Phasic REM: Across Night Behavior and Transitions to Wake
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aserinsky, E.; Kleitman, N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 1953, 118, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Dement, W.C.; Kleitman, N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr. Clin. Neurophysiol. 1957, 9, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Roffwarg, H.P.; Dement, W.C.; Muzio, J.N.; Fisher, C. Dream imagery: Relationship to rapid eye movements of sleep. Arch. Gen. Psychiatry 1962, 7, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Andrillon, T.; Nir, Y.; Cirelli, C.; Tononi, G.; Fried, I. Single-neuron activity and eye movements during human REM sleep and awake vision. Nat. Commun. 2015, 6, 7884. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senzai, Y.; Scanziani, M. A cognitive process occurring during sleep is revealed by rapid eye movements. Science 2022, 377, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Arnulf, I. The ‘scanning hypothesis’ of rapid eye movements during REM sleep: A review of the evidence. Arch. Ital. Biol. 2011, 149, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, E.; Berger, R.J. Rapid eye movements and dream imagery: Are they related? Nature 1969, 224, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.H.; Foulkes, D.; Schmidt, M. The structure of laboratory dream reports in blind and sighted subjects. J. Nerv. Ment. Dis. 1982, 170, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.M.; Mathis, J.L.; Jabbour, J.T. Dream patterns in narcoleptic and hydranencephalic patients. Am. J. Psychiatry 1965, 122, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Harmon, R.J.; Emde, R.N. Spontaneous REM behaviors in a microcephalic infant. Percept. Mot. Ski. 1972, 34, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Benoit, O.; Parot, S.; Garma, L. Evolution during the night of REM sleep in man. Electroencephalogr. Clin. Neurophysiol. 1974, 36, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P. Rapid eye movements in REM sleep—More evidence for a periodic organization. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, I. Sleep electroencephalographic and eye-movement patterns in patients with schizophrenia and with chronic brain syndrome. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 1967, 45, 211–240. [Google Scholar] [PubMed]
- Kupfer, D.J.; Ehlers, C.L. Two roads to rapid eye movement latency. Arch. Gen. Psychiatry 1989, 46, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Simor, P.; van der Wijk, G.; Nobili, L.; Peigneux, P. The microstructure of REM sleep: Why phasic and tonic? Sleep Med. Rev. 2020, 52, 101305. [Google Scholar] [CrossRef] [PubMed]
- De Carli, F.; Proserpio, P.; Morrone, E.; Sartori, I.; Ferrara, M.; Gibbs, S.A.; De Gennaro, L.; Lo Russo, G.; Nobili, L. Activation of the motor cortex during phasic rapid eye movement sleep. Ann. Neurol. 2016, 79, 326–730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aserinsky, E. Rapid eye movement density and pattern in the sleep of normal young adults. Psychophysiology 1971, 8, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Krynicki, V. Time trends and periodic cycles in REM sleep eye movements. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Salzarulo, P. Variations with time of the quantity of eye movements during fast sleep in man. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Usami, K.; Matsumoto, R.; Kobayashi, K.; Hitomi, T.; Matsuhashi, M.; Shimotake, A.; Kikuchi, T.; Yoshida, K.; Kunieda, T.; Mikuni, N.; et al. Phasic REM Transiently Approaches Wakefulness in the Human Cortex-A Single-Pulse Electrical Stimulation Study. Sleep 2017, 40, zsx077. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.S.; Cha, K.S.; Byun, J.I.; Kim, T.J.; Jun, J.S.; Lim, J.A.; Lee, S.T.; Jung, K.H.; Park, K.I.; Chu, K.; et al. Abnormal activation of motor cortical network during phasic REM sleep in idiopathic REM sleep behavior disorder. Sleep 2019, 42, zsy227. [Google Scholar] [CrossRef] [PubMed]
- Price, L.J.; Kremen, I. Variations in behavioral response threshold within the REM period of human sleep. Psychophysiology 1980, 17, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ermis, U.; Krakow, K.; Voss, U. Arousal thresholds during human tonic and phasic REM sleep. J. Sleep Res. 2010, 19, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, R.; Kaufmann, C.; Wetter, T.C.; Holsboer, F.; Auer, D.P.; Pollmächer, T.; Czisch, M. Functional microstates within human REM sleep: First evidence from fMRI of a thalamocortical network specific for phasic REM periods. Eur. J. Neurosci. 2007, 25, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Wehr, T.A.; Moul, D.E.; Barbato, G.; Giesen, H.A.; Seidel, J.A.; Barker, C.; Bender, C. Conservation of photoperiod-responsive mechanisms in humans. Am. J. Physiol. 1993, 265, R846–R857. [Google Scholar] [CrossRef] [PubMed]
- Rechtschaffen, A.; Kales, A. (Eds.) A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects; Public Health Service, US Government Printing Office: Washington, DC, USA, 1968.
