The Challenge of Diagnosing Labyrinthine Stroke—A Critical Review
Abstract
1. Introduction
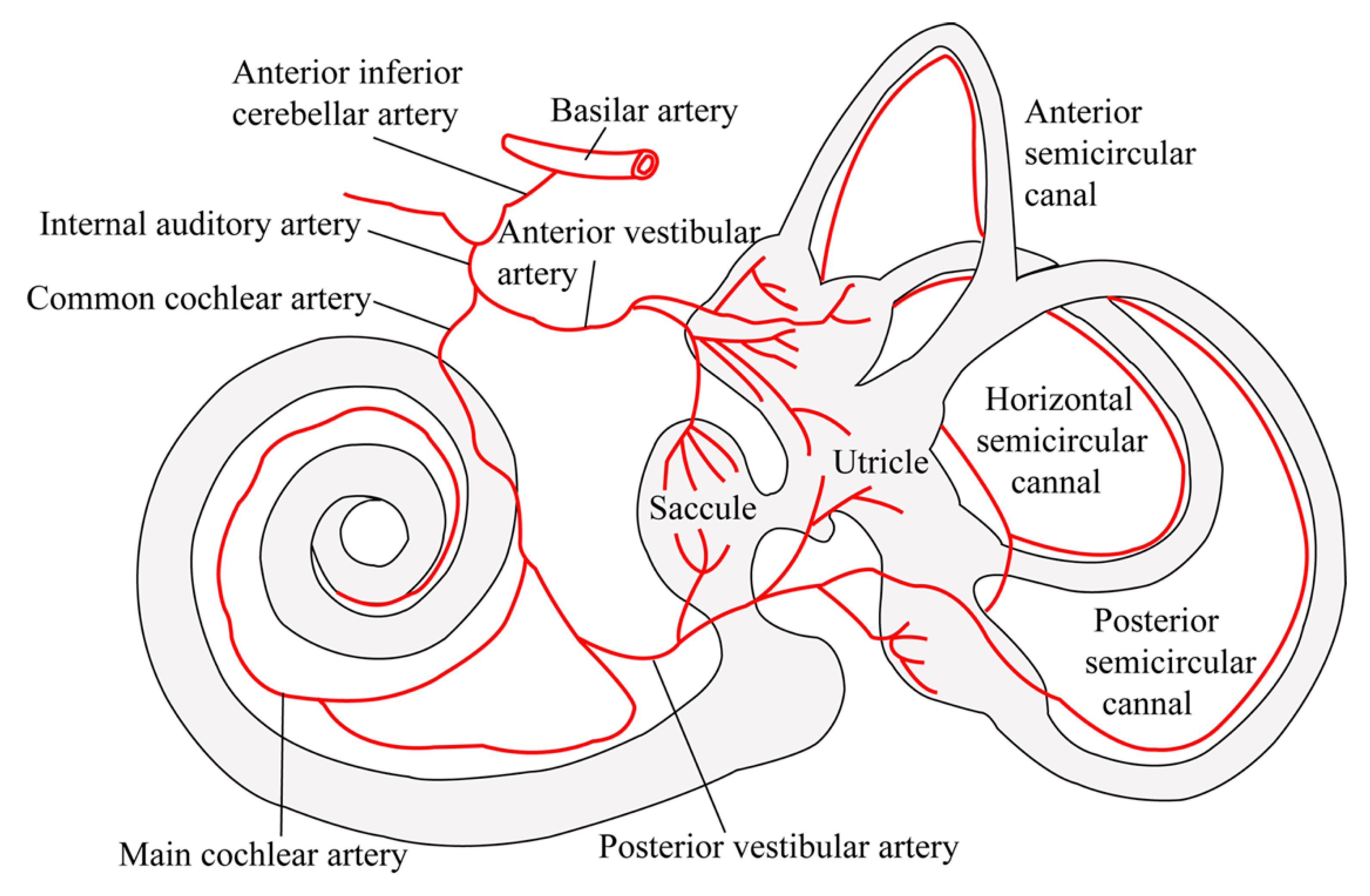
2. Inner Ear Anatomy and Vascular Supply
3. Methodology and Results of the Literature Review Performed
4. Results from the Literature Review
5. Clinical Features Distinguishing an Inner Ear Pathology from a Central Vertebrobasilar Lesion Location
6. The Value of Quantitative Audiovestibular Testing
7. The Role of MR Imaging in Identifying Vertebrobasilar Lesions
8. The Value of MR Imaging in Localizing Vascular Pathologies to the Inner Ear
9. Labyrinthine Stroke as a Warning Sign of Vertebrobasilar Stroke
10. The Spectrum of Other Causes of SSNHL and Acute Audiovestibular Loss and the Role of Imaging
11. Treatment Options and the Prognosis of Labyrinthine Stroke
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AICA | anterior inferior cerebellar artery |
| AVA | anterior vestibular artery |
| aVOR | angular vestibulo-ocular reflex |
| AVS | acute vestibular syndrome |
| DWI | diffusion-weighted imaging |
| ER | emergency room |
| FIESTA | fast imaging employing steady-state acquisition |
| FLAIR | fluid-attenuated inversion recovery |
| HINTS | head-impulse, nystagmus, test of skew |
| HIT | head-impulse test |
| IAA | internal auditory artery |
| MCA | main cochlear artery |
| MRI | magnetic resonance imaging |
| PICA | posterior inferior cerebellar artery |
| SSNHL | sudden sensorineural hearing loss |
| SD | standard deviation |
| Sx | symptoms |
| TIA | transient ischemic attack |
| TiTrATE | timing, triggers, and targeted examination |
| VCA | vestibulo-cochlear artery |
| VISTA | volume isotropic turbo spin-echo acquisition |
References
- Newman-Toker, D.E.; Della Santina, C.C.; Blitz, A.M. Vertigo and hearing loss. Handb. Clin. Neurol. 2016, 136, 905–921. [Google Scholar] [CrossRef] [PubMed]
- Newman-Toker, D.E.; Hsieh, Y.H.; Camargo, C.A., Jr.; Pelletier, A.J.; Butchy, G.T.; Edlow, J.A. Spectrum of dizziness visits to US emergency departments: Cross-sectional analysis from a nationally representative sample. Mayo Clin. Proc. 2008, 83, 765–775. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Cannon, L.M.; Stofferahn, M.E.; Rothman, R.E.; Hsieh, Y.H.; Zee, D.S. Imprecision in patient reports of dizziness symptom quality: A cross-sectional study conducted in an acute care setting. Mayo Clin. Proc. 2007, 82, 1329–1340. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.S.; Mak, P.S.; Manley, K.V.; Lam, J.M.; Tsang, A.Y.; Chan, H.M.; Rainer, T.H.; Graham, C.A. Predictors of important neurological causes of dizziness among patients presenting to the emergency department. Emerg. Med. J. 2010, 27, 517–521. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Edlow, J.A. TiTrATE: A Novel, Evidence-Based Approach to Diagnosing Acute Dizziness and Vertigo. Neurol. Clin. 2015, 33, 577–599, viii. [Google Scholar] [CrossRef]
- Kim, J.S.; Newman-Toker, D.E.; Kerber, K.A.; Jahn, K.; Bertholon, P.; Waterston, J.; Lee, H.; Bisdorff, A.; Strupp, M. Vascular vertigo and dizziness: Diagnostic criteria. J. Vestib. Res. 2022, 32, 205–222. [Google Scholar] [CrossRef]
- ICD-11 (Mortality and Morbidity Statistics). Available online: https://icd.who.int/dev11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f1462112221 (accessed on 20 November 2024).
- Tarnutzer, A.A.; Berkowitz, A.L.; Robinson, K.A.; Hsieh, Y.H.; Newman-Toker, D.E. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ 2011, 183, E571–E592. [Google Scholar] [CrossRef]
