Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Patients with aMCI (MCI)
2.1.2. Healthy Older Controls (HOCs)
2.2. Experimental Procedure
The Attention Network Test (ANT)
2.3. Statistical Analysis
3. Results
3.1. Raw RTs
3.2. Z-Scores
3.3. Attentional Network Indices
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ADL | Activities of Daily Living |
| aMCI | amnestic Mild Cognitive Impairment |
| ANOVA | Analysis of Variance |
| ANT | Attention Network Test |
| ASST | Azienda Socio-Sanitaria Territoriale |
| DBBS | Department of Brain and Behavioral Sciences |
| F | Females |
| FAB | Frontal Assessment Battery |
| FCSRT | Free and Cued Selective Reminding Test |
| GCP | Good Clinical Practice |
| GLMM | Generalized Linear Mixed Model |
| HOCs | Healthy Older Controls |
| IADL | Instrumental Activities of Daily Living |
| M | Males |
| MCI | Mild Cognitive Impairment |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| ms | Milliseconds |
| MTCF | Modified Taylor Complex Figure |
| PEBL | Psychology Experiment Building Language |
| REML | Restricted Maximum Likelihood |
| RTs | Reaction Times |
| SDMT | Symbol Digit Modalities Test |
| TMT | Trial Making Test |
References
- Posner, M.I.; Petersen, S.E. The attention system of the human brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; McCandliss, B.D.; Sommer, T.; Raz, A.; Posner, M.I. Testing the Efficiency and Independence of Attentional Networks. J. Cogn. Neurosci. 2002, 14, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Rothbart, M.K. Research on Attention Networks as a Model for the Integration of Psychological Science. Annu. Rev. Psychol. 2007, 58, 1–23. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I.M.; Wood, M.M.; Miller, W.S. A Review on the Trajectory of Attentional Mechanisms in Aging and the Alzheimer’s Disease Continuum through the Attention Network Test. Yale J. Biol. Med. 2019, 92, 37–51. [Google Scholar]
- Van Dam, N.T.; Sano, M.; Mitsis, E.M.; Grossman, H.T.; Gu, X.; Park, Y.; Hof, P.R.; Fan, J. Functional Neural Correlates of Attentional Deficits in Amnestic Mild Cognitive Impairment. PLoS ONE 2013, 8, e54035. [Google Scholar] [CrossRef] [PubMed]
