Predicting State Anxiety Level Change Using EEG Parameters: A Pilot Study in Two Museum Settings
Abstract
1. Introduction
1.1. The Healing Gaze: Art Fruition, Stress Relief, and the Brain
1.2. Healing Spaces: Psychological and Neural Effects of Museum Collections
1.3. Electrophysiological Markers of Anxiety
1.4. The Present Study
2. Materials and Methods
2.1. Participants
- -
- Being 18 years old or older.
- -
- Accepting the conditions for participation and having signed the informed written consent.
- -
- Insufficient language skills in understanding verbal assignments and interacting with presenters and peers.
- -
- Current diagnosis of a disease of psychiatric or neurological nature.
- -
- Uncorrected visual or hearing impairment.
- -
- Another medical condition that could negatively affect the activities to be performed.
2.2. Procedure
2.2.1. Procedure Phase 1: Welcoming Procedures
2.2.2. Procedure Phase 2: Pre-Visit Assessment
2.2.3. Procedure Phase 3: The Visit
2.2.4. Procedure Phase 4: Debate
2.2.5. Procedure Phase 5: Post-Treatment Assessment
2.2.6. Procedure Phase 6: Debriefing
2.3. Instruments
2.3.1. Recruitment Questionnaire
2.3.2. Pre- and Post-Experience Assessment Questionnaires
3. Results
3.1. Descriptive Statistics
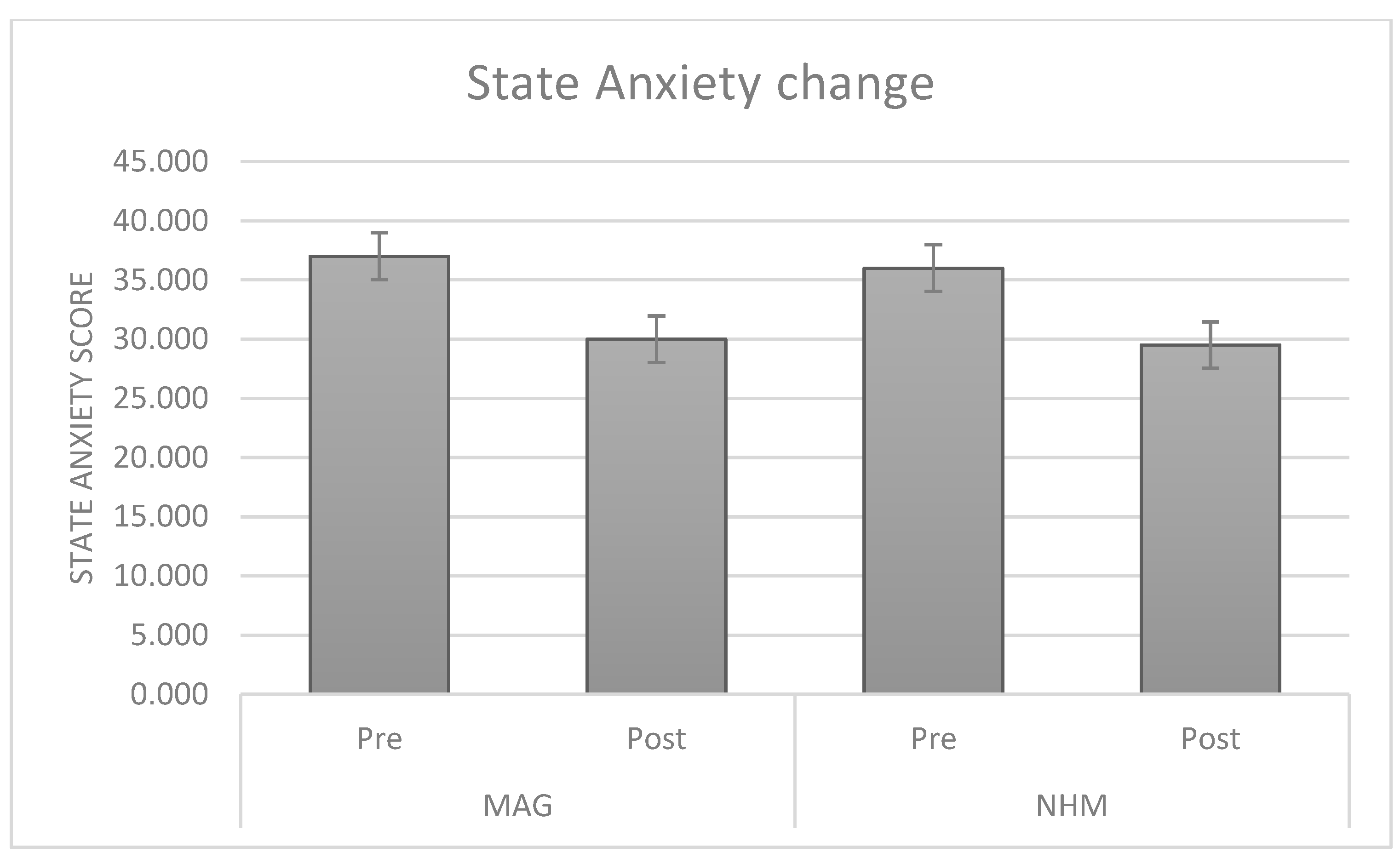
3.2. Changes in Anxiety Levels After the Visit
3.3. BCI and Psychological Measures Pre and Post Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, N.R.; Clark, D.P.A. The Observing Facet of Trait Mindfulness Predicts Frequency of Aesthetic Experiences Evoked by the Arts. Mindfulness 2016, 7, 971–978. [Google Scholar] [CrossRef]
- Mastandrea, S.; Fagioli, S.; Biasi, V. Art and Psychological Well-Being: Linking the Brain to the Aesthetic Emotion. Front. Psychol. 2019, 10, 739. [Google Scholar] [CrossRef]
- Leckey, J. The Therapeutic Effectiveness of Creative Activities on Mental Well-being: A Systematic Review of the Literature. J. Psychiatr. Ment. Health Nurs. 2011, 18, 501–509. [Google Scholar] [CrossRef]
- Lucchiari, C.; Vanutelli, M.E.; Ferrara, V.; Folgieri, R.; Banzi, A. Promoting Well-Being in the Museum: The ASBA Project Research Protocol. Int. J. Health Wellness Soc. 2024, 14, 73–88. [Google Scholar] [CrossRef]
