[18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Pain Intensity Scores
2.3. [18F]FDG PET-CT Scan Acquisition
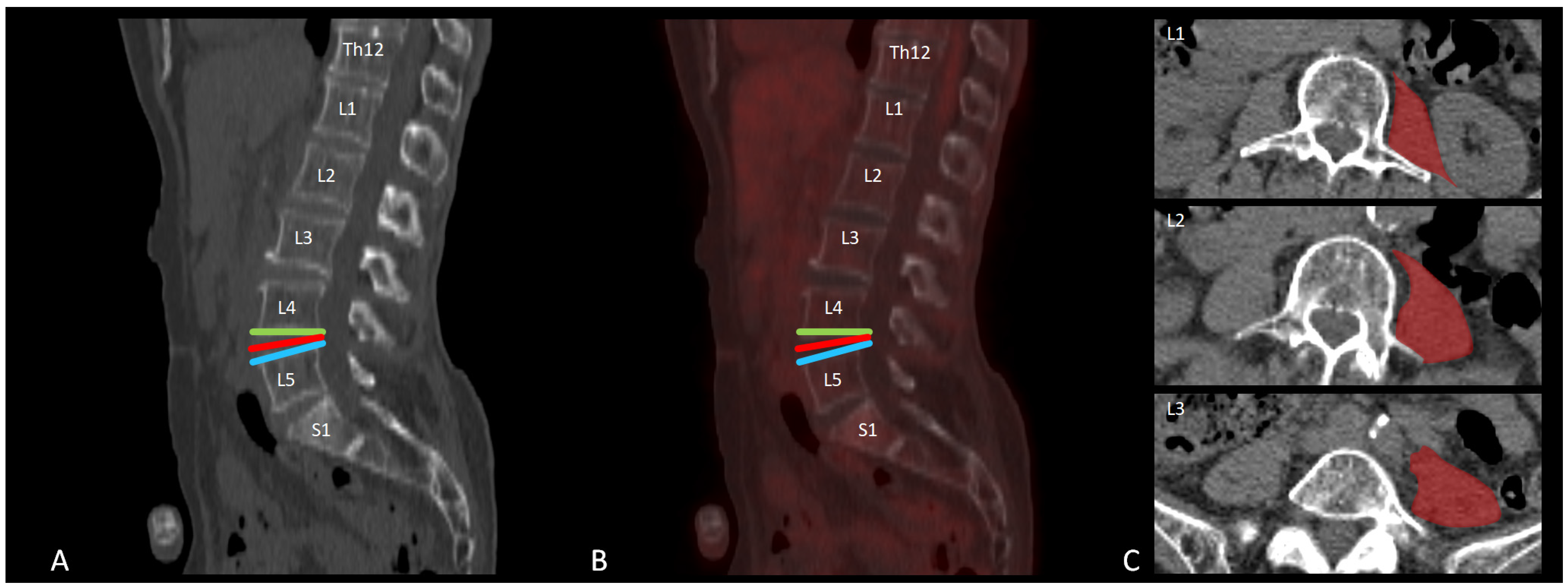
2.4. [18F]FDG PET-CT Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PSPS-T2 | Persistent Spinal Pain Syndrome Type 2 |
| [18F]FDG PET-CT | Fluorine-18 Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography |
| ROI | Region of interest |
| SUV | Standardized uptake value |
| NRS | Numerical Rating Scale |
| [99mTC]HDP SPECT-CT | Technetium-99m Hydroxymethylene Disphosphonate Single Photon Emission Computed Tomography-Computed Tomography |
| [18F]NaF PET-CT | Fluorine-18 Sodium Fluoride Positron Emission Tomography-Computed Tomography |
| SCS | Spinal cord stimulation |
| NaCl | Sodium Chloride |
| VOI | Volume of interest |
| FAP | Fibroblast-activation protein |
| [68Ga]FAPI PET-CT | Gallium-68 Fibroblast Activation Inhibitor Positron Emission Tomography-Computed Tomography |
References
- Clancy, C.; Quinn, A.; Wilson, F. The aetiologies of Failed Back Surgery Syndrome: A systematic review. J. Back Musculoskelet. Rehabil. 2017, 30, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Christelis, N.; Simpson, B.; Russo, M.; Stanton-Hicks, M.; Barolat, G.; Thomson, S.; Schug, S.; Baron, R.; Buchser, E.; Carr, D.B.; et al. Persistent Spinal Pain Syndrome: A Proposal for Failed Back Surgery Syndrome and ICD-11. Pain Med. 2021, 22, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Manca, A.; Eldabe, S.; Buchser, E.; Kumar, K.; Taylor, R.S. Relationship between health-related quality of life, pain, and functional disability in neuropathic pain patients with failed back surgery syndrome. Value Health 2010, 13, 95–102. [Google Scholar] [CrossRef]
- Thomson, S.; Jacques, L. Demographic characteristics of patients with severe neuropathic pain secondary to failed back surgery syndrome. Pain Pract. 2009, 9, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J. Failed back surgery syndrome-terminology, etiology, prevention, evaluation, and management: A narrative review. J. Yeungnam Med. Sci. 2024, 41, 166–178. [Google Scholar] [CrossRef]
- Henschke, N.; Maher, C.G.; Refshauge, K.M.; Herbert, R.D.; Cumming, R.G.; Bleasel, J.; York, J.; Das, A.; McAuley, J.H. Prevalence of and screening for serious spinal pathology in patients presenting to primary care settings with acute low back pain. Arthritis Rheum. 2009, 60, 3072–3080. [Google Scholar] [CrossRef]
- Dhagat, P.K.; Jain, M.; Singh, S.N.; Arora, S.; Leelakanth, K. Failed Back Surgery Syndrome: Evaluation with Magnetic Resonance Imaging. J. Clin. Diagn. Res. 2017, 11, Tc06–Tc09. [Google Scholar] [CrossRef]