- Tarnutzer, A.A.; Gold, D.; Wang, Z.; Robinson, K.A.; Kattah, J.C.; Mantokoudis, G.; Saber Tehrani, A.S.; Zee, D.S.; Edlow, J.A.; Newman-Toker, D.E. Impact of Clinician Training Background and Stroke Location on Bedside Diagnostic Test Accuracy in the Acute Vestibular Syndrome—A Meta-Analysis. Ann. Neurol. 2023, 94, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kattah, J.C.; Talkad, A.V.; Wang, D.Z.; Hsieh, Y.H.; Newman-Toker, D.E. HINTS to diagnose stroke in the acute vestibular syndrome: Three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009, 40, 3504–3510. [Google Scholar] [CrossRef]
- Newman-Toker, D.E.; Kerber, K.A.; Hsieh, Y.H.; Pula, J.H.; Omron, R.; Saber Tehrani, A.S.; Mantokoudis, G.; Hanley, D.F.; Zee, D.S.; Kattah, J.C. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad. Emerg. Med. 2013, 20, 986–996. [Google Scholar] [CrossRef]
- Chandrasekhar, S.S.; Tsai Do, B.S.; Schwartz, S.R.; Bontempo, L.J.; Faucett, E.A.; Finestone, S.A.; Hollingsworth, D.B.; Kelley, D.M.; Kmucha, S.T.; Moonis, G.; et al. Clinical Practice Guideline: Sudden Hearing Loss (Update). Otolaryngol. Head Neck Surg. 2019, 161, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.K.; Lin, J.R.; Atashband, S.; Irvine, R.A.; Westerberg, B.D. Systematic review of the evidence for the etiology of adult sudden sensorineural hearing loss. Laryngoscope 2010, 120, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Baloh, R.W. Sudden deafness in vertebrobasilar ischemia: Clinical features, vascular topographical patterns and long-term outcome. J. Neurol. Sci. 2005, 228, 99–104. [Google Scholar] [CrossRef]
- Tsuzuki, N.; Wasano, K. Idiopathic sudden sensorineural hearing loss: A review focused on the contribution of vascular pathologies. Auris Nasus Larynx 2024, 51, 747–754. [Google Scholar] [CrossRef]
- Mei, X.; Atturo, F.; Wadin, K.; Larsson, S.; Agrawal, S.; Ladak, H.M.; Li, H.; Rask-Andersen, H. Human inner ear blood supply revisited: The Uppsala collection of temporal bone-an international resource of education and collaboration. Ups. J. Med. Sci. 2018, 123, 131–142. [Google Scholar] [CrossRef]
- Mazzoni, A. The vascular anatomy of the vestibular labyrinth in man. Acta Otolaryngol. Suppl. 1990, 472, 1–83. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, H. Inner ear dysfunction due to vertebrobasilar ischemic stroke. Semin. Neurol. 2009, 29, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Francis, D.A.; Bronstein, A.M.; Rudge, P.; du Boulay, E.P. The site of brainstem lesions causing semicircular canal paresis: An MRI study. J. Neurol. Neurosurg. Psychiatry 1992, 55, 446–449. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.M.; Schuknecht, B.; Tarnutzer, A.A. Vestibular and Ocular Motor Properties in Lateral Medullary Stroke Critically Depend on the Level of the Medullary Lesion. Front. Neurol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Pickles, J.O. Auditory pathways: Anatomy and physiology. Handb. Clin. Neurol. 2015, 129, 3–25. [Google Scholar] [CrossRef]
- Lownie, S.P.; Parnes, L.S. Isolated vestibulocochlear dysfunction of central or peripheral vascular origin. Laryngoscope 1991, 101, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Drulovic, B.; Ribaric-Jankes, K.; Kostic, V.S.; Sternic, N. Sudden hearing loss as the initial monosymptom of multiple sclerosis. Neurology 1993, 43, 2703–2705. [Google Scholar] [CrossRef]
- Doyle, K.J.; Fowler, C.; Starr, A. Audiologic findings in unilateral deafness resulting from contralateral pontine infarct. Otolaryngol. Head Neck Surg. 1996, 114, 482–486. [Google Scholar] [CrossRef][Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, Y.W. Auditory disturbance as a prodrome of anterior inferior cerebellar artery infarction. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1644–1648. [Google Scholar] [CrossRef]
- Lee, H.; Sohn, S.I.; Jung, D.K.; Cho, Y.W.; Lim, J.G.; Yi, S.D.; Lee, S.R.; Sohn, C.H.; Baloh, R.W. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke 2002, 33, 2807–2812. [Google Scholar] [CrossRef]
