From Gamma Coherence to Theta-Phase Synchronization: Task-Dependent Interhemispheric Integration in Boundary-Free Multiple-Object Tracking
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Setup
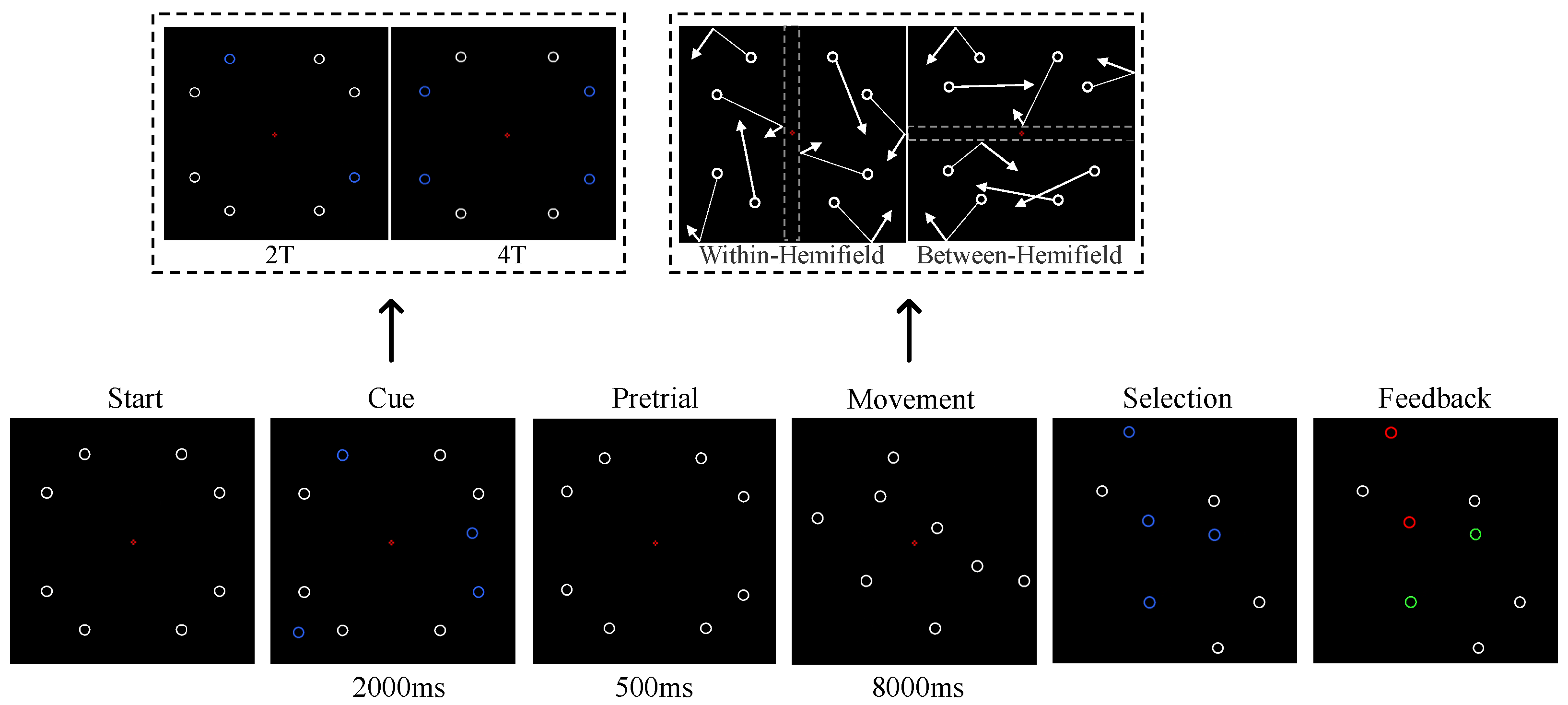
2.3. MOT Task
2.4. EEG Recording and Preprocessing
2.5. Functional Brain Network
2.5.1. Coherence
2.5.2. PLV
2.5.3. Network Properties
2.6. Statistical Analysis
3. Results
3.1. Behavior Performance
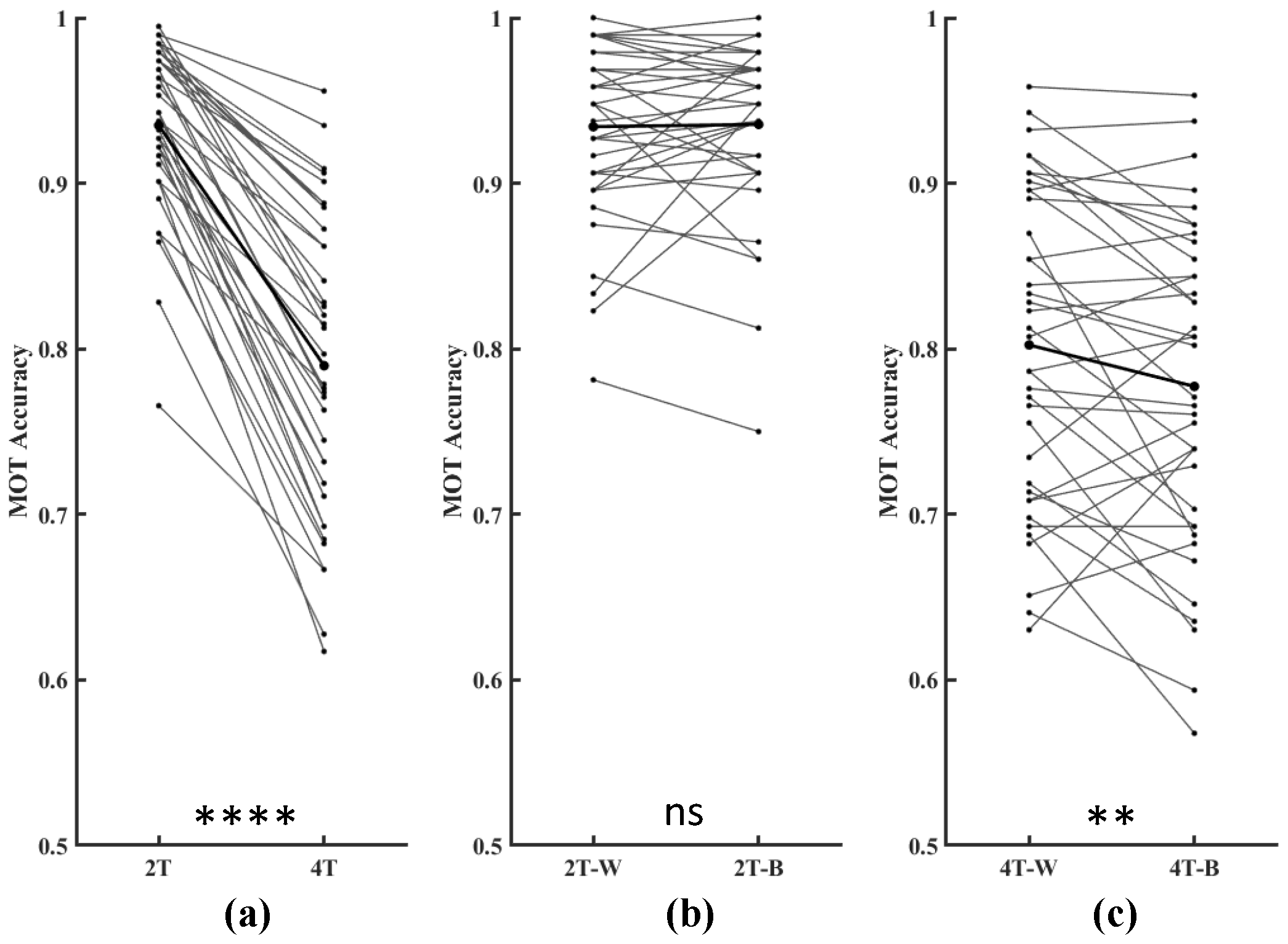
3.1.1. Accuracy in the MOT Task
3.1.2. Sex Differences in Mean Accuracy and Mean Response Click Time in MOT Task
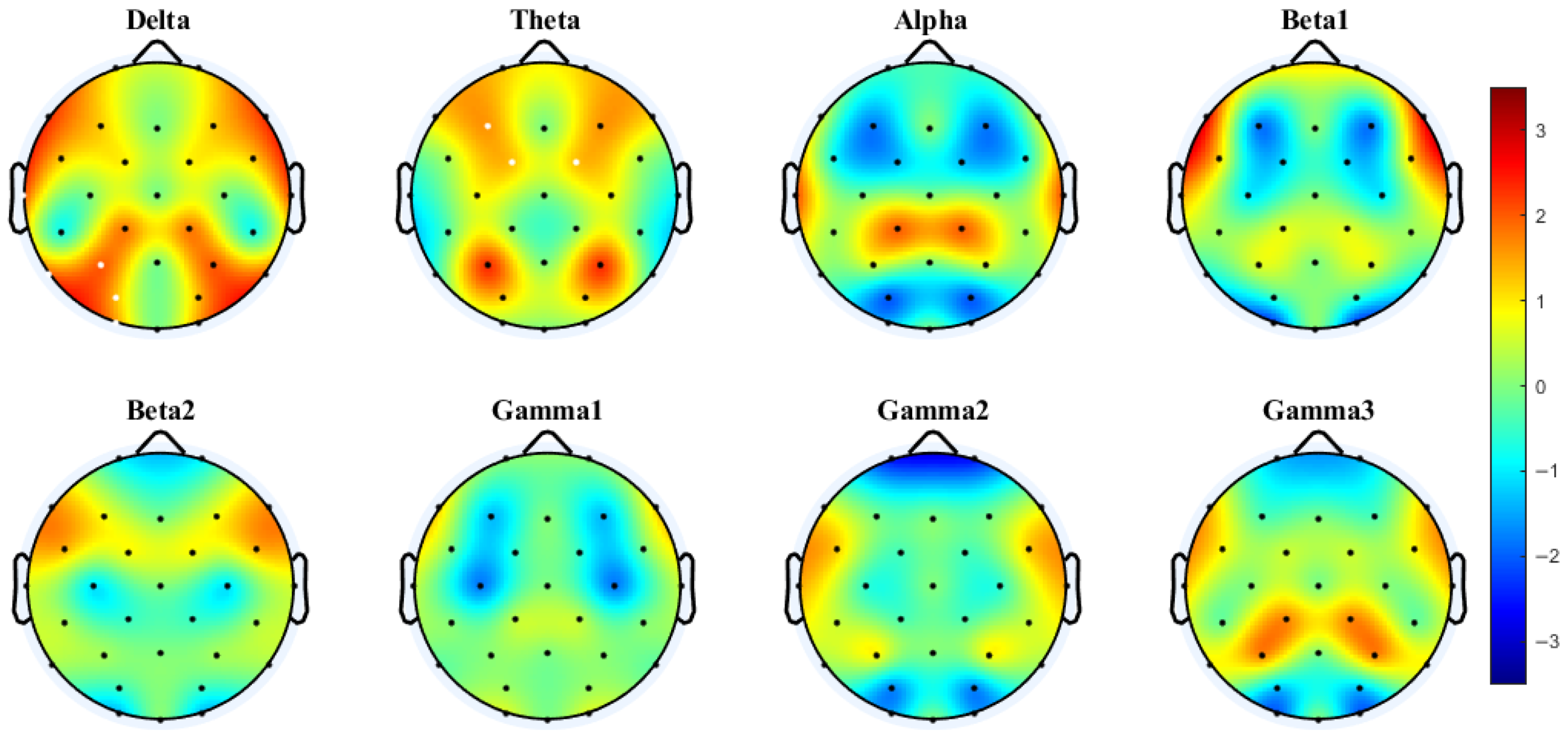
3.2. Interhemispheric Coherence and PLV Based on Paired Electrodes
3.3. Network Differences
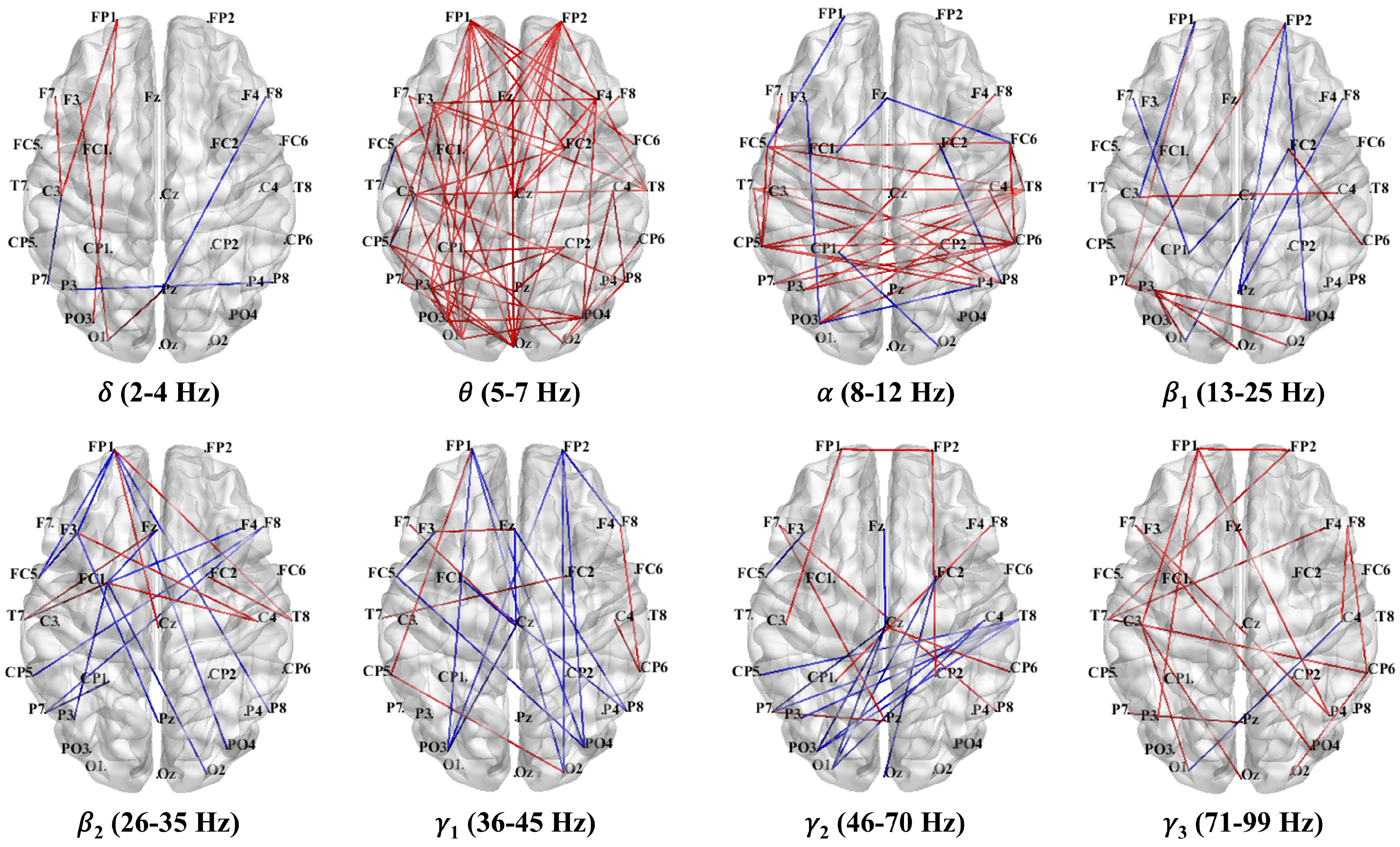
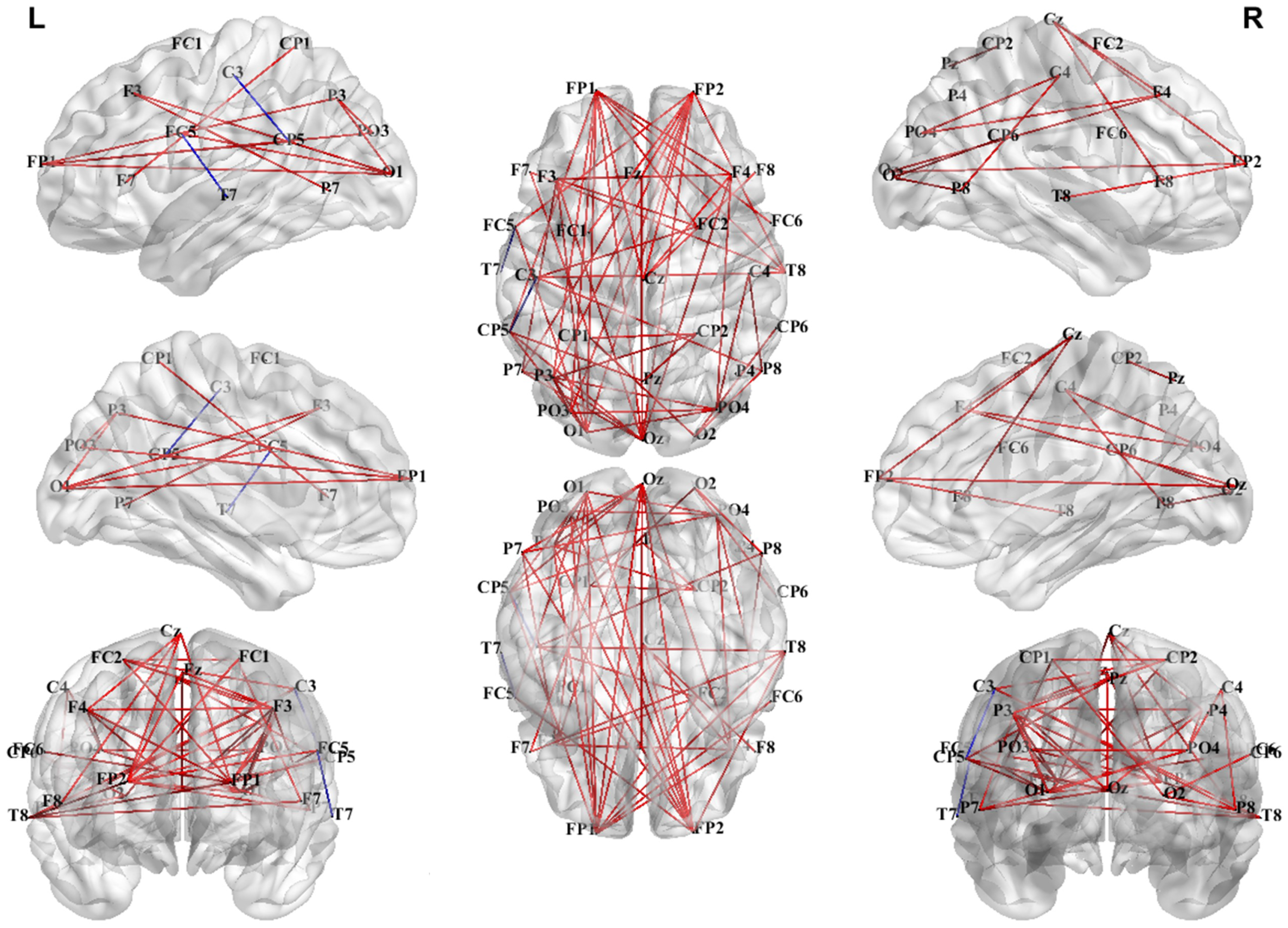