- Hall, A.M.; Aubrey-Bassler, K.; Thorne, B.; Maher, C.G. Do not routinely offer imaging for uncomplicated low back pain. BMJ 2021, 372, n291. [Google Scholar] [CrossRef]
- Witkam, R.L.; Buckens, C.F.; van Goethem, J.W.M.; Vissers, K.C.P.; Henssen, D. The current role and future directions of imaging in failed back surgery syndrome patients: An educational review. Insights Imaging 2022, 13, 117. [Google Scholar] [CrossRef]
- Babińska, A.; Wawrzynek, W.; Czech, E.; Skupiński, J.; Szczygieł, J.; Łabuz-Roszak, B. No association between MRI changes in the lumbar spine and intensity of pain, quality of life, depressive and anxiety symptoms in patients with low back pain. Neurol. Neurochir. Pol. 2019, 53, 74–82. [Google Scholar] [CrossRef]
- Huang, B.-R.; Peng, B.-R.; Pan, L.-K.; Chen, C.-Y. Potential Usefulness of Single Photon Emission Computed Tomography/Computed Tomography in Management of Patients with Failed Back Surgery Syndrome. J. Med. Imaging Health Inform. 2019, 9, 1386–1392. [Google Scholar] [CrossRef]
- Pouldar, D.; Bakshian, S.; Matthews, R.; Rao, V.; Manzano, M.; Dardashti, S. Utility of 18F sodium fluoride PET/CT imaging in the evaluation of postoperative pain following surgical spine fusion. Musculoskelet. Surg. 2017, 101, 159–166. [Google Scholar] [CrossRef]
- Peters, M.; Willems, P.; Weijers, R.; Wierts, R.; Jutten, L.; Urbach, C.; Arts, C.; van Rhijn, L.; Brans, B. Pseudarthrosis after lumbar spinal fusion: The role of 18F-fluoride PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Head, J.; Mouchtouris, N.; Hines, K.; Shea, P.; Schmidt, R.; Hoelscher, C.; Stricsek, G.; Harrop, J.; Sharan, A. The Implications of Paraspinal Muscle Atrophy in Low Back Pain, Thoracolumbar Pathology, and Clinical Outcomes After Spine Surgery: A Review of the Literature. Global Spine J. 2020, 10, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The role of the lumbar multifidus in chronic low back pain: A review. PM&R 2010, 2, 142–146; quiz 141 p following 167. [Google Scholar] [CrossRef]
- Panjabi, M.M. The stabilizing system of the spine. Part II. Neutral zone and instability hypothesis. J. Spinal Disord. 1992, 5, 390–396; discussion 397. [Google Scholar] [CrossRef]
- Sheldon, B.L.; DiMarzio, M.; Chung, S.H.; Tram, J.; Khazen, O.; Staudt, M.D.; Bondoc, M.; Pilitsis, J.G. Association of Outcomes of Spinal Cord Stimulation for Chronic Low Back Pain and Psoas Measurements Based on Size of Iliopsoas Muscles. Neuromodulation 2022, 25, 121–127. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Glaudemans, A.W.; de Vries, E.F.; Galli, F.; Dierckx, R.A.; Slart, R.H.; Signore, A. The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin. Dev. Immunol. 2013, 2013, 623036. [Google Scholar] [CrossRef]