- Lee, H.; Whitman, G.T.; Lim, J.G.; Lee, S.D.; Park, Y.C. Bilateral sudden deafness as a prodrome of anterior inferior cerebellar artery infarction. Arch. Neurol. 2001, 58, 1287–1289. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Lee, B.C.; Hong, J.H.; Yeo, C.K.; Yi, H.A.; Lee, H. Long-term prognosis for hearing recovery in stroke patients presenting vertigo and acute hearing loss. J. Neurol. Sci. 2014, 339, 176–182. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Chung, E.J.; Yi, H.A.; Chung, I.S.; Lee, S.R.; Shin, J.Y. Infarction in the territory of anterior inferior cerebellar artery: Spectrum of audiovestibular loss. Stroke 2009, 40, 3745–3751. [Google Scholar] [CrossRef]
- Aimoni, C.; Bianchini, C.; Borin, M.; Ciorba, A.; Fellin, R.; Martini, A.; Scanelli, G.; Volpato, S. Diabetes, cardiovascular risk factors and idiopathic sudden sensorineural hearing loss: A case-control study. Audiol. Neurootol. 2010, 15, 111–115. [Google Scholar] [CrossRef]
- Berrettini, S.; Seccia, V.; Fortunato, S.; Forli, F.; Bruschini, L.; Piaggi, P.; Canapicchi, R. Analysis of the 3-dimensional fluid-attenuated inversion-recovery (3D-FLAIR) sequence in idiopathic sudden sensorineural hearing loss. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Blanco Pareja, M.; Liano Esteso, G.; Suarez-Vega, V.; Manrique-Huarte, R.; Dominguez, P.; Perez-Fernandez, N. Congruence and incongruence on the radiological and functional examination of inner ear hemorrhage. Acta Otolaryngol. 2023, 143, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, G.; Cianfoni, A.; Agostino, S.; Scipione, S.; Tartaglione, T.; Galli, J.; Colosimo, C. Magnetic resonance imaging findings in sudden sensorineural hearing loss. J. Otolaryngol. 2006, 35, 310–316. [Google Scholar] [CrossRef]
- Chen, K.; Wen, L.; Zong, L.; Liu, M.; Sun, J.; Wu, X. Audiological outcomes in sudden sensorineural hearing loss with presumed inner ear hemorrhage. Am. J. Otolaryngol. 2019, 40, 274–278. [Google Scholar] [CrossRef]
- Chen, X.H.; Zeng, C.J.; Fang, Z.M.; Zhang, R.; Cheng, J.M.; Lin, C. The Natural History of Labyrinthine Hemorrhage in Patients With Sudden Sensorineural Hearing Loss. Ear Nose Throat J. 2019, 98, E13–E20. [Google Scholar] [CrossRef]
- Chen, K.; Sun, J.; Huang, B.; Liang, Y.; Liu, M.; Wu, X. Labyrinthine lesions in presumed inner ear hemorrhage-related sudden deafness. Am. J. Otolaryngol. 2022, 43, 103331. [Google Scholar] [CrossRef]
- Cho, J.; Cheon, H.; Park, J.H.; Lee, H.J.; Kim, H.J.; Choi, H.G.; Koo, J.W.; Hong, S.K. Sudden sensorineural hearing loss associated with inner ear lesions detected by magnetic resonance imaging. PLoS ONE 2017, 12, e0186038. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.S.; Jia, X.; Ma, X.; Bi, J.; Shu, Q. Diagnostic value of 3D-FLAIR MRI in children with sudden deafness caused by inner ear hemorrhage. World J. Pediatr. Surg. 2021, 4, e000280. [Google Scholar] [CrossRef]
- Gerace, C.; Pianura, C. Sudden deafness without vertigo as a sole manifestation of AICA infarct. Neurol. Sci. 2008, 29, 371–372. [Google Scholar] [CrossRef]
- Jrad, M.; Zlitni, H.; Boumediene, M.; Nasr, A.B.; Bouzrara, M. Intracochlear Hemorrhage: A Rare Cause of Sudden Sensorineural Hearing Loss. Case Rep. Radiol. 2021, 2021, 1072047. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, D.W.; Kim, T.Y.; Lee, S.; Lee, Y.J.; Lee, J.Y.; Lee, S.H.; Chung, J.H. Characteristic MR findings suggesting presumed labyrinthine hemorrhage. Acta Otolaryngol. 2017, 137, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, Y.A.; Park, S.M.; Kong, T.H.; Park, S.Y.; Bong, J.P.; Park, D.J.; Seo, Y.J. Clinical Features and Prognosis of Sudden Sensorineural Hearing Loss Secondary to Intralabyrinthine Hemorrhage. J. Audiol. Otol. 2016, 20, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Martines, F.; Dispenza, F.; Gagliardo, C.; Martines, E.; Bentivegna, D. Sudden sensorineural hearing loss as prodromal symptom of anterior inferior cerebellar artery infarction. ORL J. Otorhinolaryngol. Relat. Spec. 2011, 73, 137–140. [Google Scholar] [CrossRef]
