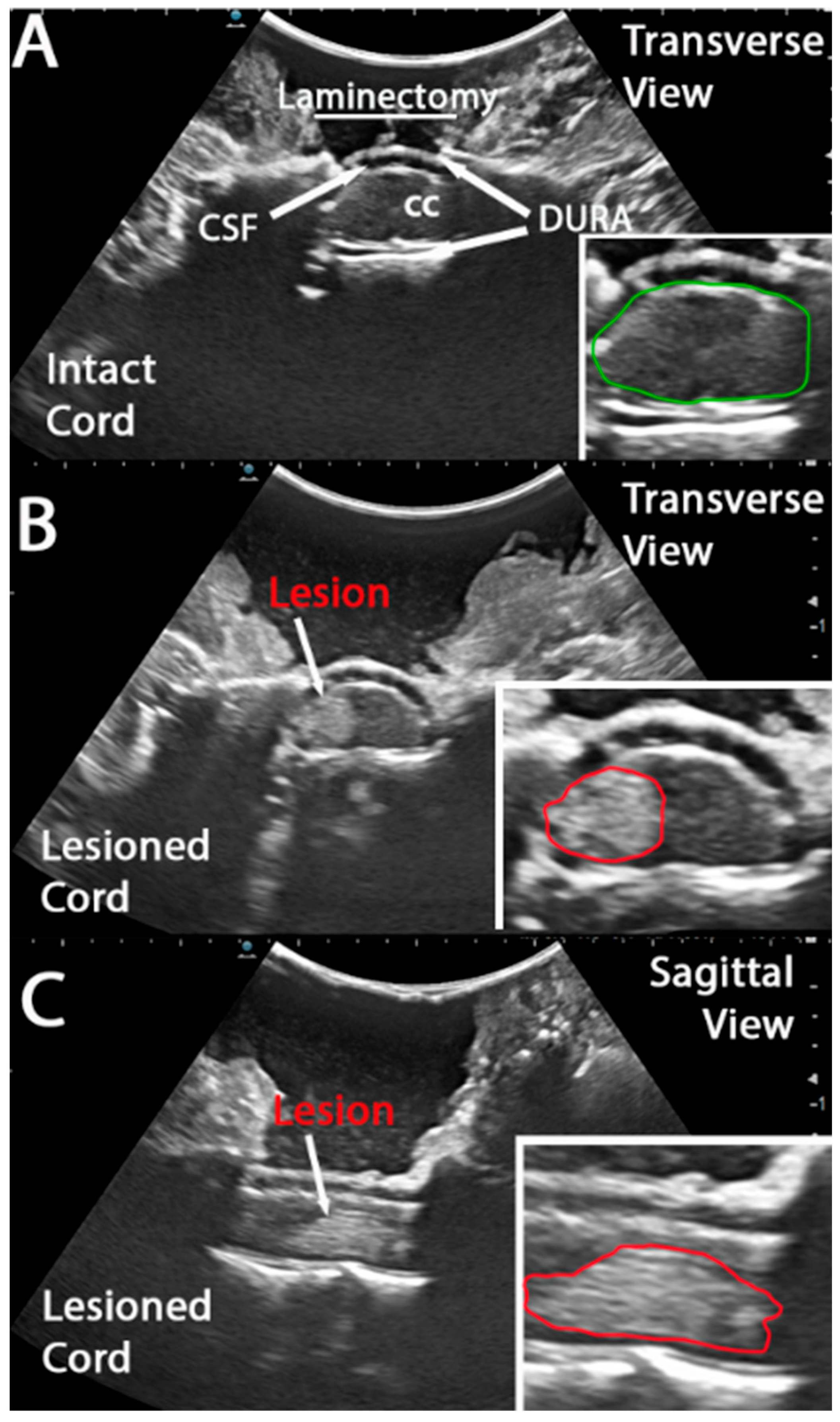
2.3. Intraoperative Ultrasound Image Acquisition and Analysis
2.4. Magnetic Resonance Imaging Acquisition and Analysis
2.5. Data Analysis
3. Results
3.1. Intra Operative Ultrasound to Visualize a Hyper Acute Timepoint in a Hemicontusion
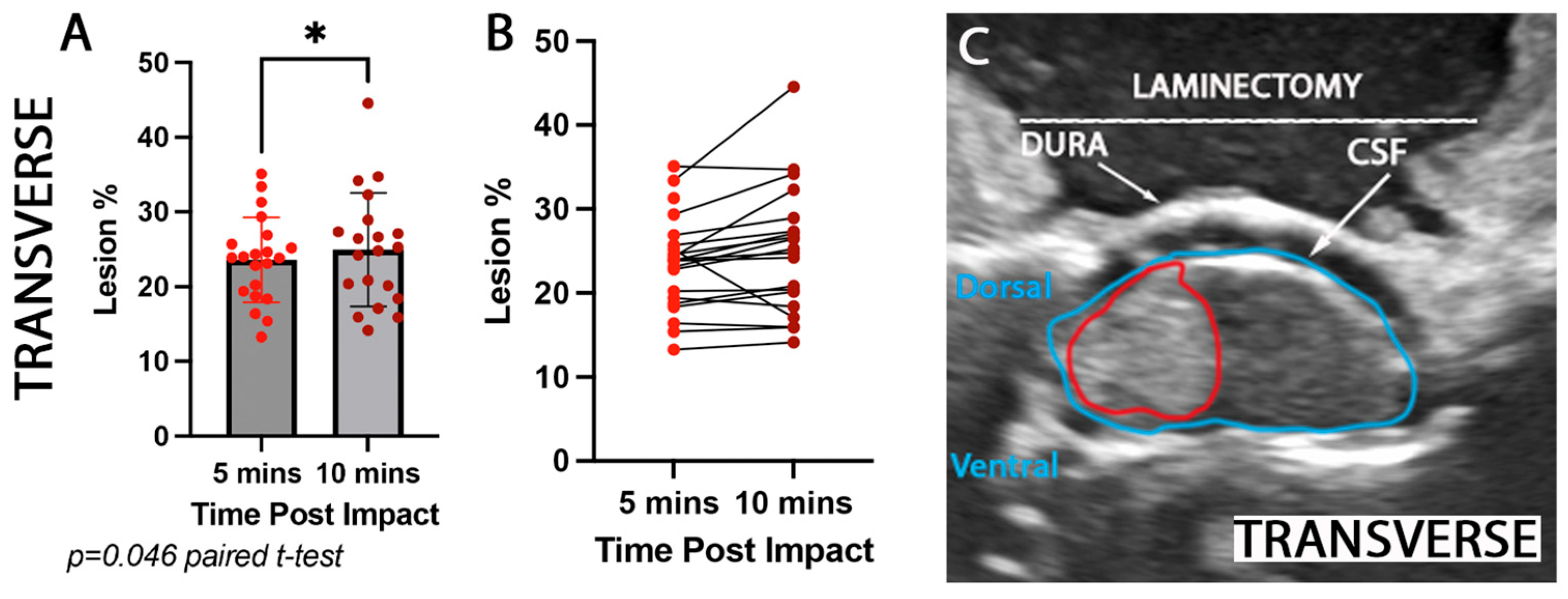
3.2. Rostrocaudal and Mediolateral Lesion Extent Increases Significantly Hyper Acutely Post Impact
3.3. Mediolateral Lesion Extent Increases Significantly at 3 Weeks Post Impact
4. Discussion
4.1. Strengths/Limitations
4.2. Comparison with Other Contusion Injury Models Using US to Characterize Lesions or Preinjury Morphology
4.3. Accessibility of IOU vs. MRI and Its Potential Utility in Preclinical Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IOU | Intraoperative ultrasound |
| NHP | Non-human primate |
| SCI | Spinal cord injury |
References
- Hussain, O.; Kaushal, M.; Agarwal, N.; Kurpad, S.; Shabani, S. The Role of Magnetic Resonance Imaging and Computed Tomography in Spinal Cord Injury. Life 2023, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Malomo, T.A.; Brown, A.A.; Bale, K.; Yung, A.; Kozlowski, P.; Heran, M.K.; Streijger, F.; Kwon, B.K. Quantifying Intraparenchymal Hemorrhage after Traumatic Spinal Cord Injury: A Review of Methodology. J. Neurotrauma 2022, 39, 1603–1635. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, J.-H.; Lee, C.-H.; Kim, S.; Kim, Y.-R.; Kim, K.-T.; Kim, J.-H.; Rhee, J.M.; Jo, W.-Y.; Oh, H.; et al. The utility of intraoperative ultrasonography for spinal cord surgery. PLoS ONE 2024, 19, e0305694. [Google Scholar] [CrossRef] [PubMed]
- Lubner, M.G.; Gettle, L.M.; Kim, D.H.; Ziemlewicz, T.J.; Dahiya, N.; Pickhardt, P. Diagnostic and procedural intraoperative ultrasound: Technique, tips and tricks for optimizing results. Br. J. Radiol. 2021, 94, 20201406. [Google Scholar] [CrossRef] [PubMed]