- Dijk, D.J.; Brunner, D.P.; Borbély, A.A. Time course of EEG power density during long sleep in humans. Am. J. Physiol. 1990, 258, R650–R661. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G.; Barker, C.; Bender, C.; Giesen, H.A.; Wehr, T.A. Extended sleep in humans in 14 hour nights (LD 10:14): Relationship between REM density and spontaneous awakening. Electroencephalogr. Clin. Neurophysiol. 1994, 90, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Heninger, G.R. REM activity as a correlate of mood changes throughout the night. Electroencephalographic sleep patterns in a patient with a 48-hour cyclic mood disorder. Arch. Gen. Psychiatry 1972, 27, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Moruzzi, G. Active processes in the brain stem during sleep. Harvey Lect. 1963, 58, 233–297. [Google Scholar] [PubMed]
- Antonioli, M.; Solano, L.; Torre, A.; Violani, C.; Costa, M.; Bertini, M. Independence of REM density from other REM sleep parameters before and after REM deprivation. Sleep 1981, 4, 221–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aserinsky, E. The maximal capacity for sleep: Rapid eye movement density as an index of sleep satiety. Biol. Psychiatry 1969, 1, 147–159. [Google Scholar] [PubMed]
- Zimmerman, J.C.; Czeisler, C.A.; Laxminarayan, S.; Knauer, R.S.; Weitzman, E.D. REM density is dissociated from REM sleep timing during free-running sleep episodes. Sleep 1980, 2, 409–415. [Google Scholar] [CrossRef]
- Barbato, G.; Wehr, T.A. Homeostatic regulation of REM sleep in humans during extended sleep. Sleep 1998, 21, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Franken, P. Long-term vs. short-term processes regulating REM sleep. J. Sleep Res. 2002, 11, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.; Dijk, D.J. Sleep and circadian rhythmicity as entangled processes serving homeostasis. Nat. Rev. Neurosci. 