- Min, S.; Wang, J.; Zhao, H.; Chi, F.L.; Gao, N. MRI evidence of inner ear hemorrhage in prognostic assessment of sudden sensorineural hearing loss. Am. J. Otolaryngol. 2025, 46, 104620. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Jeong, S.W.; Lee, J.W.; Han, S.J. A Case of Sudden Deafness with Intralabyrinthine Hemorrhage Intralabyrinthine Hemorrhage and Sudden Deafness. J. Audiol. Otol. 2015, 19, 178–181. [Google Scholar] [CrossRef]
- Poh, A.C.; Tan, T.Y. Sudden deafness due to intralabyrinthine haemorrhage: A possible rare late complication of head and neck irradiation. Ann. Acad. Med. Singap. 2007, 36, 78–82. [Google Scholar] [CrossRef]
- Todic, J.; Guinand, N.; Lenoir, V.; Senn, P.; Becker, M. Diagnostic value and prognostic significance of MRI findings in sudden sensorineural hearing loss. Laryngoscope Investig. Otolaryngol. 2022, 7, 1575–1583. [Google Scholar] [CrossRef]
- Risoud, M.; Toulemonde, P.; Beck, C.; Charley, Q.; Suzzoni, E.; Vincent, C.; Dubrulle, F. MRI-confirmed cochlear artery infarct clinically diagnosed in a patient with sickle cell disease: A case report. Eur. Arch. Otorhinolaryngol. 2024, 281, 6699–6703. [Google Scholar] [CrossRef]
- Wang, M.; Hu, N.; Wang, Y.; Sun, X.; Fan, Z.; Wang, H. Clinical Value of 3D-FLAIR MRI in Idiopathic Sudden Sensorineural Hearing Loss. ACS Chem. Neurosci. 2022, 13, 151–157. [Google Scholar] [CrossRef]
- Yoshida, T.; Sugiura, M.; Naganawa, S.; Teranishi, M.; Nakata, S.; Nakashima, T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope 2008, 118, 1433–1437. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, H.; Liu, G.; Liu, J.; Mo, J.J.; Zhao, X.; Ju, Y. Early detection of stroke at the sudden sensorineural hearing loss stage. Front. Neurol. 2023, 14, 1293102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Ou, Y.; Fu, J.; Zhang, Y.; Xiong, H.; Xu, Y. A comparison of inner ear imaging features at different time points of sudden sensorineural hearing loss with three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging. Eur. Arch. Otorhinolaryngol. 2015, 272, 2659–2665. [Google Scholar] [CrossRef] [PubMed]
- Ryu, I.S.; Yoon, T.H.; Ahn, J.H.; Kang, W.S.; Choi, B.S.; Lee, J.H.; Shim, M.J. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging in sudden sensorineural hearing loss: Correlations with audiologic and vestibular testing. Otol. Neurotol. 2011, 32, 1205–1209. [Google Scholar] [CrossRef]
- Gattringer, T.; Enzinger, C.; Birner, A.; Wunsch, G.; Niederkorn, K.; Walch, C.; Fazekas, F. Acute unilateral hearing loss as an early symptom of lateral cerebral sinus venous thrombosis. Arch. Neurol. 2012, 69, 1508–1511. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kee, H.J.; Park, J.H.; Kim, H.J.; Kim, J.S. Combined peripheral and central vestibulopathy. J. Vestib. Res. 2014, 24, 443–451. [Google Scholar] [CrossRef]
- El Bouhmadi, K.; Darouich, S.; Youbi, M.; Anajar, S.; Essaadi, M.; Snoussi, K.; Hajjij, A. A case report of labyrinthine infarction: A ‘central’ cause of vertigo with ‘peripheral’ presentation. Ann. Med. Surg. 2024, 86, 6788–6793. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.J.; Koo, J.W.; Kim, J.S. Superior divisional vestibular paresis in anterior inferior cerebellar artery infarction. J. Neurol. Sci. 2009, 285, 250–253. [Google Scholar] [CrossRef]
- Martins, A.I.; Figueiredo, C.; Melancia, D.; Jorge, A.; Amorim, A.M.; Pereira, D.; Nunes, C.; Silva, F.; Lemos, J. Labyrinthine haemorrhage secondary to cerebral venous thrombosis. Eur. J. Neurol. 2021, 28, 4258–4260. [Google Scholar] [CrossRef]
