Invasive and Non-Invasive Neuromodulation for the Treatment of Substance Use Disorders: A Review of Reviews
Abstract
1. Introduction
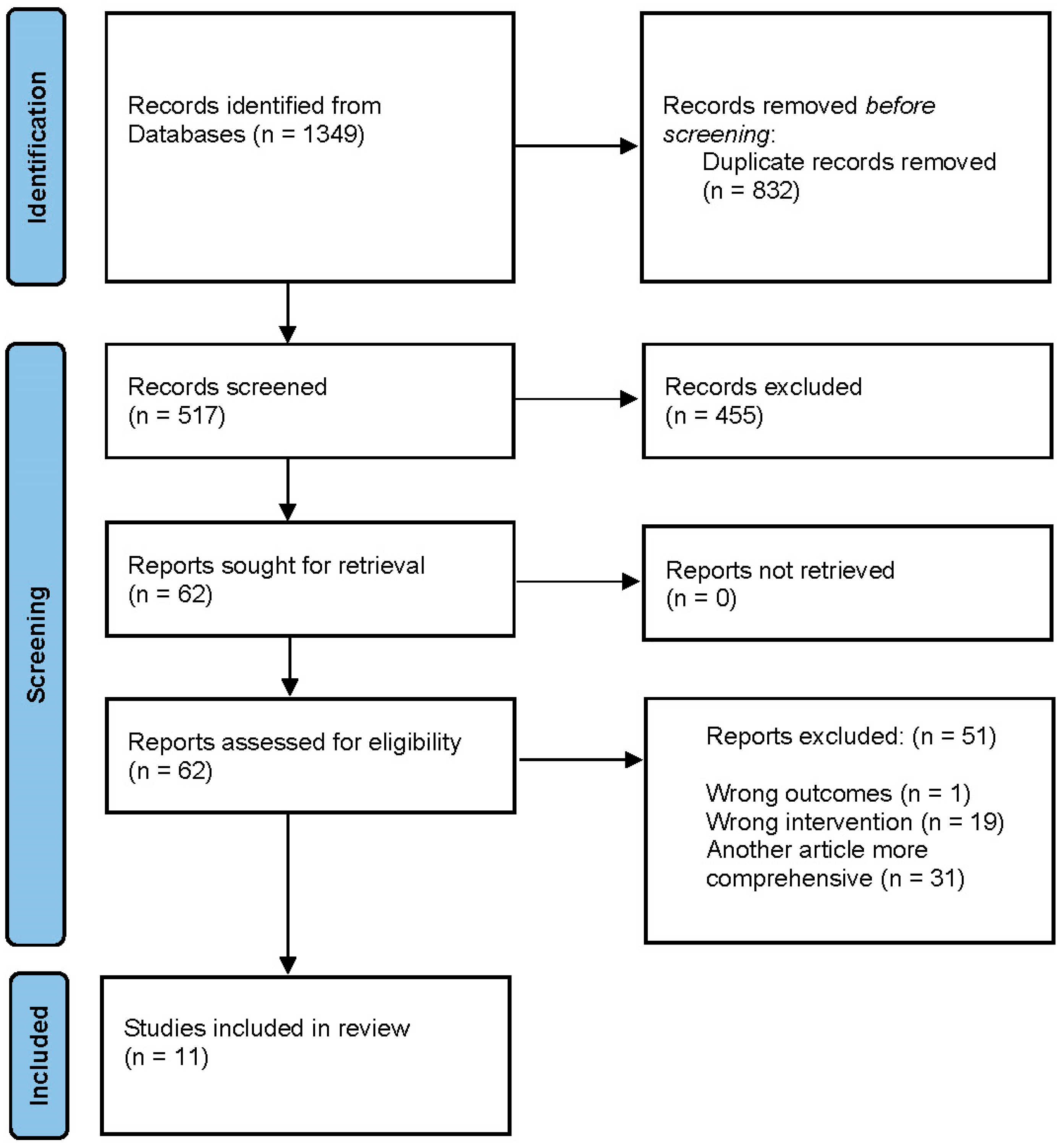
2. Materials and Methods
3. Results
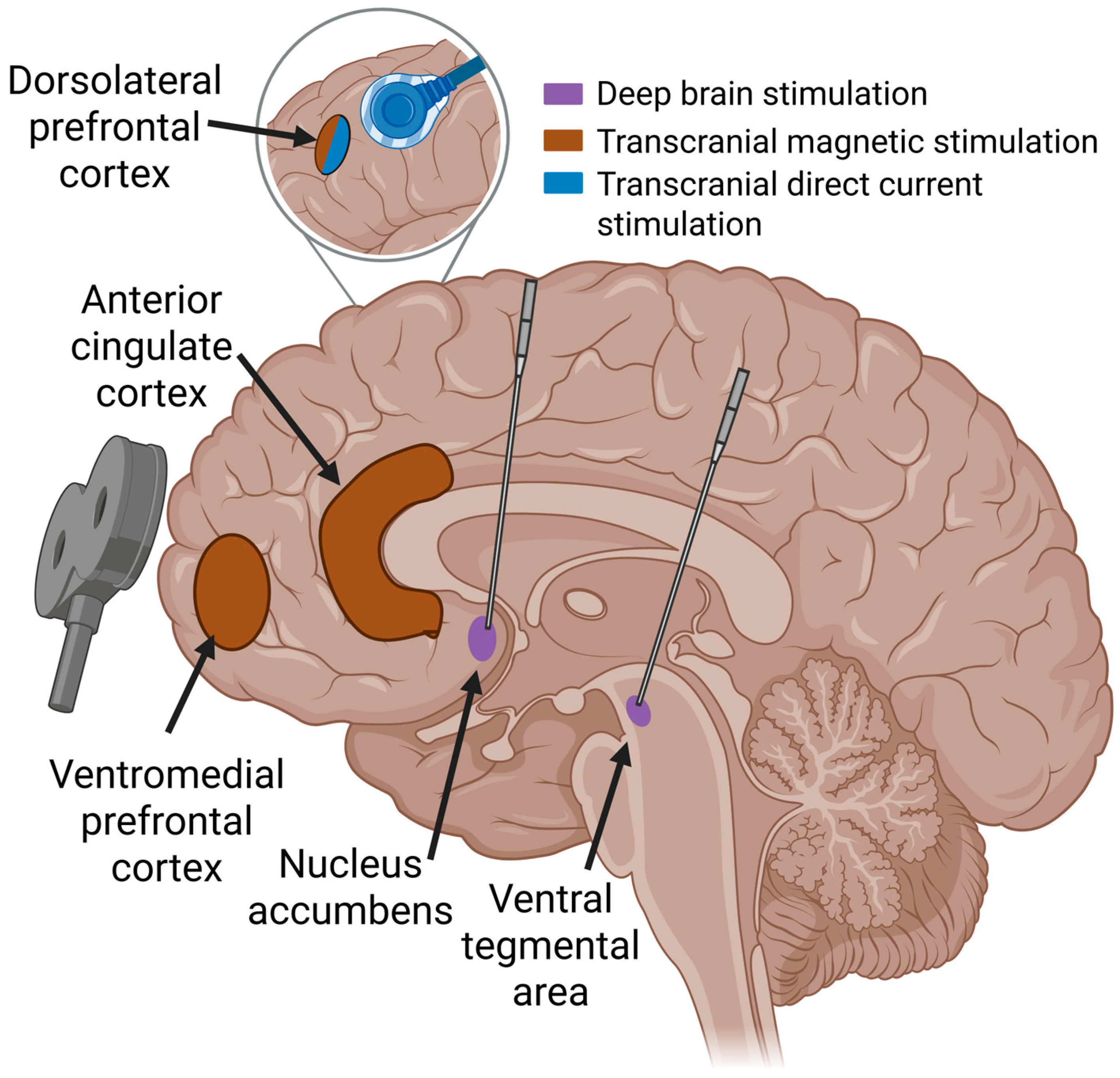
3.1. Non-Invasive Neuromodulation
3.2. Invasive Neuromodulation for SUD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endler, N.S. The Origins of Electroconvulsive Therapy (ECT). Convuls. Ther. 1988, 4, 5–23. [Google Scholar] [PubMed]
- Bormann, N.L.; Burson, J.R.; Burson, E.M.; McGinnis, M.; Karpyak, V.; Coombes, B.J.; Gold, M.; Oesterle, T.S. How Treatment-Refractory Addiction Is Defined: A Scoping Review. J. Addict. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.E.; Nagel, S.K. Addiction and Moralization: The Role of the Underlying Model of Addiction. Neuroethics 2017, 10, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic Advances from the Brain Disease Model of Addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Fowler, J.S.; Tomasi, D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 321–336. [Google Scholar] [CrossRef]
- Williams, N.R.; Taylor, J.J.; Kerns, S.; Short, E.B.; Kantor, E.M.; George, M.S. Interventional psychiatry: Why now? J. Clin. Psychiatry 2014, 75, 895–897. [Google Scholar] [CrossRef]
- Bormann, N.L.; Trapp, N.T.; Narayanan, N.S.; Boes, A.D. Developing Precision Invasive Neuromodulation for Psychiatry. J. Neuropsychiatry Clin. Neurosci. 2021, 33, 201–209. [Google Scholar] [CrossRef]
- Hyde, J.; Carr, H.; Kelley, N.; Seneviratne, R.; Reed, C.; Parlatini, V.; Garner, M.; Solmi, M.; Rosson, S.; Cortese, S.; et al. Efficacy of neurostimulation across mental disorders: Systematic review and meta-analysis of 208 randomized controlled trials. Mol. Psychiatry 2022, 27, 2709–2719. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
- Hallett, M. Transcranial magnetic stimulation: A primer. Neuron 2007, 55, 187–199. [Google Scholar] [CrossRef]
- Fitzgerald, P.B.; Fountain, S.; Daskalakis, Z.J. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 2006, 117, 2584–2596. [Google Scholar] [CrossRef]
- Amerio, A.; Baccino, C.; Breda, G.S.; Cortesi, D.; Spiezio, V.; Magnani, L.; De Berardis, D.; Conio, B.; Costanza, A.; De Paola, G.; et al. Effects of transcranial magnetic stimulation on cocaine addiction: A systematic review of randomized controlled trials. Psychiatry Res. 2023, 329, 115491. [Google Scholar] [CrossRef] [PubMed]