- Sarrias-Arrabal, E.; Izquierdo-Ayuso, G.; Vázquez-Marrufo, M. Attentional Networks in Neurodegenerative Diseases: Anatomical and Functional Evidence from the Attention Network Test. Neurología (Engl. Ed.) 2023, 38, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Posner, M. Human Attentional Networks. Psychiatr. Prax. 2004, 31, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Mccandliss, B.; Fossella, J.; Flombaum, J.; Posner, M. The Activation of Attentional Networks. NeuroImage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.J.; Campoy, G.; García Santos, J.M.; Antequera, M.M.; García-Sevilla, J.; Castillo, A.; Antúnez, C.; Fuentes, L.J. Is There a Specific Pattern of Attention Deficit in Mild Cognitive Impairment with Subcortical Vascular Features? Evidence from the Attention Network Test. Dement. Geriatr. Cogn. Disord. 2011, 31, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Festa-Martino, E.; Ott, B.R.; Heindel, W.C. Interactions Between Phasic Alerting and Spatial Orienting: Effects of Normal Aging and Alzheimer′s Disease. Neuropsychology 2004, 18, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Deiber, M.-P.; Ibañez, V.; Missonnier, P.; Rodriguez, C.; Giannakopoulos, P. Age-Associated Modulations of Cerebral Oscillatory Patterns Related to Attention Control. NeuroImage 2013, 82, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Tales, A.; Muir, J.; Jones, R.; Bayer, A.; Snowden, R.J. The Effects of Saliency and Task Difficulty on Visual Search Performance in Ageing and Alzheimer’s Disease. Neuropsychologia 2004, 42, 335–345. [Google Scholar] [CrossRef]
- Martella, D.; Manzanares, S.; Campoy, G.; Roca, J.; Antúnez, C.; Fuentes, L.J. Phasic and Tonic Alerting in Mild Cognitive Impairment: A Preliminary Study. Exp. Gerontol. 2014, 49, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, H.; Liang, K.; Wang, H.; Kong, S.; Hu, J.; Wu, F.; Sun, G. Functional Degeneration in Dorsal and Ventral Attention Systems in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: An fMRI Study. Neurosci. Lett. 2015, 585, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Duque, D.; Black, S.E. Attentional Networks in Normal Aging and Alzheimer′s Disease. Neuropsychology 2006, 20, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Wang, X.; Cui, Y.; Jin, J.; Sun, Y.; Tang, Y.; Bai, Y.; Wang, Y.; Zhou, L. Application of Attention Network Test and Demographic Information to Detect Mild Cognitive Impairment via Combining Feature Selection with Support Vector Machine. Comput. Methods Programs Biomed. 