- Mastandrea, S.; Maricchiolo, F.; Carrus, G.; Giovannelli, I.; Giuliani, V.; Berardi, D. Visits to Figurative Art Museums May Lower Blood Pressure and Stress. Arts Health 2019, 11, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, H.L.; Nobel, J. The Connection between Art, Healing, and Public Health: A Review of Current Literature. Am. J. Public Health 2010, 100, 254–263. [Google Scholar] [CrossRef]
- Chatterjee, H.J.; Noble, G. Museums, Health and Well-Being; Routledge: New York, NY, USA, 2016; ISBN 1317092716. [Google Scholar]
- Thomson, L.J.; Lockyer, B.; Camic, P.M.; Chatterjee, H.J. Effects of a Museum-Based Social Prescription Intervention on Quantitative Measures of Psychological Wellbeing in Older Adults. Perspect. Public Health 2018, 138, 28–38. [Google Scholar] [CrossRef]
- Ander, E.E.; Thomson, L.J.M.; Blair, K.; Noble, G.; Menon, U.; Lanceley, A.; Chatterjee, H.J. Using Museum Objects to Improve Wellbeing in Mental Health Service Users and Neurological Rehabilitation Clients. Br. J. Occup. Ther. 2013, 76, 208–216. [Google Scholar] [CrossRef]
- Camic, P.M.; Chatterjee, H.J. Museums and Art Galleries as Partners for Public Health Interventions. Perspect. Public Health 2013, 133, 66–71. [Google Scholar] [CrossRef]
- Chiang, K.-J.; Chu, H.; Chang, H.-J.; Chung, M.-H.; Chen, C.-H.; Chiou, H.-Y.; Chou, K.-R. The Effects of Reminiscence Therapy on Psychological Well-Being, Depression, and Loneliness among the Institutionalized Aged. Int. J. Geriatr. Psychiatry 2010, 25, 380–388. [Google Scholar] [CrossRef]
- Eekelaar, C.; Camic, P.M.; Springham, N. Art Galleries, Episodic Memory and Verbal Fluency in Dementia: An Exploratory Study. Psychol. Aesthetics, Creat. Arts 2012, 6, 262–272. [Google Scholar] [CrossRef]
- Bolwerk, A.; Mack-Andrick, J.; Lang, F.R.; Dörfler, A.; Maihöfner, C. How Art Changes Your Brain: Differential Effects of Visual Art Production and Cognitive Art Evaluation on Functional Brain Connectivity. PLoS ONE 2014, 9, e101035. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Zeki, S. Neural Correlates of Beauty. J. Neurophysiol. 2004, 91, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Lacey, S.; Hagtvedt, H.; Patrick, V.M.; Anderson, A.; Stilla, R.; Deshpande, G.; Hu, X.; Sato, J.R.; Reddy, S.; Sathian, K. Art for Reward’s Sake: Visual Art Recruits the Ventral Striatum. Neuroimage 2011, 55, 420–433. [Google Scholar] [CrossRef]