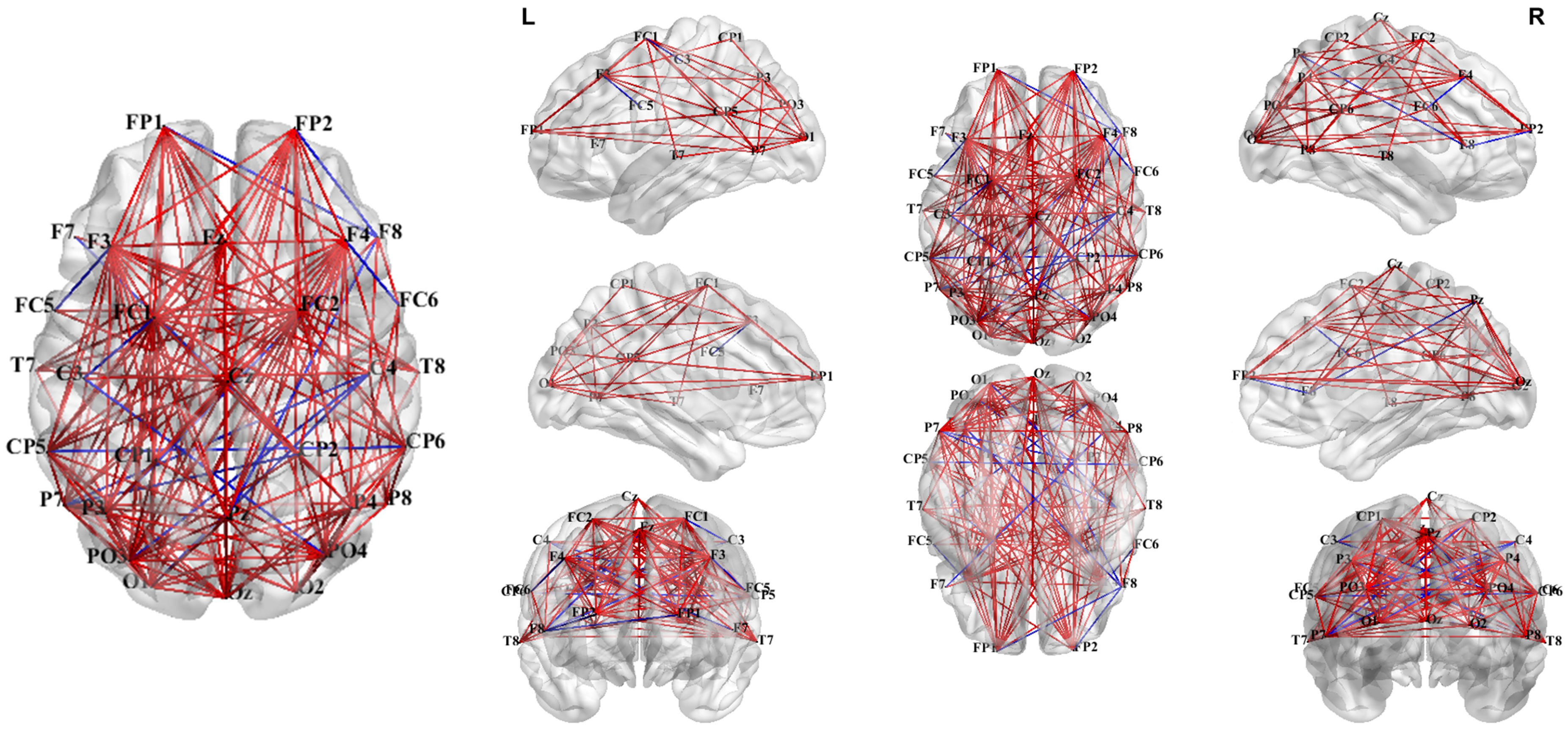
3.3.1. PLV Network Analysis in Theta Band
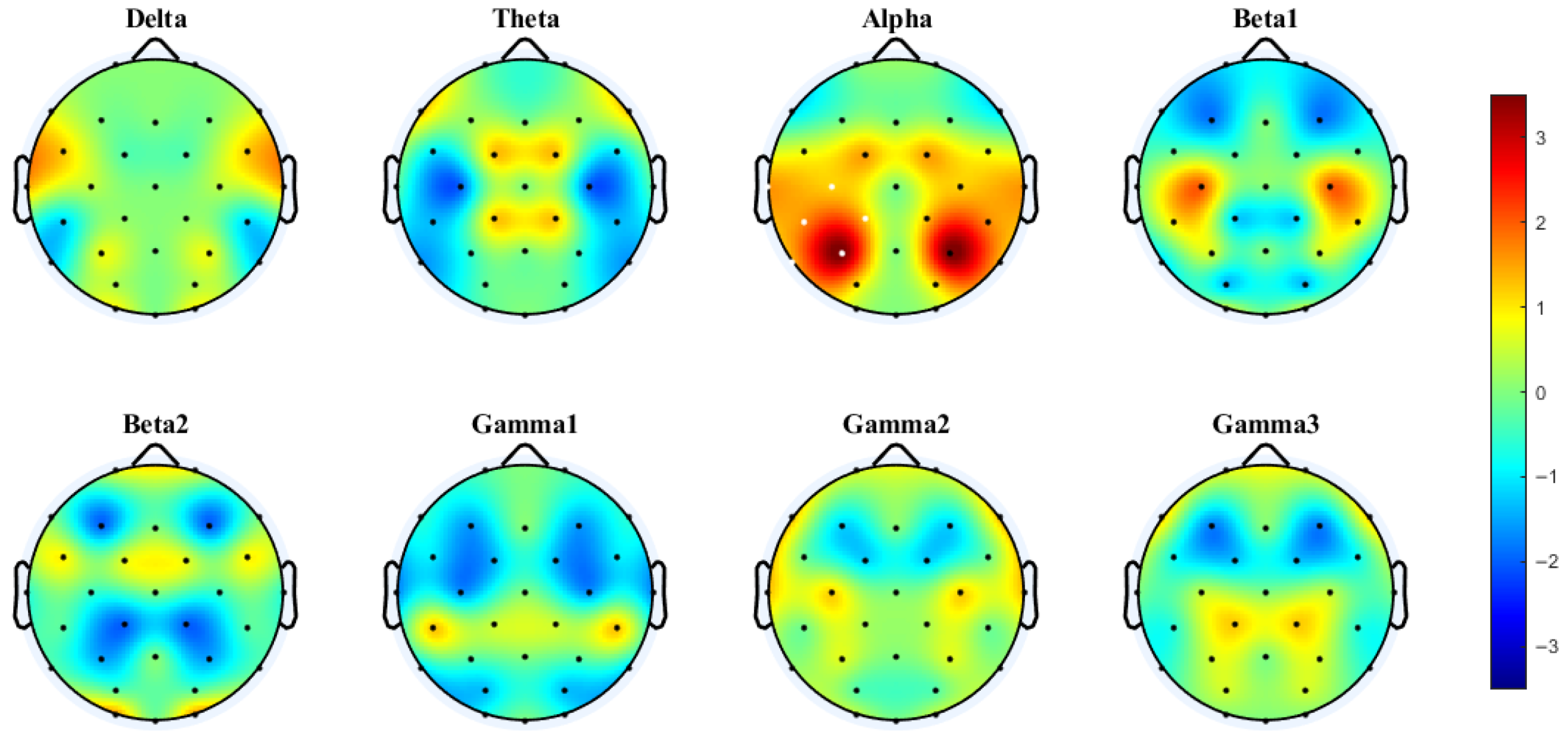
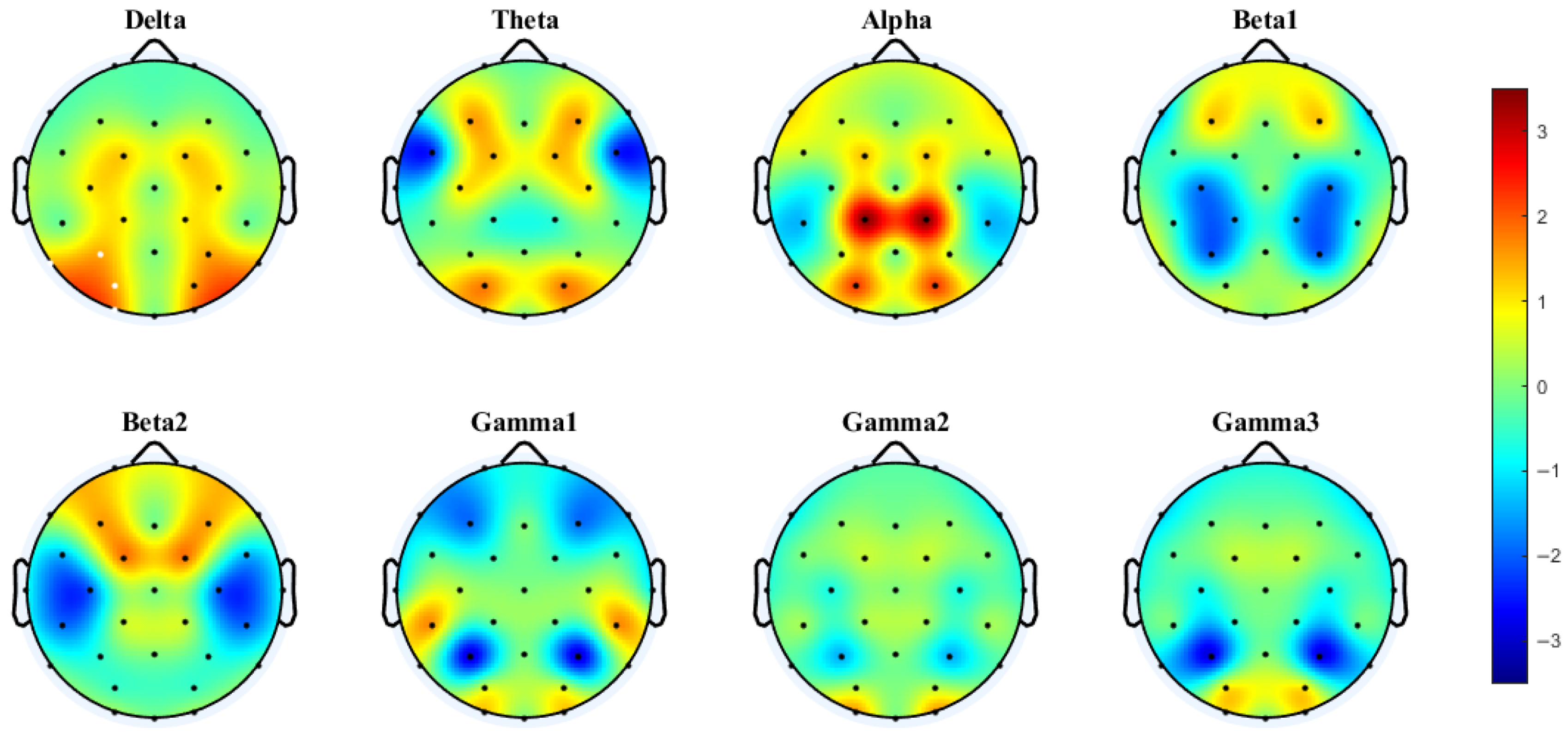
3.3.2. PLV Networks Across Frequency Bands
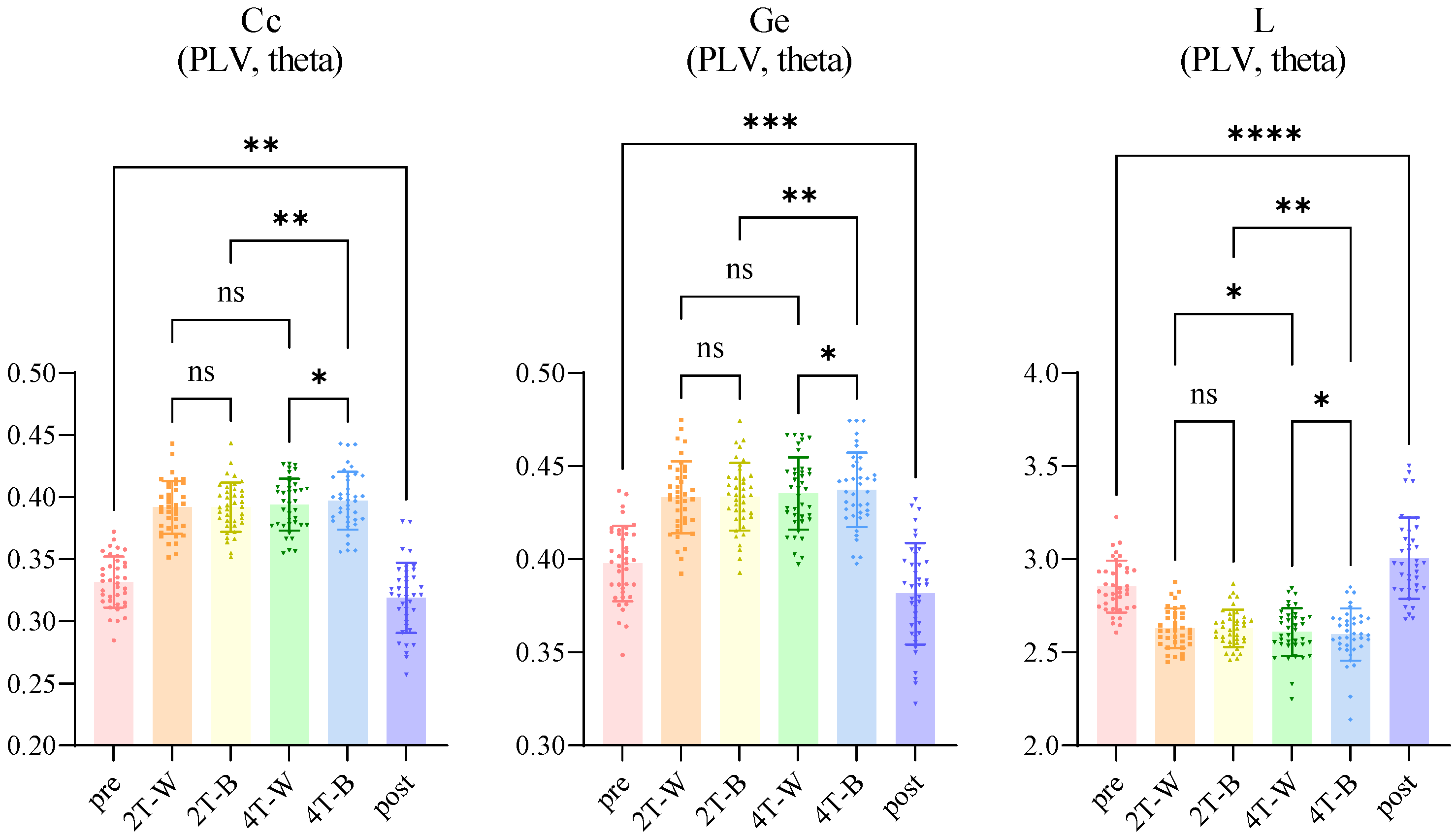
3.3.3. Network Properties
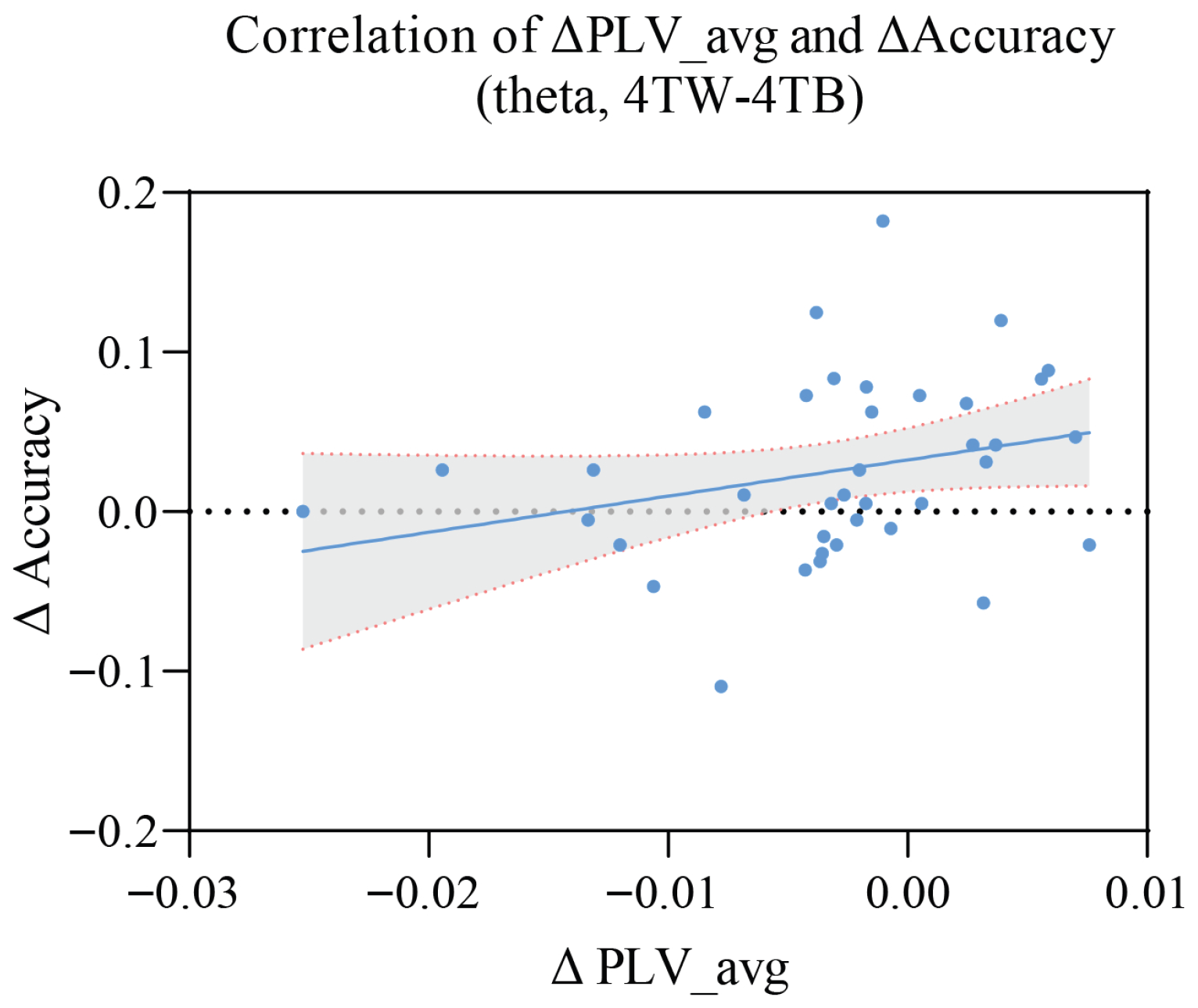
3.4. Correlation Between PLV and Accuracy
3.5. Analysis of Temporal Trends in Accuracy and Click Time Across Blocks
4. Discussion
4.1. Behavioral Findings in the Boundary-Free MOT Task
4.2. Frequency-Dependent Neural Mechanisms: Gamma Coherence Versus Theta-Band Phase Synchronization
4.3. Functional Brain Network Dynamics
4.4. Network Properties Analyses
4.5. Correlation Between Neural Synchronization and Behavioral Performance
4.6. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MOT | Multiple-object tracking |