- Bormann, N.L.; Oesterle, T.S.; Arndt, S.; Karpyak, V.M.; Croarkin, P.E. Systematic review and meta-analysis: Combining transcranial magnetic stimulation or direct current stimulation with pharmacotherapy for treatment of substance use disorders. Am. J. Addict. 2024, 33, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, M.; Yu, Y.; Zhong, X.; Dai, S.; Dai, Y.; Jiang, C. Is Transcranial Direct Current Stimulation Effective for Cognitive Dysfunction in Substance Use Disorders? A Systematic Review. Brain Sci. 2024, 14, 754. [Google Scholar] [CrossRef]
- Sahaf, S.M.S.; Heydari Yazdi, A.S.; Ramezani, F.; Kamrani, M. Effectiveness of Transcranial-Direct Current Stimulation in Individuals with Methamphetamine Use Disorder: A Systematic Review and Meta-Analysis. Int. J. High. Risk Behav. Addict. 2024, 13, e146021. [Google Scholar] [CrossRef]
- Chan, Y.H.; Chang, H.M.; Lu, M.L.; Goh, K.K. Targeting cravings in substance addiction with transcranial direct current stimulation: Insights from a meta-analysis of sham-controlled trials. Psychiatry Res. 2024, 331, 115621. [Google Scholar] [CrossRef]
- Davidson, B.; Bhattacharya, A.; Sarica, C.; Darmani, G.; Raies, N.; Chen, R.; Lozano, A.M. Neuromodulation techniques—From non-invasive brain stimulation to deep brain stimulation. Neurotherapeutics 2024, 21, e00330. [Google Scholar] [CrossRef] [PubMed]
- Hariz, M.; Lees, A.J.; Blomstedt, Y.; Blomstedt, P. Serendipity and Observations in Functional Neurosurgery: From James Parkinson’s Stroke to Hamani’s & Lozano’s Flashbacks. Stereotact. Funct. Neurosurg. 2022, 100, 201–209. [Google Scholar] [CrossRef]
- dos Santos, P.L.M.; Curti, R.O.; da Silva, L.J. Neurosurgical Treatment for Drug Addiction: Systematic Review. Arq. Bras. Neurocir. 2020, 39, 116–124. [Google Scholar] [CrossRef]
- Hassan, O.; Phan, S.; Wiecks, N.; Joaquin, C.; Bondarenko, V. Outcomes of deep brain stimulation surgery for substance use disorder: A systematic review. Neurosurg. Rev. 2021, 44, 1967–1976. [Google Scholar] [CrossRef]
- Navarro, P.A.; Paranhos, T.; Lovo, E.; De Oliveira-Souza, R.; Gorgulho, A.A.; De Salles, A.; Lopez, W.O.C. Safety and Feasibility of Nucleus Accumbens Surgery for Drug Addiction: A Systematic Review. Neuromodulation 2022, 25, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, M.; Eskandari, K.; Sayehmiri, F.; Kuhn, J.; Haghparast, A. Deep brain stimulation for opioid use disorder: A systematic review of preclinical and clinical evidence. Brain Res. Bull. 2022, 187, 39–48. [Google Scholar] [CrossRef]