- Nadal, M.; Munar, E.; Angel Capo, M.; Rossello, J.; Cela-Conde, C.J. Towards a Framework for the Study of the Neural Correlates of Aesthetic Preference. Spat. Vis. 2008, 21, 379–396. [Google Scholar] [CrossRef]
- Banzi, A. The Brain-Friendly Museum: Using Psychology and Neuroscience to Improve the Visitor Experience; Taylor & Francis: New York, NY, USA, 2022; ISBN 1000684164. [Google Scholar]
- Chatterjee, H.J.; Camic, P.M. The Health and Well-Being Potential of Museums and Art Galleries. Arts Health 2015, 7, 183–186. [Google Scholar] [CrossRef]
- Thomson, L.J.; Chatterjee, H.J. Measuring the Impact of Museum Activities on Well-Being: Developing the Museum Well-Being Measures Toolkit. Mus. Manag. Curatorsh. 2015, 30, 44–62. [Google Scholar] [CrossRef]
- Goodman-Casanova, J.M.; Guzman-Parra, J.; Duran-Jimenez, F.J.; Garcia-Gallardo, M.; Cuesta-Lozano, D.; Mayoral-Cleries, F. Effectiveness of Museum-Based Participatory Arts in Mental Health Recovery. Int. J. Ment. Health Nurs. 2023, 32, 1416–1428. [Google Scholar] [CrossRef]
- Todd, C.; Camic, P.M.; Lockyer, B.; Thomson, L.J.M.; Chatterjee, H.J. Museum-Based Programs for Socially Isolated Older Adults: Understanding What Works. Health Place 2017, 48, 47–55. [Google Scholar] [CrossRef]
- Binnie, J. Does Viewing Art in the Museum Reduce Anxiety and Improve Wellbeing? Mus. Soc. Issues 2010, 5, 191–201. [Google Scholar] [CrossRef]
- Brown, K.; Mairesse, F. The Definition of the Museum through Its Social Role. Curator Mus. J. 2018, 61, 525–539. [Google Scholar] [CrossRef]
- Kaplan, S.; Bardwell, L.V.; Slakter, D.B. The Museum as a Restorative Environment. Environ. Behav. 1993, 25, 725–742. [Google Scholar] [CrossRef]
- Konlaan, B.B.; Bygren, L.O.; Johansson, S.-E. Visiting the Cinema, Concerts, Museums or Art Exhibitions as Determinant of Survival: A Swedish Fourteen-Year Cohort Follow-Up. Scand. J. Public Health 2000, 28, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Lackoi, K.; Patsou, M.; Chatterjee, H.J. Museums for Health and Wellbeing: A Preliminary Report, National Alliance for Museums, Health and Wellbeing. Available online: https://museumsandwellbeingalliance.wordpress.com (accessed on 8 April 2025).