| EEG | Electroencephalogram |
| PLV | Phase locking value |
| EO | Eyes open |
| EC | Eyes closed |
| 2T-W | Two-target within-hemifield condition |
| 2T-B | Two-target between-hemifield condition |
| 4T-W | Four-target within-hemifield condition |
| 4T-B | Four-target between-hemifield condition |
References
- Faubert, J.; Sidebottom, L. Perceptual-cognitive training of athletes. J. Clin. Sport Psychol. 2012, 6, 85–102. [Google Scholar] [CrossRef]
- Meyerhoff, H.S.; Papenmeier, F.; Huff, M. Studying visual attention using the multiple object tracking paradigm: A tutorial review. Atten. Percept. Psychophys. 2017, 79, 1255–1274. [Google Scholar] [CrossRef]
- Pylyshyn, Z.W.; Storm, R.W. Tracking multiple independent targets: Evidence for a parallel tracking mechanism. Spat. Vis. 1988, 3, 179–197. [Google Scholar] [CrossRef]
- Pylyshyn, Z. Some puzzling findings in multiple object tracking: I. tracking without keeping track of object identities. Vis. Cogn. 2004, 11, 801–822. [Google Scholar] [CrossRef]
- Liang, S.; Guo, Y.; Cheng, S.; Wu, S.; Wang, X.; Wang, X.; Lu, D.; Liu, X. Experimental studies of the attention processing model in multiple object tracking task. Brain Sci. 2022, 12, 1686. [Google Scholar] [CrossRef]
- Alvarez, G.A.; Cavanagh, P. Independent resources for attentional tracking in the left and right visual hemifields. Psychol. Sci. 2005, 16, 637–643. [Google Scholar] [CrossRef]
- Delvenne, J.-F. The capacity of visual short-term memory within and between hemifields. Cognition 2005, 96, B79–B88. [Google Scholar] [CrossRef]