2024, 25, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Czeisler, C.A.; Zimmerman, J.C.; Ronda, J.M.; Moore-Ede, M.C.; Weitzman, E.D. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep 1980, 2, 329–346. [Google Scholar] [CrossRef]
- Zulley, J. Distribution of REM sleep in entrained 24 hour and free-running sleep--wake cycles. Sleep 1980, 2, 377–389. [Google Scholar] [PubMed]
- Feinberg, I.; Baker, T.; Leder, R.; March, J.D. Response of delta (0–3 Hz) EEG and eye movement density to a night with 100 minutes of sleep. Sleep 1988, 11, 473–487. [Google Scholar] [PubMed]
- Barbato, G.; Barker, C.; Bender, C.; Wehr, T.A. Spontaneous sleep interruptions during extended nights. Relationships with NREM and REM sleep phases and effects on REM sleep regulation. Clin. Neurophysiol. 2002, 113, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G. Is REM Density a Measure of Arousal during Sleep? Brain Sci. 2023, 13, 378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalsa, S.B.; Conroy, D.A.; Duffy, J.F.; Czeisler, C.A.; Dijk, D.J. Sleep- and circadian-dependent modulation of REM density. J. Sleep Res. 2002, 11, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.T.; Andersen, M.L.; Luciano, Y.M.; Kakazu, V.A.; Tufik, S.; Pires, G.N. Methods for REM Sleep Density Analysis: A Scoping Review. Clocks Sleep 2023, 5, 793–805. [Google Scholar] [CrossRef]
- Arfken, C.L.; Joseph, A.; Sandhu, G.R.; Roehrs, T.; Douglass, A.B.; Boutros, N.N. The status of sleep abnormalities as a diagnostic test for major depressive disorder. J. Affect. Disord. 2014, 156, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Atsumi, Y. Precise measurement of individual rapid eye movements in REM sleep of humans. Sleep 1997, 20, 743–752. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ktonas, P.; Nygren, A.; Frost, J., Jr. Two-minute rapid eye movement (REM) density fluctuations in human REM sleep. Neurosci. Lett. 2003, 353, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.A.E.; Kempfner, L.; Leonthin, H.L.; Hvidtfelt, M.; Nikolic, M.; Kornum, B.R.; Jennum, P. Novel method for evaluation of eye movements in patients with narcolepsy. Sleep Med. 2017, 33, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yetton, B.D.; Niknazar, M.; Duggan, K.A.; McDevitt, E.A.; Whitehurst, L.N.; Sattari, N.; Mednick, S.C. Automatic detection of rapid eye movements (REMs): A machine learning approach. J. Neurosci. Methods. 2016, 259, 72–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, R.; Takeuchi, T.; Laroche, S.; Gotman, J. Detection of rapid-eye movements in sleep studies. IEEE Trans. Biomed. Eng. 2005, 52, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Betta, M.; Laurino, M.; Gemignani, A.; Landi, A.; Menicucci, D. A Classification method for eye movements direction during REM sleep trained on wake electro-oculographic recordings. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 370–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petre-Quadens, O.; De Lee, C. Eye-movements during sleep: A common criterion of learning capacities and endocrine activity. Dev. Med. Child Neurol. 1970, 12, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Dement, W.C. Sleep studies on a 90-minute day. Electroencephalogr. Clin. Neurophysiol. 1975, 39, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Duncan, W.C.; Barbato, G.; Fagioli, I.; Garcia-Borreguero, D.; Wehr, T.A. A biphasic daily pattern of slow wave activity during a two-day 90-minute sleep wake schedule. Arch. Ital. Biol. 2009, 147, 117–130. [Google Scholar] [PubMed]