- Murakami, T.; Nakayasu, H.; Doi, M.; Fukada, Y.; Hayashi, M.; Suzuki, T.; Takeuchi, Y.; Nakashima, K. Anterior and posterior inferior cerebellar artery infarction with sudden deafness and vertigo. J. Clin. Neurosci. 2006, 13, 1051–1054. [Google Scholar] [CrossRef]
- Nam, H.W.; Yoo, D.; Lee, S.U.; Choi, J.Y.; Yu, S.; Kim, J.S. Pearls & Oy-sters: Labyrinthine Infarction Mimicking Vestibular Neuritis. Neurology 2021, 97, 787–790. [Google Scholar] [CrossRef]
- Aiba, Y.; Sakakibara, R.; Yamaguchi, T.; Tateno, F. Inner-Ear Symptom May Herald Basilar Artery Occlusion. Case Rep. Neurol. 2021, 13, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Anizar Rodriguez, C.B.; Mendoza Ugalde, D.M.; Garcia-Tecpa, R.A. Case Report: Mixed-Cause Vertigo and Sudden Sensorineural Hearing Loss as Presentations of Vertebrobasilar Dolichoectasia. Cureus 2022, 14, e28136. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Martins, J.; Melo, P.; Ribeiro, C.; Barros, E. Recovery of cochlear and vestibular function after labyrinthine haemorrhage. Acta Med. Port. 2014, 27, 649–651. [Google Scholar] [CrossRef]
- Braverman, I.; Ben David, J.; Shupak, A. MTHFR polymorphism: Associated intralabyrinthine hemorrhage. Otolaryngol. Head Neck Surg. 2009, 141, 541–542. [Google Scholar] [CrossRef]
- Byun, S.; Lee, J.Y.; Kim, B.G.; Hong, H.S. Acute vertigo and sensorineural hearing loss from infarction of the vestibulocochlear nerve: A case report. Medicine 2018, 97, e12777. [Google Scholar] [CrossRef]
- Castellucci, A.; Pepponi, E.; Bertellini, A.; Senesi, C.; Bettini, M.; Botti, C.; Martellucci, S.; Malara, P.; Delmonte, S.; Crocetta, F.M.; et al. Case Report: Filling Defect in Posterior Semicircular Canal on MRI With Balanced Steady-State Gradient-Echo Sequences After Labyrinthine Ischemia in the Common Cochlear Artery Territory as an Early Sign of Fibrosis. Front. Neurol. 2020, 11, 608838. [Google Scholar] [CrossRef]
- Cervantes, S.S.; Barrs, D.M. Sudden Sensorineural Hearing Loss Associated with Intralabyrinthine Hemorrhage. Otol. Neurotol. 2015, 36, e134–e135. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Y.; Zhan, T. Anterior inferior cerebellar artery occlusion accompanied by hemorheology-documented increased blood viscosity: A case report. J. Int. Med. Res. 2023, 51, 3000605231169435. [Google Scholar] [CrossRef]
- Chern, A.; Famuyide, A.O.; Moonis, G.; Lalwani, A.K. Bilateral Sudden Sensorineural Hearing Loss and Intralabyrinthine Hemorrhage in a Patient With COVID-19. Otol. Neurotol. 2021, 42, e10–e14. [Google Scholar] [CrossRef]
- Harrison, P.; Blazak, J.; Richmond, J.; Fraser-Kirk, K.; Hoffmann, A.; Collins, G.; Tsang, B.K. Sudden unilateral audiovestibular loss due to acute labyrinthine haemorrhage can be missed on early MRI brain sequences: Case report. BMJ Neurol. Open 2024, 6, e000563. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, K.H.; Lee, H. Isolated labyrinthine infarction as a harbinger of anterior inferior cerebellar artery territory infarction with normal diffusion-weighted brain MRI. J. Neurol. Sci. 2009, 278, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lopez, I.; DiPatre, P.L.; Liu, F.; Ishiyama, A.; Baloh, R.W. Internal auditory artery infarction: Clinicopathologic correlation. Neurology 1999, 52, 40–44. [Google Scholar] [CrossRef]
- Kong, J.; Lee, S.U.; Park, E.; Kim, J.S. Labyrinthine Infarction Documented on Magnetic Resonance Imaging. Stroke 2024, 55, e277–e280. [Google Scholar] [CrossRef]