- Regelsberger, J.; Fritzsche, E.; Langer, N.; Westphal, M. Intraoperative sonography of intra- and extramedullary tumors. Ultrasound Med. Biol. 2005, 31, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, V.S.; Abd-El-Barr, M.; Pompeu, Y.A.; Karhade, A.; Groff, M.W.; Lu, Y. Use of Intraoperative Ultrasound During Spinal Surgery. Glob. Spine J. 2017, 7, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Routkevitch, D.; Soulé, Z.; Kats, N.; Baca, E.; Hersh, A.M.; Kempski-Leadingham, K.M.; Menta, A.K.; Bhimreddy, M.; Jiang, K.; Davidar, A.D.; et al. Non-contrast ultrasound image analysis for spatial and temporal distribution of blood flow after spinal cord injury. Sci. Rep. 2024, 14, 714. [Google Scholar] [CrossRef] [PubMed]
- Salegio, E.A.; Bresnahan, J.C.; Sparrey, C.J.; Camisa, W.; Fischer, J.; Leasure, J.; Buckley, J.; Nout-Lomas, Y.S.; Rosenzweig, E.S.; Moseanko, R.; et al. A Unilateral Cervical Spinal Cord Contusion Injury Model in Non-Human Primates (Macaca mulatta). J. Neurotrauma 2016, 33, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Yari, D.; Saberi, A.; Salmasi, Z.; Ghoreishi, S.A.; Etemad, L.; Movaffagh, J.; Ganjeifar, B. Recent Advances in the Treatment of Spinal Cord Injury. Arch. Bone Jt. Surg. 2024, 12, 380–399. [Google Scholar] [PubMed]
- Kim, M.; Hong, S.K.; Jeon, S.R.; Roh, S.W.; Lee, S. Early (≤48 H) versus Late (>48 H) Surgery in Spinal Cord Injury: Treatment Outcomes and Risk Factors for Spinal Cord Injury. World Neurosurg. 2018, 118, e513–e525. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Yue, J.K.; Ngwenya, L.B.; Winkler, E.A.; Talbott, J.F.; Pan, J.Z.; Ferguson, A.R.; Beattie, M.S.; Bresnahan, J.C.; Haefeli, J.; et al. Ultra-Early (<12 Hours) Surgery Correlates with Higher Rate of American Spinal Injury Association Impairment Scale Conversion After Cervical Spinal Cord Injury. Neurosurgery 2019, 85, 199–203. [Google Scholar] [PubMed]