- McSweeney, K.; Kavanagh, J. Museum Participation: New Directions for Audience Collaboration; MuseumsEtc: Edinburgh, UK, 2016; ISBN 1910144789. [Google Scholar]
- Gerger, G.; Leder, H. Titles Change the Esthetic Appreciations of Paintings. Front. Hum. Neurosci. 2015, 9, 464. [Google Scholar] [CrossRef] [PubMed]
- Putman, P.; Verkuil, B.; Arias-Garcia, E.; Pantazi, I.; van Schie, C. EEG Theta/Beta Ratio as a Potential Biomarker for Attentional Control and Resilience against Deleterious Effects of Stress on Attention. Cogn. Affect. Behav. Neurosci. 2014, 14, 782–791. [Google Scholar] [CrossRef]
- Angelidis, A.; van der Does, W.; Schakel, L.; Putman, P. Frontal EEG Theta/Beta Ratio as an Electrophysiological Marker for Attentional Control and Its Test-Retest Reliability. Biol. Psychol. 2016, 121, 49–52. [Google Scholar] [CrossRef]
- Morillas-Romero, A.; Tortella-Feliu, M.; Bornas, X.; Putman, P. Spontaneous EEG Theta/Beta Ratio and Delta–Beta Coupling in Relation to Attentional Network Functioning and Self-Reported Attentional Control. Cogn. Affect. Behav. Neurosci. 2015, 15, 598–606. [Google Scholar] [CrossRef]
- Putman, P.; van Peer, J.; Maimari, I.; van der Werff, S. EEG Theta/Beta Ratio in Relation to Fear-Modulated Response-Inhibition, Attentional Control, and Affective Traits. Biol. Psychol. 2010, 83, 73–78. [Google Scholar] [CrossRef]
- Sultanov, M.; İsmailova, K. EEG Rhythms in Prefrontal Cortex as Predictors of Anxiety among Youth Soccer Players. Transl. Sport. Med. 2019, 2, 203–208. [Google Scholar] [CrossRef]
- Cruz-Garza, J.G.; Brantley, J.A.; Nakagome, S.; Kontson, K.; Megjhani, M.; Robleto, D.; Contreras-Vidal, J.L. Deployment of Mobile EEG Technology in an Art Museum Setting: Evaluation of Signal Quality and Usability. Front. Hum. Neurosci. 2017, 11, 527. [Google Scholar] [CrossRef]
- Abdelrahman, Y.; Hassib, M.; Marquez, M.G.; Funk, M.; Schmidt, A. Implicit Engagement Detection for Interactive Museums Using Brain-Computer Interfaces. In Proceedings of the 17th International Conference on Human-Computer Interaction with Mobile Devices and Services Adjunct, Copenhagen, Denmark, 24–27 August 2015; pp. 838–845. [Google Scholar]
- Cuypers, K.; Krokstad, S.; Holmen, T.L.; Knudtsen, M.S.; Bygren, L.O.; Holmen, J. Patterns of Receptive and Creative Cultural Activities and Their Association with Perceived Health, Anxiety, Depression and Satisfaction with Life among Adults: The HUNT Study, Norway. J. Epidemiol. Community Health 2012, 66, 698–703. [Google Scholar] [CrossRef]
- Spielberg, C.D.; Gorsuch, R.L.; Lushene, R.E. STAI Manual for the State-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A Global Measure of Perceived Stress. J. Health Soc. Behav. 1983, 24, 386–396. [Google Scholar] [CrossRef]
- Field, T.; Diego, M.; Delgado, J.; Medina, L. Yoga and Social Support Reduce Prenatal Depression, Anxiety and Cortisol. J. Bodyw. Mov. Ther. 2013, 17, 397–403. [Google Scholar] [CrossRef]
- Mackay, G.J.; Neill, J.T. The Effect of “Green Exercise” on State Anxiety and the Role of Exercise Duration, Intensity, and Greenness: A Quasi-Experimental Study. Psychol. Sport Exerc. 2010, 11, 238–245. [Google Scholar] [CrossRef]