- Kothari, M.; Knopp, E.; Jonas, S.; Levine, D. Presumed vestibular hemorrhage secondary to warfarin. Neuroradiology 1995, 37, 324–325. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, B.H.; Baloh, R.W. Sudden deafness with vertigo as a sole manifestation of anterior inferior cerebellar artery infarction. J. Neurol. Sci. 2004, 222, 105–107. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.J.; Koo, J.W.; Kim, J.S. Progression of acute cochleovestibulopathy into anterior inferior cerebellar artery infarction. J. Neurol. Sci. 2009, 278, 119–122. [Google Scholar] [CrossRef]
- Meunier, A.; Clavel, P.; Aubry, K.; Lerat, J. A sudden bilateral hearing loss caused by inner ear hemorrhage. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Ishihara, S.; Iwano, S.; Sone, M.; Nakashima, T. Detection of presumed hemorrhage in the ampullar endolymph of the semicircular canal: A case report. Magn. Reson. Med. Sci. 2009, 8, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Nagaratnam, N.; Mak, J.; Phan, T.A.; Kalouche, H. Sudden permanent hearing loss following anterior inferior cerebellar artery infarction. Int. J. Clin. Pract. 2002, 56, 153–154. [Google Scholar] [CrossRef]
- Nicoucar, K.; Sakbani, K.; Vukanovic, S.; Guyot, J.P. Intralabyrinthine haemorrhage following cocaine consumption. Acta Otolaryngol. 2005, 125, 899–901. [Google Scholar] [CrossRef]
- Okada, T.; Makimoto, K.; Yoshii, R.; Yoshimoto, K.; Moinuddin, F.M.; Yamashita, M.; Arita, K. Dissecting aneurysm of the anterior inferior cerebellar artery in the internal auditory canal presenting with deafness without hemorrhage: A case report and literature review. Surg. Neurol. Int. 2022, 13, 88. [Google Scholar] [CrossRef]
- Ori, M.; Faralli, M.; Ricci, G. Cochleovestibular Transient Ischemic Attack as a Manifestation of Patent Foramen Ovale. J. Int. Adv. Otol. 2017, 13, 422–425. [Google Scholar] [CrossRef]
- Pogson, J.M.; Taylor, R.L.; Young, A.S.; McGarvie, L.A.; Flanagan, S.; Halmagyi, G.M.; Welgampola, M.S. Vertigo with sudden hearing loss: Audio-vestibular characteristics. J. Neurol. 2016, 263, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Rosado, W.M., Jr.; Palacios, E. Sudden onset of sensorineural hearing loss secondary to intralabyrinthine hemorrhage: MRI findings. Ear Nose Throat J. 2008, 87, 130–131. [Google Scholar] [CrossRef]
- Shinohara, S.; Yamamoto, E.; Saiwai, S.; Tsuji, J.; Muneta, Y.; Tanabe, M.; Sakamoto, T.; Kim, T. Clinical features of sudden hearing loss associated with a high signal in the labyrinth on unenhanced T1-weighted magnetic resonance imaging. Eur. Arch. Otorhinolaryngol. 2000, 257, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Naganawa, S.; Teranishi, M.; Sato, E.; Kojima, S.; Nakashima, T. Inner ear hemorrhage in systemic lupus erythematosus. Laryngoscope 2006, 116, 826–828. [Google Scholar] [CrossRef]
- Vakkalanka, S.; Ey, E.; Goldenberg, R.A. Inner ear hemorrhage and sudden sensorineural hearing loss. Am. J. Otol. 2000, 21, 764–765. [Google Scholar] [PubMed]
- Valente, P.; Pinto, I.; Aguiar, C.; Castro, E.; Conde, A.; Larangeiro, J. Acute vestibular syndrome and hearing loss mimicking labyrinthitis as initial presentation of multiple sclerosis. Int. J. Pediatr. Otorhinolaryngol. 2020, 134, 110048. [Google Scholar] [CrossRef]
- Vivas, E.X.; Panella, N.J.; Baugnon, K.L. Spontaneous Labyrinthine Hemorrhage: A Case Series. Otolaryngol. Head Neck Surg. 2018, 159, 908–913. [Google Scholar] [CrossRef]
- Whitehead, R.E.; MacDonald, C.B.; Melhem, E.R.; McMahon, L. Spontaneous labyrinthine hemorrhage in sickle cell disease. AJNR Am. J. Neuroradiol. 1998, 19, 1437–1440. [Google Scholar]
- Yoshida, T.; Ikemiyagi, Y.; Ikemiyagi, F.; Tamura, Y.; Suzuki, M.; Tsuyusaki, Y. Anterior Inferior cerebellar artery infarction misdiagnosed as inner ear disease. B-ENT 2016, 12, 143–147. [Google Scholar] [PubMed]