- Nout, Y.S.; Mihai, G.; Tovar, C.A.; Schmalbrock, P.; Bresnahan, J.C.; Beattie, M.S. Hypertonic saline attenuates cord swelling and edema in experimental spinal cord injury: A study utilizing magnetic resonance imaging. Crit. Care Med. 2009, 37, 2160–2166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quarato, C.M.I.; Lacedonia, D.; Salvemini, M.; Tuccari, G.; Mastrodonato, G.; Villani, R.; Fiore, L.A.; Scioscia, G.; Mirijello, A.; Saponara, A.; et al. A Review on Biological Effects of Ultrasounds: Key Messages for Clinicians. Diagnostics 2023, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Streijger, F.; Hill, C.E.; Anderson, A.J.; Bacon, M.; Beattie, M.S.; Blesch, A.; Bradbury, E.J.; Brown, A.; Bresnahan, J.C.; et al. Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp. Neurol. 2015, 269, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.J.; Benavides, F.D.; Padgett, K.R.; Guada, L.G.; Nunez-Gomez, Y.; Solano, J.P.; Guest, J.D. Dichotomous locomotor recoveries are predicted by acute changes in segmental blood flow after thoracic spinal contusion injuries in pigs. J. Neurotrauma 2019, 36, 1399–1415. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, T.; Zhang, Z.; Xiao, X.; Chen, L.; Wei, G.; Wang, Y.; Yang, K.; Jin, H.; Zhu, Y. Standalone ultrasound-based highly visualized volumetric spine imaging for surgical navigation. Sci. Rep. 2025, 15, 4922. [Google Scholar] [CrossRef] [PubMed]


| Subject | Age | Impactor Head Size | Max Cord Displacement (mm) | Impactor Head Placement Beyond Cord Midline | Impact Force | MRI |
|---|---|---|---|---|---|---|
| 1 | 15 yrs | 5 mm | 4.01 | 1 mm | 13.7 N | YES |
| 2 | 8 yrs | 5 mm | 3.73 | 1 mm | 27.02 N | YES |
| 3 | 11 yrs | 5 mm | 3.97 | 1 mm | 19.9 N | YES |
| 4 | 7 yrs | 5 mm | 3.75 | 1 mm | 29 N | YES |
| 5 | 11 yrs | 5 mm | 3.70 | 1 mm | 26.5 N | YES |
| 6 | 6 yrs | 4 mm | 3.86 | 0.7 mm | 26.9 N | YES |
| 7 | 9 yrs | 5 mm | 3.67 | 1 mm | 23.4 N | YES |
| 8 | 12.9 yrs | 5 mm | 3.92 | 1 mm | 9.9 N | NO |
| 9 | 8 yrs | 5 mm | 3.64 | 1 mm | 24.5 N | NO |
| 10 | 6 yrs | 5 mm | 3.99 | 1 mm | 5.6 N | NO |
| 11 | 7.5 yrs | 5 mm | 3.96 | 1 mm | 20.8 N | YES |