2010, 97, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.R.; Howard, J.H.; Howard, D.V. Not Just Scenery: Viewing Nature Pictures Improves Executive Attention in Older Adults. Exp. Aging Res. 2014, 40, 513–530. [Google Scholar] [CrossRef] [PubMed]
- Gamboz, N.; Zamarian, S.; Cavallero, C. Age-Related Differences in the Attention Network Test (ANT). Exp. Aging Res. 2010, 36, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.M.; Dagenbach, D.; Engle, C.M.; Funke, L.J. Age-Related Changes and the Attention Network Task: An Examination of Alerting, Orienting, and Executive Function. Aging Neuropsychol. Cogn. 2007, 14, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Westlye, L.T.; Grydeland, H.; Walhovd, K.B.; Fjell, A.M. Associations between Regional Cortical Thickness and Attentional Networks as Measured by the Attention Network Test. Cereb. Cortex 2011, 21, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Mather, M. Look Out—It′s Your Off-Peak Time of Day! Time of Day Matters More for Alerting than for Orienting or Executive Attention. Exp. Aging Res. 2013, 39, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.S.; Biel, A.L.; Wegier, P.; Lapp, L.K.; Dyson, B.J.; Spaniol, J. Age Differences in the Attention Network Test: Evidence from Behavior and Event-Related Potentials. Brain Cogn. 2016, 102, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, T. Alzheimer’s Disease Neuroimaging Initiative Gender Differences in Elderly With Subjective Cognitive Decline. Front. Aging Neurosci. 2018, 10, 166. [Google Scholar] [CrossRef]
- Sundermann, E.E.; Biegon, A.; Rubin, L.H.; Lipton, R.B.; Mowrey, W.; Landau, S.; Maki, P.M. Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology 2016, 86, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Schumacher Dimech, A.; Santuccione Chadha, A.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex Differences in Alzheimer Disease—the Gateway to Precision Medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lee, C.; Torres, E.R.S.; Carling, G.; Gan, L. Mechanisms of Sex Differences in Alzheimer’s Disease. Neuron 2024, 112, 1208–1221. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Morris, J.C. Mild Cognitive Impairment as a Clinical Entity and Treatment Target. Arch. Neurol. 2005, 62, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Christa Maree Stephan, B.; Minett, T.; Pagett, E.; Siervo, M.; Brayne, C.; McKeith, I.G. Diagnosing Mild Cognitive Impairment (MCI) in Clinical Trials: A Systematic Review. BMJ Open 2013, 3, e001909. [Google Scholar] [CrossRef] [PubMed]
- Salemme, S.; Lombardo, F.L.; Lacorte, E.; Sciancalepore, F.; Remoli, G.; Bacigalupo, I.; Piscopo, P.; Zamboni, G.; Rossini, P.M.; Cappa, S.F.; et al. The Prognosis of Mild Cognitive Impairment: A Systematic Review and Meta-analysis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2025, 17, e70074. [Google Scholar] [CrossRef] [PubMed]
- Measso, G.; Cavarzeran, F.; Zappalà, G.; Lebowitz, B.D.; Crook, T.H.; Pirozzolo, F.J.; Amaducci, L.A.; Massari, D.; Grigoletto, F. The Mini-mental State Examination: Normative Study of an Italian Random Sample. Dev. Neuropsychol. 1993, 9, 77–85. [Google Scholar] [CrossRef]
- Katz, S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. J. Am. Geriat. Soc. 1983, 31, 721–727. [Google Scholar] [CrossRef]
- Grober, E.; Buschke, H. Genuine Memory Deficits in Dementia. Dev. Neuropsychol. 1987, 3, 13–36. [Google Scholar] [CrossRef]
- Frasson, P.; Ghiretti, R.; Catricalà, E.; Pomati, S.; Marcone, A.; Parisi, L.; Rossini, P.M.; Cappa, S.F.; Mariani, C.; Vanacore, N. Free and Cued Selective Reminding Test: An Italian Normative Study. Neurol. Sci. 2011, 32, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Papagno, C.; Capitani, E.; Laiacona, M. Tre Test Clinici Di Memoria Verbale a Lungo Termine: Taratura Su Soggetti Normali. Archiv. PNP 1986, 47, 278–296. [Google Scholar]
- Sartori, G.; Job, R. The Oyster with Four Legs: A Neuropsychological Study on the Interaction of Visual and Semantic Information. Cogn. Neuropsychol. 1988, 5, 105–132. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and Backward Span for Verbal and Visuo-Spatial Data: Standardization and Normative Data from an Italian Adult Population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Casarotti, A.; Papagno, C.; Zarino, B. Modified Taylor Complex Figure: Normative Data from 290 Adults. J. Neuropsychol. 