- Shaheen, N.; Shaheen, A.; Sarica, C.; Singh, A.; Zanaty, M.; Johari, K.; Yang, A.; Zesiewicz, T.; Dalm, B.; Bezchlibnyk, Y.; et al. Deep brain stimulation for substance use disorder: A systematic review and meta-analysis. Front. Psychiatry 2023, 14, 1231760. [Google Scholar] [CrossRef]
- Zammit Dimech, D.; Zammit Dimech, A.A.; Hughes, M.; Zrinzo, L. A systematic review of deep brain stimulation for substance use disorders. Transl. Psychiatry 2024, 14, 361. [Google Scholar] [CrossRef] [PubMed]
- Naish, K.R.; Vedelago, L.; MacKillop, J.; Amlung, M. Effects of neuromodulation on cognitive performance in individuals exhibiting addictive behaviors: A systematic review. Drug Alcohol. Depend. 2018, 192, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Razza, L.B.; Afonso Dos Santos, L.; Borrione, L.; Bellini, H.; Branco, L.C.; Cretaz, E.; Duarte, D.; Ferrao, Y.; Galhardoni, R.; Quevedo, J.; et al. Appraising the effectiveness of electrical and magnetic brain stimulation techniques in acute major depressive episodes: An umbrella review of meta-analyses of randomized controlled trials. Braz. J. Psychiatry 2021, 43, 514–524. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Moffa, A.H.; Fregni, F.; Palm, U.; Padberg, F.; Blumberger, D.M.; Daskalakis, Z.J.; Bennabi, D.; Haffen, E.; Alonzo, A.; et al. Transcranial direct current stimulation for acute major depressive episodes: Meta-analysis of individual patient data. Br. J. Psychiatry 2016, 208, 522–531. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Fenoy, A.J.; Carvalho, A.F.; Soares, J.C.; Quevedo, J. Deep brain stimulation for treatment-resistant depression: An integrative review of preclinical and clinical findings and translational implications. Mol. Psychiatry 2018, 23, 1094–1112. [Google Scholar] [CrossRef]
- Raviv, N.; Staudt, M.D.; Rock, A.K.; MacDonell, J.; Slyer, J.; Pilitsis, J.G. A Systematic Review of Deep Brain Stimulation Targets for Obsessive Compulsive Disorder. Neurosurgery 2020, 87, 1098–1110. [Google Scholar] [CrossRef]
- Bormann, N.L.; Weber, A.N.; Miskle, B.; Arndt, S.; Lynch, A.C. Recovery Capital Gains May Precede Craving Reduction in Opioid Use Disorder. Subst. Abuse Rehabil. 2023, 14, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.N.; Trebach, J.; Brenner, M.A.; Thomas, M.M.; Bormann, N.L. Managing Opioid Withdrawal Symptoms During the Fentanyl Crisis: A Review. Subst. Abuse Rehabil. 2024, 15, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.R.; Moosa, S.; Dallapiazza, R.F.; Elias, W.J.; Lynch, W.J. Deep brain stimulation for the treatment of drug addiction. Neurosurg. Focus 2018, 45, E11. [Google Scholar] [CrossRef] [PubMed]