- Drew, T.; Vogel, E.K. Neural measures of individual differences in selecting and tracking multiple moving objects. J. Neurosci. 2008, 28, 4183–4191. [Google Scholar] [CrossRef]
- Tootell, R.B.; Hadjikhani, N.; Hall, E.K.; Marrett, S.; Vanduffel, W.; Vaughan, J.T.; Dale, A.M. The retinotopy of visual spatial attention. Neuron 1998, 21, 1409–1422. [Google Scholar] [CrossRef]
- Serrien, D.J.; Ivry, R.B.; Swinnen, S.P. Dynamics of hemispheric specialization and integration in the context of motor control. Nat. Rev. Neurosci. 2006, 7, 160–166. [Google Scholar] [CrossRef]
- Banich, M.T. The missing link: The role of interhemispheric interaction in attentional processing. Brain Cogn. 1998, 36, 128–157. [Google Scholar] [CrossRef]
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Herrmann, C.S.; Engel, A.K.; Schneider, T.R. Different coupling modes mediate cortical cross-frequency interactions. Neuroimage 2016, 140, 76–82. [Google Scholar] [CrossRef]
- Buzsáki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Buzsaki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef]
- Von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Doesburg, S.M.; Roggeveen, A.B.; Kitajo, K.; Ward, L.M. Large-scale gamma-band phase synchronization and selective attention. Cereb. Cortex 2009, 19, 807–817. [Google Scholar] [CrossRef]
- Singer, W.; Gray, C.M. Visual feature integration and the temporal correlation hypothesis. Annu. Rev. Neurosci. 1995, 18, 555–586. [Google Scholar] [CrossRef]
- Sauseng, P.; Klimesch, W.; Schabus, M.; Doppelmayr, M. Fronto-parietal eeg coherence in theta and upper alpha reflect central executive functions of working memory. Int. J. Psychophysiol. 2005, 57, 97–103. [Google Scholar] [CrossRef]