- Piri, R.; Nøddeskou-Fink, A.H.; Gerke, O.; Larsson, M.; Edenbrandt, L.; Enqvist, O.; Høilund-Carlsen, P.F.; Stochkendahl, M.J. PET/CT imaging of spinal inflammation and microcalcification in patients with low back pain: A pilot study on the quantification by artificial intelligence-based segmentation. Clin. Physiol. Funct. Imaging 2022, 42, 225–232. [Google Scholar] [CrossRef]
- Sharma, D.N.; Yerramneni, V.K.; Srivastava, M.K.; Yerragunta, T.; Akurati, S. Role of magnetic resonance imaging and 18-fluorodeoxyglucose positron emission tomography-computed tomography in identifying pain generators in patients with chronic low back pain. J. Craniovertebral Junction Spine 2023, 14, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Rieger, A.C.; Jafarvand, M.G.; Menon, N.; Farolfi, A.; Benz, M.R.; Calais, J. Non-oncologic incidental uptake on FAPI PET/CT imaging. Br. J. Radiol. 2023, 96, 20220463. [Google Scholar] [CrossRef] [PubMed]
- Lubina, Z.I.; Baranovic, S.; Karlak, I.; Novacic, K.; Potocki-Karacic, T.; Lovrić, D. The grading model for the assessment of the total amount of epidural fibrosis in postoperative lumbar spine. Eur. Spine J. 2013, 22, 892–897. [Google Scholar] [CrossRef]
- Guner, D.; Asik, I.; Ozgencil, G.E.; Peker, E.; Erden, M.I. The Correlation of Epidural Fibrosis with Epiduroscopic and Radiologic Imaging for Chronic Pain after Back Surgery. Pain Physician 2021, 24, E1219–E1226. [Google Scholar]
- Yee, A.; Lam, M.P.; Tam, V.; Chan, W.C.; Chu, I.K.; Cheah, K.S.; Cheung, K.M.; Chan, D. Fibrotic-like changes in degenerate human intervertebral discs revealed by quantitative proteomic analysis. Osteoarthr. Cartil. 2016, 24, 503–513. [Google Scholar] [CrossRef]
- Oegema, T.R., Jr.; Johnson, S.L.; Aguiar, D.J.; Ogilvie, J.W. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine (Phila Pa 1976) 2000, 25, 2742–2747. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Huang, G.S.; Tang, C.T.; Chang, W.C.; Hsu, Y.C. Case Report: Nerve Root Entrapment Due to Epidural Fibrosis in a Patient with Failed Back Surgery Syndrome: Value of 2-18F-Fluorodeoxyglucose Simultaneous Positron Emission Tomography-Magnetic Resonance Imaging. Front. Med. 2022, 9, 860545. [Google Scholar] [CrossRef]
- Ji, H.; Song, X.; Lv, X.; Shao, F.; Long, Y.; Song, Y.; Song, W.; Qiao, P.; Gai, Y.; Jiang, D.; et al. [68Ga]FAPI PET for Imaging and Treatment Monitoring in a Preclinical Model of Pulmonary Fibrosis: Comparison to [18F]FDG PET and CT. Pharmaceuticals 2024, 17, 726. [Google Scholar] [CrossRef]