| Authors | Type of Study | Type of Neuromodulation | Target Location | Target Substance of Abuse | Findings |
|---|---|---|---|---|---|
| Amerio et al. [13] | Systematic review | rTMS | Left DLPFC | Cocaine | High-frequency rTMS significantly reduced self-reported cue-induced cocaine craving and impulsivity |
| Chan et al. [17] | Meta-analysis | tDCS | Right DLPFC | Alcohol, opioids, methamphetamine, cocaine, tobacco, cannabis | tDCS led to a moderate reduction in cravings for opioids, methamphetamine, cocaine, and tobacco |
| Sahaf et al. [16] | Systematic review and meta-analysis | tDCS | DLPFC | Methamphetamine | tDCS significantly reduced craving |
| Zhang et al. [15] | Systematic review | tDCS | DLPFC | Alcohol, nicotine, cocaine, methamphetamine, opioids, cannabis | tDCS can improve cognitive functions in SUD patients |
| Bormann et al. [14] | Systematic review and meta-analysis | TMS or tDCS | DLPFC | Opioids, tobacco | Combining TMS or tDCS with MAT significantly reduced craving-related measures compared to sham stimulation, with a Hedges’ g effect size of −0.42 (95% confidence interval: −0.73 to −0.11, p < 0.01). |
| Author | Substance Use Disorder | Inclusion/Exclusion Criteria | Intervention | Results |
|---|---|---|---|---|
| dos Santos (2020) [20] | Various—heroin (62%), alcohol (33%), cocaine (5%), others | Inclusion: DBS in patients with SUDs, with no restriction on gender, age, ethnicity, or substance Exclusion: DBS without SUD as main focus; discontinued clinical trials | High-frequency DBS to NAc ± additional stim to ALIC, VS/VS, BNST | Reduced craving in most patients. In total, 48% achieved abstinence; relapses typically occurred with reduced severity. Some mood and quality of life improvements. |
| Hassan (2020) [21] | Various—heroin (43%), alcohol (29%), nicotine (14%), cocaine, meth | Inclusion: Human studies targeting NAc Exclusion: follow-up less than 6 mos. | High-frequency DBS to NAc ± ALIC | Reduced cravings. Remission: 61% at 6 months, 53% at 1 year, 43% at 2 years; relapse common over time. |