- Vitasari, P.; Wahab, M.N.A.; Herawan, T.; Othman, A.; Sinnadurai, S.K. Re-Test of State Trait Anxiety Inventory (STAI) among Engineering Students in Malaysia: Reliability and Validity Tests. Procedia-Soc. Behav. Sci. 2011, 15, 3843–3848. [Google Scholar] [CrossRef]
- Vanutelli, M.E.; Grigis, C.; Lucchiari, C. Breathing Right… or Left! The Effects of Unilateral Nostril Breathing on Psychological and Cognitive Wellbeing: A Pilot Study. Brain Sci. 2024, 14, 302. [Google Scholar] [CrossRef]
- McFarland, D.J.; Wolpaw, J.R. Brain-Computer Interfaces for Communication and Control. Commun. ACM 2011, 54, 60–66. [Google Scholar] [CrossRef]
- Flesher, S.; Downey, J.; Collinger, J.; Foldes, S.; Weiss, J.; Tyler-Kabara, E.; Bensmaia, S.; Schwartz, A.; Boninger, M.; Gaunt, R. Intracortical Microstimulation as a Feedback Source for Brain-Computer Interface Users. In Brain-Computer Interface Research: A State-of-the-Art Summary 6; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 43–54. [Google Scholar] [CrossRef]
- Bockbrader, M. Upper Limb Sensorimotor Restoration through Brain–Computer Interface Technology in Tetraparesis. Curr. Opin. Biomed. Eng. 2019, 11, 85–101. [Google Scholar] [CrossRef]
- Hosseini, S.M.H.; Pritchard-Berman, M.; Sosa, N.; Ceja, A.; Kesler, S.R. Task-Based Neurofeedback Training: A Novel Approach toward Training Executive Functions. Neuroimage 2016, 134, 153–159. [Google Scholar] [CrossRef]
- Maksimenko, V.; Lüttjohann, A.; van Heukelum, S.; Kelderhuis, J.; Makarov, V.; Hramov, A.; Koronovskii, A.; van Luijtelaar, G. Brain-Computer Interface for the Epileptic Seizures Prediction and Prevention. In Proceedings of the 2020 8th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Republic of Korea, 26–28 February 2020; IEEE: New York, NY, USA, 2020; pp. 1–5. [Google Scholar]
- Schwerdtfeger, A.R.; Rominger, C.; Weber, B.; Aluani, I. A Brief Positive Psychological Intervention Prior to a Potentially Stressful Task Facilitates More Challenge-like Cardiovascular Reactivity in High Trait Anxious Individuals. Psychophysiology 2021, 58, e13709. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Bogdan, R.; Ratner, K.G.; Jahn, A.L. Increased Perceived Stress Is Associated with Blunted Hedonic Capacity: Potential Implications for Depression Research. Behav. Res. Ther. 2007, 45, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Vanutelli, M.E.; Lucchiari, C. “Hyperfeedback” as a Tool to Assess and Induce Interpersonal Synchrony: The Role of Applied Social Neurosciences for Research, Training, and Clinical Practice. J. Health Med. Sci. 2022, 5, 11–18. [Google Scholar] [CrossRef]
- Vanutelli, M.E.; Salvadore, M.; Lucchiari, C. BCI Applications to Creativity: Review and Future Directions, from Little-c to C2. Brain Sci. 2023, 13, 665. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; Karamacoska, D.; Johnstone, S.J. The EEG theta/beta ratio: A marker of arousal or cognitive processing capacity? Appl. Psychophysiol. Biofeedback 2019, 44, 123–129. [Google Scholar] [CrossRef]