- Mallio, C.A.; Russo, F.; Vadalà, G.; Papalia, R.; Pileri, M.; Mancuso, V.; Bernetti, C.; Volpecina, M.; Di Gennaro, G.; Beomonte Zobel, B.; et al. The importance of psoas muscle on low back pain: A single-center study on lumbar spine MRI. N. Am. Spine Soc. J. 2024, 18, 100326. [Google Scholar] [CrossRef]
- Goubert, D.; Oosterwijck, J.V.; Meeus, M.; Danneels, L. Structural Changes of Lumbar Muscles in Non-specific Low Back Pain: A Systematic Review. Pain Physician 2016, 19, E985–E1000. [Google Scholar]
- Seyedhoseinpoor, T.; Taghipour, M.; Dadgoo, M.; Sanjari, M.A.; Takamjani, I.E.; Kazemnejad, A.; Khoshamooz, Y.; Hides, J. Alteration of lumbar muscle morphology and composition in relation to low back pain: A systematic review and meta-analysis. Spine J. 2022, 22, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Clark, B.C.; Nalls, M.A.; Goodpaster, B.H.; Ploutz-Snyder, L.L.; Harris, T.B. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am. J. Clin. Nutr. 2007, 85, 377–384. [Google Scholar] [CrossRef]
- Pagano, A.F.; Brioche, T.; Arc-Chagnaud, C.; Demangel, R.; Chopard, A.; Py, G. Short-term disuse promotes fatty acid infiltration into skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 335–347. [Google Scholar] [CrossRef]
- Muellner, M.; Haffer, H.; Chiapparelli, E.; Dodo, Y.; Shue, J.; Tan, E.T.; Zhu, J.; Pumberger, M.; Sama, A.A.; Cammisa, F.P.; et al. Fat infiltration of the posterior paraspinal muscles is inversely associated with the fat infiltration of the psoas muscle: A potential compensatory mechanism in the lumbar spine. BMC Musculoskelet. Disord. 2023, 24, 846. [Google Scholar] [CrossRef]
- Özcan-Ekşi, E.E.; Ekşi, M.; Turgut, V.U.; Canbolat, Ç.; Pamir, M.N. Reciprocal relationship between multifidus and psoas at L4-L5 level in women with low back pain. Br. J. Neurosurg. 2021, 35, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A. Peripheral Neuroinflammation and Pain: How Acute Pain Becomes Chronic. Curr. Neuropharmacol. 2024, 22, 6–14. [Google Scholar] [CrossRef]
- Ji, R.R.; Nackley, A.; Huh, Y.; Terrando, N.; Maixner, W. Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 2018, 129, 343–366. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gong, Y.; Liu, J.; Guo, Y.; Tang, H.; Qin, S.; Zhao, Y.; Wang, S.; Xu, Z.; Chen, B. Peripheral and Central Pathological Mechanisms of Chronic Low Back Pain: A Narrative Review. J. Pain Res. 2021, 14, 1483–1494. [Google Scholar] [CrossRef]
- Rogers, A.H.; Farris, S.G. A meta-analysis of the associations of elements of the fear-avoidance model of chronic pain with negative affect, depression, anxiety, pain-related disability and pain intensity. Eur. J. Pain 2022, 26, 1611–1635. [Google Scholar] [CrossRef]