| Navarro (2020) [22] | Various –Alcohol, opioids, nicotine | Inclusion: NAc DBS or lesioning Exclusion: articles with dual NAc/ALIC DBS | High-frequency DBS to NAc vs. lesioning via RF ablation | Reduced cravings. Relapse: 38.4% with DBS and 39% for RF ablation; further studies needed |
| Fattahi (2022) [23] | Opioids | Inclusion: Studies on opioid-dependent individuals, both human and animal models | High-frequency DBS targeting NAc ± ALIC, VC/VS | Reduced opioid craving and consumption. Abstinence not consistently reported. Supports DBS as a promising alternative |
| Shaheen (2023) [24] | Various—Alcohol, heroin, tobacco | Inclusion: DBS for SUD Exclusion: did not include any scale of addiction treatment | HF DBS to NAc ± ALIC | Reduced cravings—59.6% clinical improvement; relapse rate 8%. Outcomes vary by age, substance type, and addiction duration |
| Dimech (2024) [25] | Various | Inclusion: DBS for SUD Exclusions: non-SUD | HF DBS to NAc ± ALIC | Reduced cravings. Relapse of 73.2%. Psychiatric symptom improvements noted. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oesterle, T.S.; Bormann, N.L.; Al-Soleiti, M.; Kung, S.; Singh, B.; McGinnis, M.T.; Correa da Costa, S.; Rummans, T.; Chauhan, M.; Rojas Cabrera, J.M.; et al. Invasive and Non-Invasive Neuromodulation for the Treatment of Substance Use Disorders: A Review of Reviews. Brain Sci. 2025, 15, 723. https://doi.org/10.3390/brainsci15070723
Oesterle TS, Bormann NL, Al-Soleiti M, Kung S, Singh B, McGinnis MT, Correa da Costa S, Rummans T, Chauhan M, Rojas Cabrera JM, et al. Invasive and Non-Invasive Neuromodulation for the Treatment of Substance Use Disorders: A Review of Reviews. Brain Sciences. 2025; 15(7):723. https://doi.org/10.3390/brainsci15070723
Chicago/Turabian StyleOesterle, Tyler S., Nicholas L. Bormann, Majd Al-Soleiti, Simon Kung, Balwinder Singh, Michele T. McGinnis, Sabrina Correa da Costa, Teresa Rummans, Mohit Chauhan, Juan M. Rojas Cabrera, and et al. 2025. "Invasive and Non-Invasive Neuromodulation for the Treatment of Substance Use Disorders: A Review of Reviews" Brain Sciences 15, no. 7: 723. https://doi.org/10.3390/brainsci15070723
APA StyleOesterle, T. S., Bormann, N. L., Al-Soleiti, M., Kung, S., Singh, B., McGinnis, M. T., Correa da Costa, S., Rummans, T., Chauhan, M., Rojas Cabrera, J. M., Vettleson-Trutza, S. A., Scheitler, K. M., Shin, H., Lee, K. H., & Gold, M. S. (2025). Invasive and Non-Invasive Neuromodulation for the Treatment of Substance Use Disorders: A Review of Reviews. Brain Sciences, 15(7), 723. https://doi.org/10.3390/brainsci15070723