2014, 8, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail Making Test: Normative Values from 287 Normal Adult Controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Caffarra, P.; Vezzadini, G.; Dieci, F.; Zonato, F.; Venneri, A. A Short Version of the Stroop Test: Normative Data in an Italian Population Sample. Riv. Neurol. 2002, 12, 111–115. [Google Scholar]
- Nocentini, U.; Giordano, A.; Vincenzo, S.D.; Panella, M.; Pasqualetti, P. The Symbol Digit Modalities Test—Oral Version: Italian Normative Data. Funct. Neurol. 2006, 21, 93–96. [Google Scholar] [PubMed]
- Appollonio, I.; Leone, M.; Isella, V.; Piamarta, F.; Consoli, T.; Villa, M.L.; Forapani, E.; Russo, A.; Nichelli, P. The Frontal Assessment Battery (FAB): Normative Values in an Italian Population Sample. Neurol. Sci. 2005, 26, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.; Capitani, E.; Laiacona, M. Raven′s Coloured Progressive Matrices: Normative Values on 305 Adult Normal Controls. Funct. Neurol. 1987, 2, 189–194. [Google Scholar] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Bonazzi, S.; Laiacona, M.; Masina, M.; Coralli, M.V. Montreal Cognitive Assessment (MoCA)-Italian Version: Regression Based Norms and Equivalent Scores. Neurol. Sci. 2015, 36, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Luke, S.G. Evaluating Significance in Linear Mixed-Effects Models in R. Behav. Res. 2017, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Dash, T. Linear Mixed-Model Analysis Better Captures Subcomponents of Attention in a Small Sample Size of Persons With Aphasia. Am. J. Speech. Lang. Pathol. 2023, 32, 748–761. [Google Scholar] [CrossRef] [PubMed]
- Lassen-Greene, C.L.; Steward, K.; Okonkwo, O.; Porter, E.; Crowe, M.; Vance, D.E.; Griffith, H.R.; Ball, K.; Marson, D.C.; Wadley, V.G. Mild Cognitive Impairment and Changes in Everyday Function Over Time: The Importance of Evaluating Both Speed and Accuracy. J. Geriatr. Psychiatry Neurol. 2017, 30, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Uchoa, M.F.; Moser, V.A.; Pike, C.J. Interactions between Inflammation, Sex Steroids, and Alzheimer’s Disease Risk Factors. Front. Neuroendocr. 2016, 43, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Vegeto, E.; Poletti, A.; Maggi, A. Estrogens, Neuroinflammation, and Neurodegeneration. Endocr. Rev. 2016, 37, 372–402. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Paolacci, L.; Remondini, D.; Giampieri, E.; Poli, G.; Curti, N.; Cecchetti, R.; Villa, A.; Ruggiero, C.; Brancorsini, S.; et al. Cognitive Decline and Alzheimer’s Disease in Old Age: A Sex-Specific Cytokinome Signature. JAD 2019, 72, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.T.; Klinger, H.M.; Boyle, R.; Betthauser, T.J.; Binette, A.P.; Christenson, L.; Chadwick, T.; Hansson, O.; Harrison, T.M.; Healy, B. Sex Differences in Longitudinal Tau-PET in Preclinical Alzheimer Disease: A Meta-Analysis. JAMA Neurol. 2025, 82, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Cavedo, E.; Chiesa, P.A.; Houot, M.; Ferretti, M.T.; Grothe, M.J.; Teipel, S.J.; Lista, S.; Habert, M.; Potier, M.; Dubois, B.; et al. Sex Differences in Functional and Molecular Neuroimaging Biomarkers of Alzheimer′s Disease in Cognitively Normal Older Adults with Subjective Memory Complaints. Alzheimer’s Dement. 2018, 14, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Perneczky, R.; Diehl-Schmid, J.; Förstl, H.; Drzezga, A.; Kurz, A. Male Gender Is Associated with Greater Cerebral Hypometabolism in Frontotemporal Dementia: Evidence for Sex-related Cognitive Reserve. Int. J. Geriatr. Psychiatry 2007, 22, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.; Laws, K.R.; Gale, T.M.; Kondel, T.K. Greater Cognitive Deterioration in Women than Men with Alzheimer′s Disease: A Meta Analysis. J. Clin. Exp. Neuropsychol. 2012, 34, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M. Sex and Gender Differences in Alzheimer′s Disease Dementia. Psych. Times 2019, 35, 14–17. [Google Scholar]
- Nelson, A.; O′Connor, M. Mild Cognitive Impairment: A Neuropsychological Perspective. CNS Spectrums 2008, 13, 56–64. [Google Scholar] [CrossRef]
- Gomar, J.J. Utility of Combinations of Biomarkers, Cognitive Markers, and Risk Factors to Predict Conversion From Mild Cognitive Impairment to Alzheimer Disease in Patients in the Alzheimer′s Disease Neuroimaging Initiative. Arch. Gen. Psychiatry 2011, 68, 961. [Google Scholar] [CrossRef] [PubMed]
- Reinvang, I.; Grambaite, R.; Espeseth, T. Executive Dysfunction in MCI: Subtype or Early Symptom. Int. J. Alzheimer’s Dis. 2012, 2012, 936272. [Google Scholar] [CrossRef] [PubMed]