| 12 | 9 yrs | 5 mm | 3.70 | 0.7 mm | 18.7 N | NO |
| 13 | 6.5 yrs | 5 mm | 4.04 | 1.1 mm | 13.4 N | YES |
| 14 | 8 yrs | 5 mm | 3.89 | 0.5 mm | 17.6 N | NO |
| 15 | 8 yrs | 5 mm | 3.74 | 1 mm | 14.4 N | YES |
| 16 | 7 yrs | 5 mm | 3.75 | 1 mm | 23.2 N | NO |
| 17 | 8 yrs | 5 mm | 3.99 | 1 mm | 23.1 N | YES |
| 18 | 7.5 yrs | 5 mm | 3.96 | 0.5 mm | 10.3 N | NO |
| 19 | 8 yrs | 4 mm | 4.11 | 0.55 mm | 15.3 N | NO |
| 20 | 7.5 yrs | 5 mm | 4.01 | 1 mm | 12.1 N | YES |
| 21 | 8 yrs | 5 mm | 3.85 | 1 mm | 24.3 N | NO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinopoulou, E.; Chow, M.W.; Obaid, N.; Chong, E.; Nout-Lomas, Y.S.; Wurr, R.; Macon, R.; Huie, J.R.; Ferguson, A.R.; Tuszynski, M.H.; et al. Spinal Cord Injury in Real Time: Intra-Operative Ultrasound for Acute Phase Examination in Non-Human Primates. Brain Sci. 2025, 15, 1005. https://doi.org/10.3390/brainsci15091005
Sinopoulou E, Chow MW, Obaid N, Chong E, Nout-Lomas YS, Wurr R, Macon R, Huie JR, Ferguson AR, Tuszynski MH, et al. Spinal Cord Injury in Real Time: Intra-Operative Ultrasound for Acute Phase Examination in Non-Human Primates. Brain Sciences. 2025; 15(9):1005. https://doi.org/10.3390/brainsci15091005
Chicago/Turabian StyleSinopoulou, Eleni, Michelle W. Chow, Numaira Obaid, Emily Chong, Yvette S. Nout-Lomas, Rachele Wurr, Ryan Macon, J. Russell Huie, Adam R. Ferguson, Mark H. Tuszynski, and et al. 2025. "Spinal Cord Injury in Real Time: Intra-Operative Ultrasound for Acute Phase Examination in Non-Human Primates" Brain Sciences 15, no. 9: 1005. https://doi.org/10.3390/brainsci15091005
APA StyleSinopoulou, E., Chow, M. W., Obaid, N., Chong, E., Nout-Lomas, Y. S., Wurr, R., Macon, R., Huie, J. R., Ferguson, A. R., Tuszynski, M. H., Beattie, M. S., Bresnahan, J. C., & Sparrey, C. J. (2025). Spinal Cord Injury in Real Time: Intra-Operative Ultrasound for Acute Phase Examination in Non-Human Primates. Brain Sciences, 15(9), 1005. https://doi.org/10.3390/brainsci15091005