- Tsunematsu, T. What are the neural mechanisms and physiological functions of dreams? Neurosci. Res. 2023, 189, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. REM sleep function: Mythology vs. reality. Rev. Neurol. 2023, 179, 643–648, Erratum in Rev. Neurol. 2023, 179, 1152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pagel, J.F. The Persistent Paradox of Rapid Eye Movement Sleep (REMS): Brain Waves and Dreaming. Brain Sci. 2024, 14, 622. [Google Scholar] [CrossRef]
- Morra, D.; Barbato, G. REM sleep in schizophrenia: A systematic review and meta-analysis. Sleep Med. Rev. 2025, 83, 102134. [Google Scholar] [CrossRef] [PubMed]
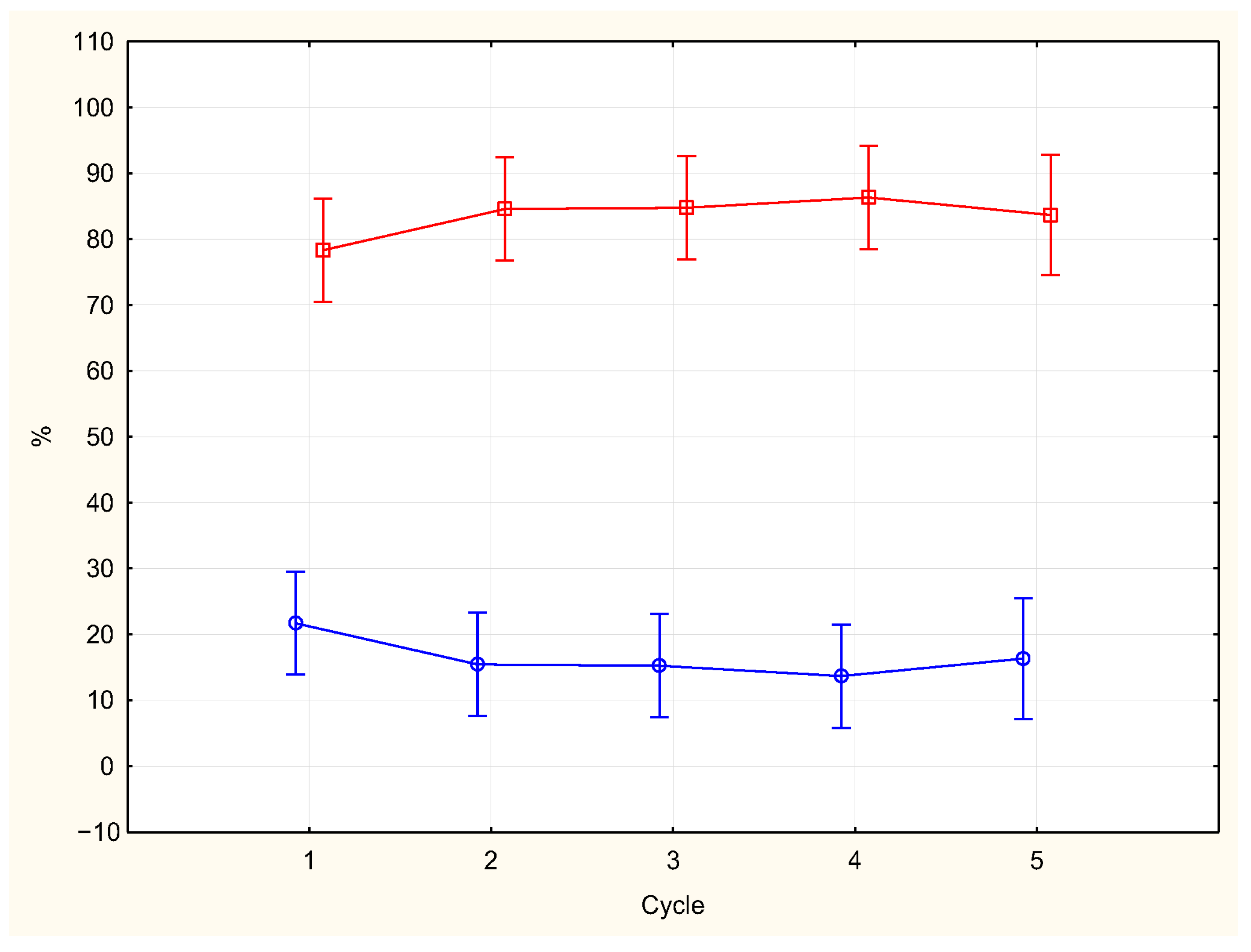


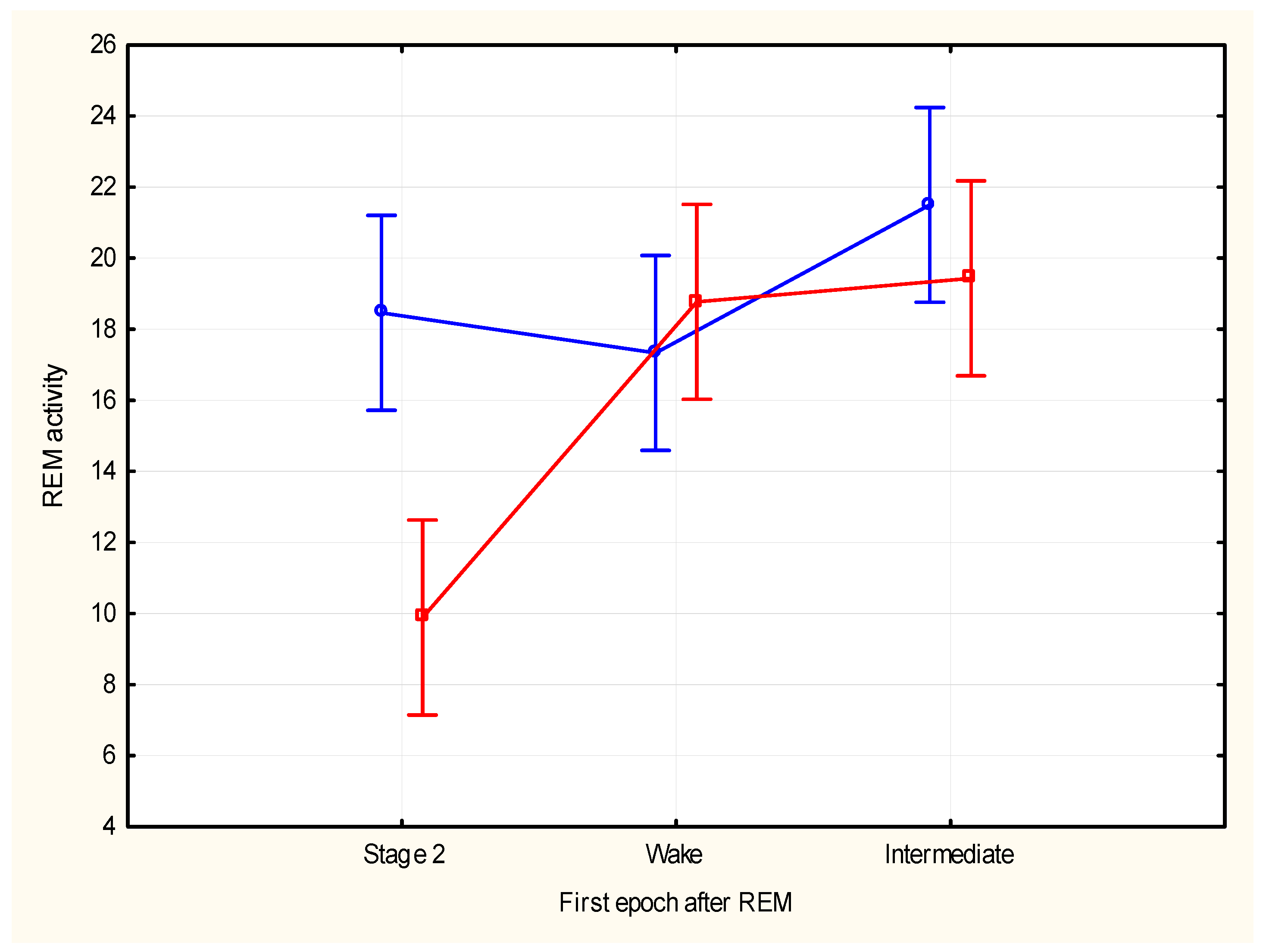
| Cycle (N) | REM Time (SD) | REM Density (SD) |
|---|---|---|
| 1 (15) | 16.41 (7.48) | 1.71 (0.66) |
| 2 (15) | 27.35 (8.66) | 2.15 (0.70) |
| 3 (15) | 30.98 (6.26) | 2.27 (0.72) |
| 4 (15) | 25.63 (9.27) | 2.16 (0.60) |
| 5 (11) | 26.78 (12.45) | 2.11 (0.76) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbato, G.; Wehr, T.A. Phasic REM: Across Night Behavior and Transitions to Wake. Brain Sci. 2025, 15, 840. https://doi.org/10.3390/brainsci15080840
Barbato G, Wehr TA. Phasic REM: Across Night Behavior and Transitions to Wake. Brain Sciences. 2025; 15(8):840. https://doi.org/10.3390/brainsci15080840
Chicago/Turabian StyleBarbato, Giuseppe, and Thomas A. Wehr. 2025. "Phasic REM: Across Night Behavior and Transitions to Wake" Brain Sciences 15, no. 8: 840. https://doi.org/10.3390/brainsci15080840
APA StyleBarbato, G., & Wehr, T. A. (2025). Phasic REM: Across Night Behavior and Transitions to Wake. Brain Sciences, 15(8), 840. https://doi.org/10.3390/brainsci15080840