- Monsell, S.E.; Mock, C.; Hassenstab, J.; Roe, C.M.; Cairns, N.J.; Morris, J.C.; Kukull, W. Neuropsychological Changes in Asymptomatic Persons with Alzheimer Disease Neuropathology. Neurology 2014, 83, 434–440. [Google Scholar] [CrossRef] [PubMed]
- König, I.R.; Fuchs, O.; Hansen, G.; Von Mutius, E.; Kopp, M.V. What Is Precision Medicine? Eur. Respir. J. 2017, 50, 1700391. [Google Scholar] [CrossRef] [PubMed]
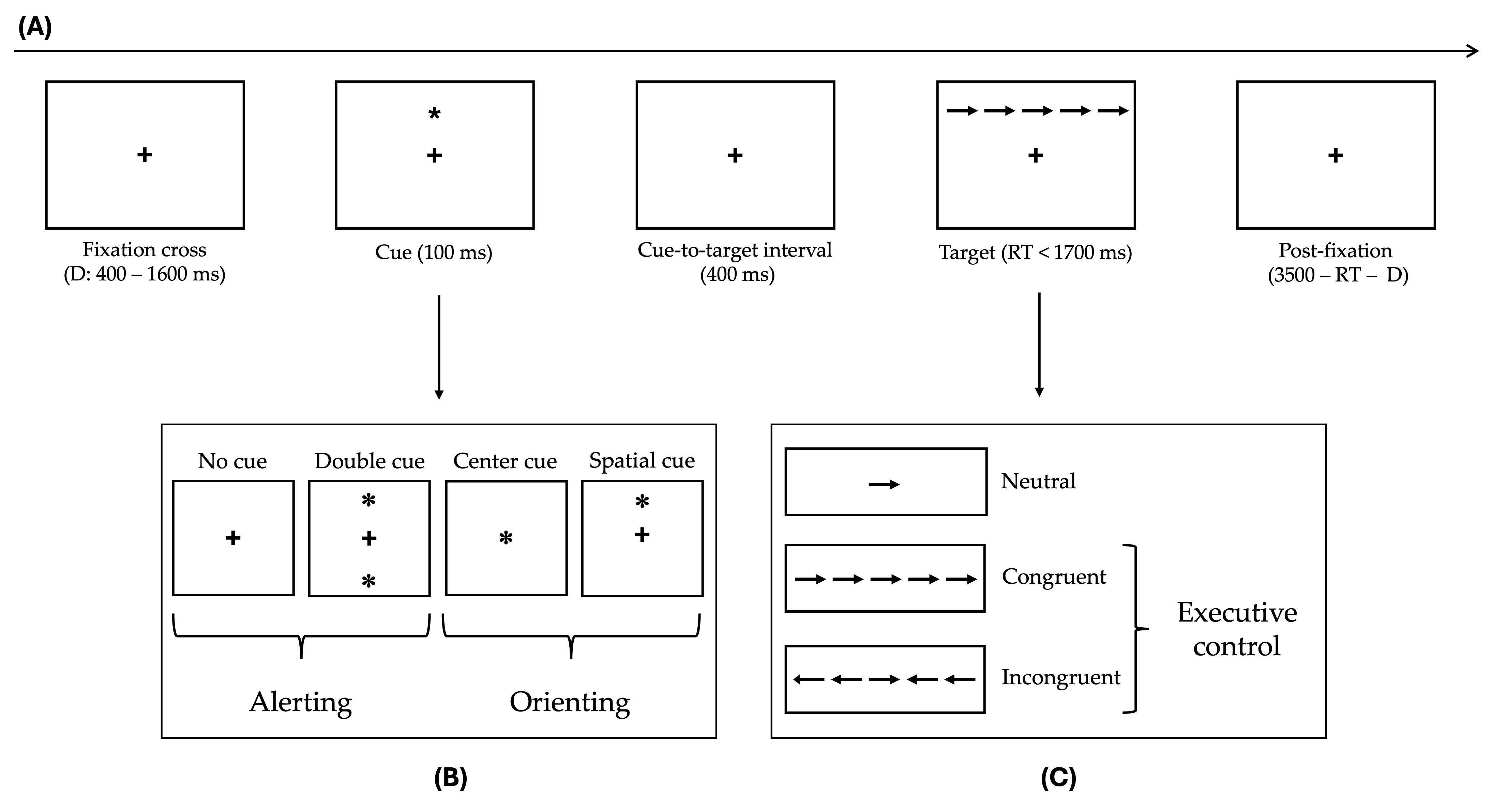

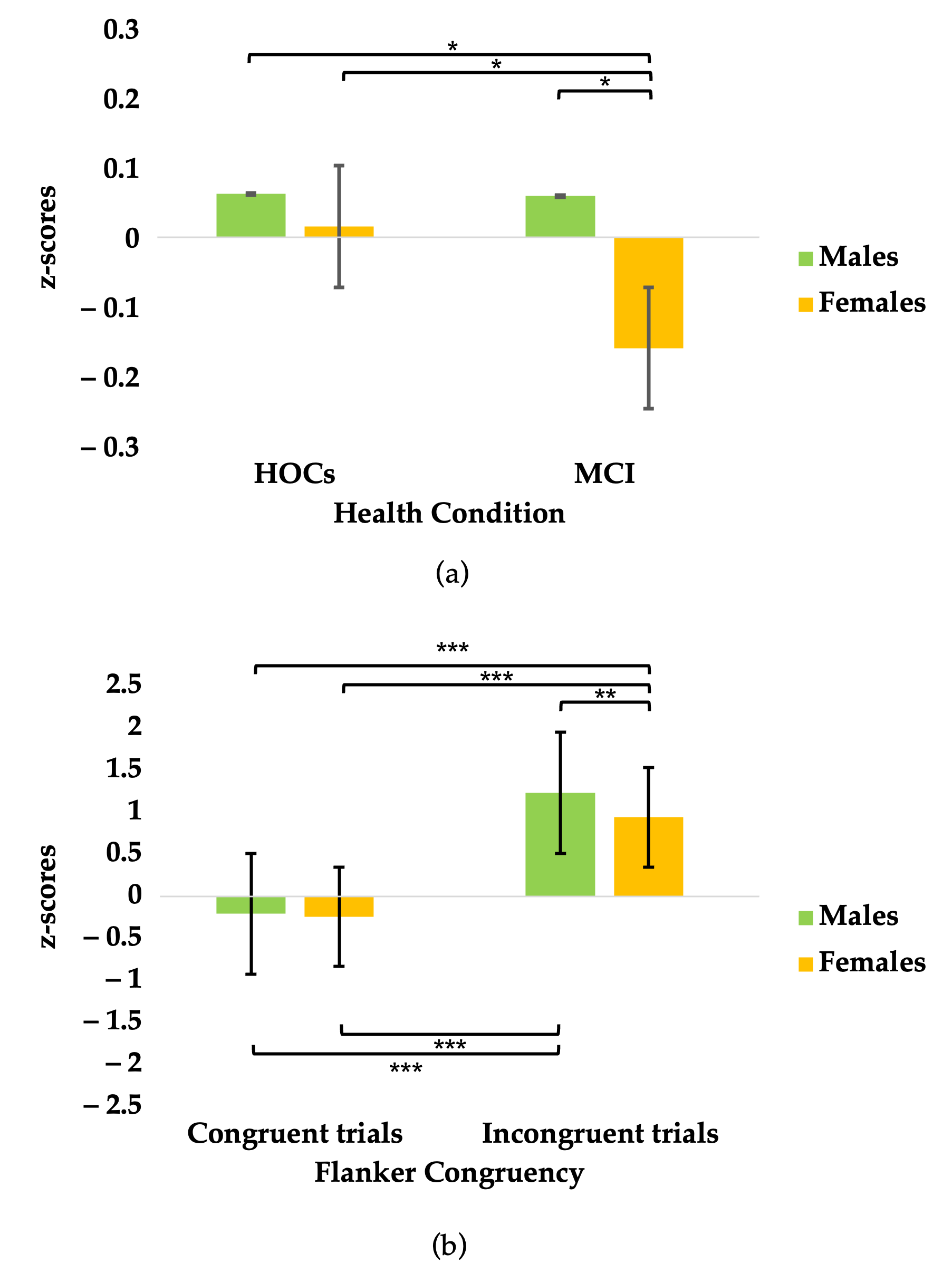
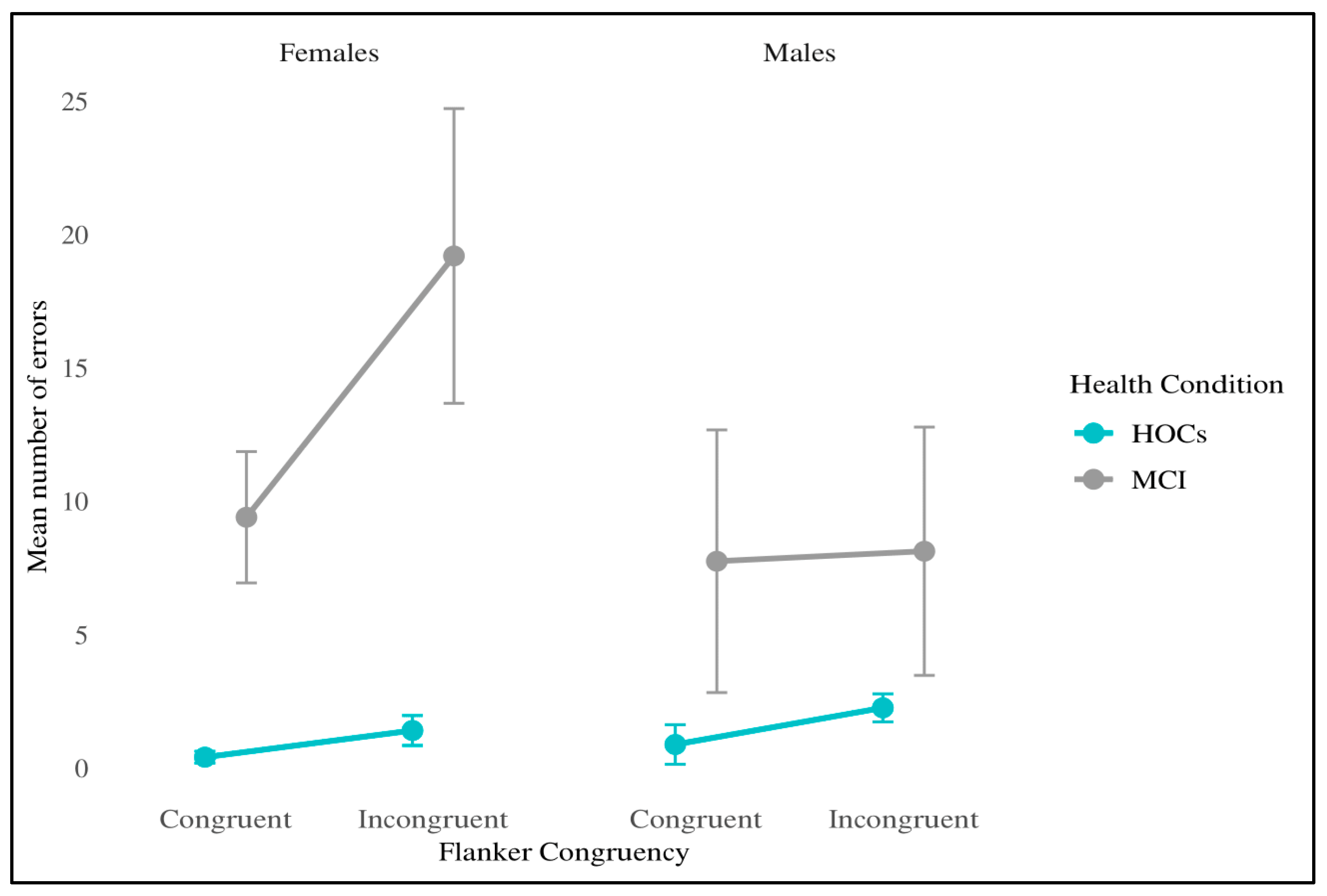
| TEST | Normative Cut-Off | MCI (n = 18) Adjusted Score |
|---|---|---|
| General cognitive status | ||
| MMSE [30] | >23.86 | 25.92 ± 1.55 (30) |
| ADL [31] | >5 | 5.94 ± 0.24 (6) |
| IADL [31] | >7 | 7.41 ± 0.87 (8) |
| Language | ||
| Verbal fluency [34] | >17.77 | 32.88 ± 10.33 |
| Semantic fluency [34] | >28.34 | 36.01 ± 7.42 |
| Visual naming test [35] | - | z score 4.53 ± 4.17 |
| Verbal memory | ||
| FCSRT [33] | ||
| - Immediate Free Recall | >21.26 | 12.52 ± 5.46 (48) |
| - Delayed Free Recall | >8.1 | 3.57 ± 3.11 (16) |
| Digit span [36] | >4.26 | 5.94 ± 1.07 (9) |
| Digit span backward [36] | >2.65 | 4.95 ± 0.89 (8) |
| Visuo-spatial memory | ||
| MTCF [37] | ||
| - Direct copy | >27.66 | 32.04 ± 4.46 (36) |
| - Delayed recall | >8.4 | 8.04 ± 3.77 (36) |
| Corsi span [36] | >3.46 | 5.59 ± 0.85 (9) |
| Corsi span backward [36] | >3.08 | 5.1 ± 0.76 (8) |
| Attention and executive functions | ||
| TMT [38] | ||
| - A | <93 | 39.9 ± 16.25 |
| - B | <282 | 110.45 ± 66.32 |
| Stroop Test [39] | ||
| - Time | <36.92 | 20.58 ± 15.55 |
| - Errors | <4.24 | 0.38 ± 1.22 |
| SDMT [40] | >34.2 | 44.4 ± 9.23 (110) |
| FAB [41] | >13.4 | 16.8 ± 3.8 (18) |
| Abstract reasoning | ||
| Raven’s colored matrices [42] | >17.50 | 34.06 ± 2.4 (36) |
| TEST | Normative Cut-Off | HOCs (n = 18) Adjusted Score |
|---|---|---|
| MoCA [44] | >17.362 | 24.23 ± 1.48 (30) |
| FCSRT [33] | ||
| - Immediate Free Recall | >19.59 | 30.24 ± 3.53 (36) |
| - Delayed Free Recall | >6.31 | 10.72 ± 1.24 (12) |
| Cue Type | ||||||
|---|---|---|---|---|---|---|
| Condition | Sex | Flanker Type | No Cue | Double Cue | Central Cue | Spatial Cue |
| MCI | Females | Congruent | 971 (101) | 941 (119) | 982 (101) | 949 (118) |