- Lee, S.J.; Lee, S.A.; Kim, B.G.; Hong, H.S.; Lee, J.Y.; Lee, J.D. Feasibility of magnetic resonance imaging in the differential diagnosis of isolated acute audiovestibular loss. J. Vestib. Res. 2018, 28, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Eliezer, M.; Toupet, M.; Guichard, J.P.; Kania, R.; Houdart, E.; Hautefort, C. Cochleovestibular artery syndrome: Consideration based on VHIT, VEMP, and inner ear MRI. J. Neurol. 2019, 266, 2327–2329. [Google Scholar] [CrossRef]
- Eliezer, M.; Verillaud, B.; Guichard, J.P.; Kania, R.; Toupet, M.; Herman, P.; Houdart, E.; Hautefort, C. Labyrinthine infarction caused by vertebral artery dissection: Consideration based on MRI. J. Neurol. 2019, 266, 2575–2577. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Park, J.H.; Kim, H.J.; Kim, J.S. Vestibulocochlear nerve infarction documented with diffusion-weighted MRI. J. Neurol. 2015, 262, 1363–1365. [Google Scholar] [CrossRef]
- Oas, J.G.; Baloh, R.W. Vertigo and the anterior inferior cerebellar artery syndrome. Neurology 1992, 42, 2274–2279. [Google Scholar] [CrossRef]
- Liqun, Z.; Park, K.H.; Kim, H.J.; Lee, S.U.; Choi, J.Y.; Kim, J.S. Acute Unilateral Audiovestibulopathy due to Embolic Labyrinthine Infarction. Front. Neurol. 2018, 9, 311. [Google Scholar] [CrossRef]
- Gempp, E.; Louge, P. Inner ear decompression sickness in scuba divers: A review of 115 cases. Eur. Arch. Otorhinolaryngol. 2013, 270, 1831–1837. [Google Scholar] [CrossRef]
- Wuthrich, M.; Wang, Z.; Martinez, C.M.; Carmona, S.; Mantokoudis, G.; Tarnutzer, A.A. Systematic review and meta-analysis of the diagnostic accuracy of spontaneous nystagmus patterns in acute vestibular syndrome. Front. Neurol. 2023, 14, 1208902. [Google Scholar] [CrossRef]
- Lee, S.U.; Tarnutzer, A.A. Usefulness of Nystagmus Patterns in Distinguishing Peripheral From Central Acute Vestibular Syndromes at the Bedside: A Critical Review. J. Clin. Neurol. 2025, 21, 161–172. [Google Scholar] [CrossRef]
- Korda, A.; Zamaro, E.; Wagner, F.; Morrison, M.; Caversaccio, M.D.; Sauter, T.C.; Schneider, E.; Mantokoudis, G. Acute vestibular syndrome: Is skew deviation a central sign? J. Neurol. 2022, 269, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Wang, Z.; Zalazar, G.; Carmona, S.; Kattah, J.; Tarnutzer, A.A. Systematic Review and Meta-Analysis of the Diagnostic Accuracy of a Graded Gait and Truncal Instability Rating in Acutely Dizzy and Ataxic Patients. Cerebellum 2024, 23, 2244–2256. [Google Scholar] [CrossRef]
- Lee, S.U.; Kim, H.J.; Choi, J.Y.; Kim, J.S. Abnormal vestibular-evoked myogenic potentials as an isolated finding of probable transient labyrinthine ischemia. J. Neurol. 2017, 264, 1523–1525. [Google Scholar] [CrossRef]
- Koohi, N.; Simonyan, S.; Joffily, L.; Simister, R.; Bamiou, D.E.; Kaski, D. Testing hearing in suspected stroke: A diagnostic opportunity. Lancet 2025, 405, 540–541. [Google Scholar] [CrossRef]
- Khosravipour, M.; Rajati, F. Sensorineural hearing loss and risk of stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 11021. [Google Scholar] [CrossRef] [PubMed]
- Yoon, R.G.; Choi, Y.; Park, H.J. Clinical usefulness of labyrinthine three-dimensional fluid-attenuated inversion recovery magnetic resonance images in idiopathic sudden sensorineural hearing loss. Curr. Opin. Otolaryngol. Head Neck Surg. 2021, 29, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Bae, Y.J.; Kim, H.J.; Choi, J.Y.; Song, J.J.; Choi, B.Y.; Choi, B.S.; Koo, J.W.; Kim, J.S. Intralabyrinthine Schwannoma: Distinct Features for Differential Diagnosis. Front. Neurol. 2019, 10, 750. [Google Scholar] [CrossRef]
- Edlow, J.A.; Tarnutzer, A.A. Intravenous thrombolysis in patients with acute dizziness or imbalance and suspected ischemic stroke-systematic review. J. Neurol. 2025, 272, 91. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
| n (Studies) | n (Patients) | |