- Tsuji, T.; Matsudaira, K.; Sato, H.; Vietri, J. The impact of depression among chronic low back pain patients in Japan. BMC Musculoskelet. Disord. 2016, 17, 447. [Google Scholar] [CrossRef]
- Meints, S.M.; Edwards, R.R. Evaluating psychosocial contributions to chronic pain outcomes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 168–182. [Google Scholar] [CrossRef] [PubMed]
| Level | Patients | Controls | p-Value * | |
|---|---|---|---|---|
| Superior endplates | L1 | 0.55 (0.15) | 0.71 (0.38) | 0.266 |
| L2 | 0.56 (0.17) | 0.75 (0.31) | 0.131 | |
| L3 | 0.56 (0.20) | 0.78 (0.31) | 0.097 | |
| L4 | 0.55 (0.17) | 0.80 (0.29) | 0.048 | |
| L5 | 0.60 (0.19) | 0.87 (0.37) | 0.074 | |
| S1 | 0.57 (0.21) | 0.78 (0.20) | 0.037 | |
| Discus | L1–L2 | 0.40 (0.10) | 0.52 (0.21) | 0.152 |
| L2–L3 | 0.39 (0.20) | 0.47 (0.10) | 0.293 | |
| L3–L4 | 0.40 (0.24) | 0.55 (0.20) | 0.182 | |
| L4–L5 | 0.49 (0.26) | 0.71 (0.15) | 0.043 | |
| L5–S1 | 0.40 (0.14) | 0.75 (0.21) | <0.001 | |
| T12–L1 | 0.44 (0.11) | 0.61 (0.31) | 0.134 | |
| Psoas left | L3 | 0.58 (0.51) | 0.43 (0.14) | 0.414 |
| L4 | 0.55 (0.48) | 0.41 (0.15) | 0.414 | |
| L5 | 0.49 (0.39) | 0.41 (0.12) | 0.573 | |
| Psoas right | L3 | 0.60 (0.57) | 0.39 (0.11) | 0.301 |
| L4 | 0.55 (0.55) | 0.44 (0.13) | 0.547 | |
| L5 | 0.46 (0.34) | 0.57 (0.29) | 0.446 | |
| Posterior endplates | L1 | 0.59 (0.13) | 0.67 (0.29) | 0.451 |
| L2 | 0.57 (0.15) | 0.66 (0.23) | 0.331 | |
| L3 | 0.56 (0.12) | 0.78 (0.37) | 0.104 | |
| L4 | 0.58 (0.16) | 0.85 (0.30) | 0.029 | |
| L5 | 0.53 (0.14) | 0.79 (0.31) | 0.036 | |
| S1 | 0.59 (0.17) | 0.77 (0.36) | 0.195 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burmeister, L.S.; Witkam, R.L.; Vissers, K.C.P.; Gotthardt, M.; Henssen, D.J.H.A. [18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis. Brain Sci. 2025, 15, 724. https://doi.org/10.3390/brainsci15070724
Burmeister LS, Witkam RL, Vissers KCP, Gotthardt M, Henssen DJHA. [18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis. Brain Sciences. 2025; 15(7):724. https://doi.org/10.3390/brainsci15070724
Chicago/Turabian StyleBurmeister, Lara S., Richard L. Witkam, Kris C. P. Vissers, Martin Gotthardt, and Dylan J. H. A. Henssen. 2025. "[18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis" Brain Sciences 15, no. 7: 724. https://doi.org/10.3390/brainsci15070724
APA StyleBurmeister, L. S., Witkam, R. L., Vissers, K. C. P., Gotthardt, M., & Henssen, D. J. H. A. (2025). [18F]FDG PET-CT Imaging of the Low Back in Persistent Spinal Pain Syndrome Type 2: A Pilot Study Towards Improved Diagnosis. Brain Sciences, 15(7), 724. https://doi.org/10.3390/brainsci15070724