| Incongruent | 1080 (114) | 1061 (117) | 1074 (122) | 1043 (103) | ||
| Neutral | 906 (80) | 869 (93) | 873 (112) | 907 (111) | ||
| Males | Congruent | 812 (49) | 729 (53) | 780 (70) | 749 (87) | |
| Incongruent | 908 (56) | 933 (107) | 895 (72) | 854 (50) | ||
| Neutral | 775 (88) | 693 (82) | 692 (82) | 718 (95) | ||
| HOCs | Females | Congruent | 758 (85) | 725 (53) | 724 (56) | 709 (73) |
| Incongruent | 836 (116) | 845 (89) | 844 (90) | 794 (82) | ||
| Neutral | 705 (69) | 668 (51) | 666 (29) | 659 (59) | ||
| Males | Congruent | 752 (115) | 690 (79) | 692 (95) | 664 (78) | |
| Incongruent | 816 (103) | 831 (111) | 824 (96) | 788 (109) | ||
| Neutral | 692 (88) | 619 (69) | 632 (99) | 618 (90) | ||
| Executive Control Index | ||||||
|---|---|---|---|---|---|---|
| RTs | ||||||
| Condition | Sex | Flanker Type | Mean RTs | Proportional RTs | Conflict Index | Number of Errors |
| MCI | Females | Congruent | 956 (101) | 0.92 (0.04) | 0.08 (0.11) | 9.4 (7.8) |
| Incongruent | 1032 (149) | 1.0 (0.15) | 19.2 (17.5) | |||
| Males | Congruent | 768 (57) | 0.94 (0.04) | 0.16 (0.02) | 7.75 (14) | |
| Incongruent | 897 (66) | 1.1 (0.05) | 8.13 (13.2) | |||
| HOCs | Females | Congruent | 729 (56) | 0.97 (0.02) | 0.14 (0.04) | 0.4 (0.7) |
| Incongruent | 835 (75) | 1.11 (0.02) | 1.4 (1.78) | |||
| Males | Congruent | 699 (89) | 0.97 (0.02) | 0.16 (0.05) | 0.88 (2.1) | |
| Incongruent | 814 (103) | 1.12 (0.03) | 2.25 (1.49) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facci, L.; Sandrini, L.; Bottini, G. Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences. Brain Sci. 2025, 15, 770. https://doi.org/10.3390/brainsci15070770
Facci L, Sandrini L, Bottini G. Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences. Brain Sciences. 2025; 15(7):770. https://doi.org/10.3390/brainsci15070770
Chicago/Turabian StyleFacci, Laura, Laura Sandrini, and Gabriella Bottini. 2025. "Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences" Brain Sciences 15, no. 7: 770. https://doi.org/10.3390/brainsci15070770
APA StyleFacci, L., Sandrini, L., & Bottini, G. (2025). Attentional Functioning in Healthy Older Adults and aMCI Patients: Results from the Attention Network Test with a Focus on Sex Differences. Brain Sciences, 15(7), 770. https://doi.org/10.3390/brainsci15070770