|---|---|---|
| Study population | ||
| Acute cochlear symptoms (SSNHL) [14,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55] | 27 | 2689 |
| Acute vertigo/dizziness (AVS) [30,56,57,58,59,60,61] | 7 | 86 |
| SSNHL and AVS [62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98] | 37 | 121 |
| SSNHL or AVS [99] | 1 | 115 |
| All | 72 | 3011 |
| Imaging performed | ||
| MRI 3D-FIESTA [67,93] | 2 | 32 |
| MRI 3D-FLAIR acute [32,35,36,37,38,39,43,50,51,54,69,70,71,79,87] | 15 | 1761 |
| MRI 3D-FLAIR acute + 4 h post contrast [42,49,53,74,94,95] | 6 | 56 |
| MRI-DWI/T1/FLAIR [14,29,30,33,34,40,41,44,45,46,47,48,52,55,56,57,58,59,60,61,62,63,64,65,66,68,72,73,75,76,77,78,80,81,82,83,84,85,86,88,89,90,91,92,96,97,98] | 47 | 907 |
| None [31,99] | 2 | 256 |
| Vestibular symptoms (vertigo, dizziness) | ||
| Yes | 1383 | |
| No | 1094 | |
| Not reported | 534 | |
| Cochlear symptoms (hearing loss) | ||
| Yes | 2894 | |
| No | 115 | |
| Not reported | 0 | |
| Focal neurologic symptoms | ||
| Yes | 150 | |
| No | 2383 | |
| Not reported | 478 | |
| Subtle oculomotor findings | ||
| Yes | 163 | |
| No | 860 | |
| Not reported | 1988 | |
| MRI-based diagnosis | ||
| Peripheral, inner ear disease | ||
| Labyrinthine ischemia confirmed on MRI (3D-FLAIR 4h post contrast [49,74,94,95] or 3D-VISTA post contrast [98]) * | 5 | |
| Ischemia of the vestibulocochlear nerve [66,96] † | 2 | |
| Labyrinthine hemorrhage [32,33,34,35,36,37,38,39,41,42,43,45,46,47,48,50,59,64,65,68,70,71,75,78,79,81,85,86,87,88,90,91] | 437 | |
| Inflammatory inner ear disease [32,34,38,48,50,84] | 106 | |
| Inner ear trauma [84] | 1 | |
| Tumor (cerebellopontine angle) ‡ | 38 | |
| Other peripheral disorders § | 550 | |
| Idiopathic [31,32,34,38,39,43,45,47,48,50,51,53,54,93] | 1552 | |
| All peripheral cases | 2691 | |
| Central diseases | ||
| Ischemic stroke | ||
| AICA territory [14,29,30,40,44,56,58,61,66,69,72,76,77,80,84,92,93,96,97] | 123 | |
| PICA territory [14,98] | 7 | |
| Multiple territories | ||
| Combined AICA and other territories [14,29,60,62] | 60 | |
| Combined, no AICA involvement [14,29,30,98] | 14 | |
| Territory not reported [52,57] | 31 | |
| Other central disorders || | 88 | |
| All central cases | 323 | |
| Findings in cases with suspected labyrinthine ischemia | ||
| Confirmed ischemic stroke (vertebrobasilar central) [14,29,30,40,44,49,52,56,57,58,60,61,62,66,69,72,76,77,80,84,89,92,93,96,97,98] | 235 | |
| Audiovestibular symptoms potentially explained by MRI lesions ¶ [14,30,76,80,92,96,97] | 34 | |
| Prodromal audiovestibular signs or symptoms # [14,52,62,72,77,97] | 46 | |
| Recovery of audiovestibular symptoms | ||
| No recovery | 223 | |
| Slight recovery | 19 | |
| Partial recovery | 395 | |
| Full recovery | 37 | |
| Full recovery within 24 h (TIA) | 1 | |
| Partial recovery of vestibular sx, no recovery of cochlear sx. | 5 | |
| Not reported | 2331 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarnutzer, A.A.; Lee, S.-U.; Kim, J.-S.; Kaski, D. The Challenge of Diagnosing Labyrinthine Stroke—A Critical Review. Brain Sci. 2025, 15, 725. https://doi.org/10.3390/brainsci15070725
Tarnutzer AA, Lee S-U, Kim J-S, Kaski D. The Challenge of Diagnosing Labyrinthine Stroke—A Critical Review. Brain Sciences. 2025; 15(7):725. https://doi.org/10.3390/brainsci15070725
Chicago/Turabian StyleTarnutzer, Alexander A., Sun-Uk Lee, Ji-Soo Kim, and Diego Kaski. 2025. "The Challenge of Diagnosing Labyrinthine Stroke—A Critical Review" Brain Sciences 15, no. 7: 725. https://doi.org/10.3390/brainsci15070725
APA StyleTarnutzer, A. A., Lee, S.-U., Kim, J.-S., & Kaski, D. (2025). The Challenge of Diagnosing Labyrinthine Stroke—A Critical Review. Brain Sciences, 15(7), 725. https://doi.org/10.3390/brainsci15070725