- Kaushik, P.; Shrivastava, P.K. Remediation of Learning Difficulty Utilizing School-Based Cognitive Behavioral Intervention Measured by EEG Theta-Alpha and Theta-Beta Ratio During Resting and Cognitive Task Performance Conditions. Clin. EEG Neurosci. 2024, 55, 426–444. [Google Scholar] [CrossRef]
- Šveb Dragija, M.; Jelinčić, D.A. Can Museums Help Visitors Thrive? Review of Studies on Psychological Wellbeing in Museums. Behav. Sci. 2022, 12, 458. [Google Scholar] [CrossRef]
- French, J.; Lunt, N.; Pearson, M. The MindLab Project. Local Museums Supporting Community Wellbeing before and after UK Lockdown. Mus. Soc. 2020, 18, 314–318. [Google Scholar] [CrossRef]

| Museum | N | Min | Max | Mean | SD | |
|---|---|---|---|---|---|---|
| MAG | Trait Anxiety | 17 | 25 | 61 | 44.57 | 9.37 |
| Perceived Stress | 17 | 7 | 37 | 19.14 | 6.53 | |
| Stress last month | 17 | 3 | 10 | 7.14 | 1.91 | |
| NHM | Trait Anxiety | 16 | 31 | 62 | 49.44 | 8.89 |
| Perceived Stress | 16 | 10 | 32 | 20.78 | 5.84 | |
| Stress last month | 16 | 2 | 9 | 8.00 | 1.71 |
| Mean | SD | Df | p | |
|---|---|---|---|---|
| SAI | −6.53 | 8.52 | 34 | <0.001 |
| Mood | 1.17 | 1.11 | 34 | <0.001 |
| Stress | −1.67 | 1.96 | 34 | <0.001 |
| Mental Clarity | 0.42 | 1.08 | 34 | 0.004 |
| Contentment | 1.08 | 1.26 | 34 | <0.001 |
| Calmness | 1.00 | 1.50 | 34 | <0.001 |
| Restlessness | −71 | 2.60 | 34 | 0.030 |
| Mean | SD | ||
|---|---|---|---|
| SAI | Pre | 36.73 | 8.4 |
| Post | 30.00 | 5.17 | |
| Mood | Pre | 7.20 | 2.52 |
| Post | 8.38 | 1.48 | |
| Stress | Pre | 4.67 | 2.24 |
| Post | 2.96 | 1.6 | |
| Calmness | Pre | 7.24 | 1.24 |
| Post | 8.28 | 0.96 | |
| Clarity of mind | Pre | 7.76 | 1.58 |
| Post | 8.20 | 1.16 | |
| Restlessness | Pre | 3.37 | 1.88 |
| Post | 2.62 | 1.28 |
| B | SE | Beta | p | ||
|---|---|---|---|---|---|
| (Constant) | −16.482 | 7.081 | −2.328 | 0.033 | |
| ΔTBR | −807 | 0.242 | −519 | −3.333 | 0.004 |
| PSS | −405 | 0.185 | −354 | −2.187 | 0.043 |
| TAI | 0.450 | 0.161 | 0.440 | −2.802 | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanutelli, M.E.; Banzi, A.; Cicirello, M.; Folgieri, R.; Lucchiari, C. Predicting State Anxiety Level Change Using EEG Parameters: A Pilot Study in Two Museum Settings. Brain Sci. 2025, 15, 855. https://doi.org/10.3390/brainsci15080855
Vanutelli ME, Banzi A, Cicirello M, Folgieri R, Lucchiari C. Predicting State Anxiety Level Change Using EEG Parameters: A Pilot Study in Two Museum Settings. Brain Sciences. 2025; 15(8):855. https://doi.org/10.3390/brainsci15080855
Chicago/Turabian StyleVanutelli, Maria Elide, Annalisa Banzi, Maria Cicirello, Raffaella Folgieri, and Claudio Lucchiari. 2025. "Predicting State Anxiety Level Change Using EEG Parameters: A Pilot Study in Two Museum Settings" Brain Sciences 15, no. 8: 855. https://doi.org/10.3390/brainsci15080855
APA StyleVanutelli, M. E., Banzi, A., Cicirello, M., Folgieri, R., & Lucchiari, C. (2025). Predicting State Anxiety Level Change Using EEG Parameters: A Pilot Study in Two Museum Settings. Brain Sciences, 15(8), 855. https://doi.org/10.3390/brainsci15080855