- Sauseng, P.; Griesmayr, B.; Freunberger, R.; Klimesch, W. Control mechanisms in working memory: A possible function of eeg theta oscillations. Neurosci. Biobehav. Rev. 2010, 34, 1015–1022. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef]
- Ahn, J.S.; Heo, J.; Oh, J.; Lee, D.; Jhung, K.; Kim, J.-J.; Park, J.Y. The functional interactions between cortical regions through theta-gamma coupling during resting-state and a visual working memory task. Brain Sci. 2022, 12, 274. [Google Scholar] [CrossRef]
- Ursino, M.; Pirazzini, G. Theta–gamma coupling as a ubiquitous brain mechanism: Implications for memory, attention, dreaming, imagination, and consciousness. Curr. Opin. Behav. Sci. 2024, 59, 101433. [Google Scholar] [CrossRef]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef]
- Kawasaki, M.; Kitajo, K.; Yamaguchi, Y. Dynamic links between theta executive functions and alpha storage buffers in auditory and visual working memory. Eur. J. Neurosci. 2010, 31, 1683–1689. [Google Scholar] [CrossRef]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef]
- Onton, J.; Delorme, A.; Makeig, S. Frontal midline eeg dynamics during working memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef]
- Genç, E.; Bergmann, J.; Singer, W.; Kohler, A. Interhemispheric connections shape subjective experience of bistable motion. Curr. Biol. 2011, 21, 1494–1499. [Google Scholar] [CrossRef]
- Bland, N.S.; Mattingley, J.B.; Sale, M.V. No evidence for phase-specific effects of 40 hz hd–tacs on multiple object tracking. Front. Psychol. 2018, 9, 304. [Google Scholar] [CrossRef]
- Bland, N.S.; Mattingley, J.B.; Sale, M.V. Gamma coherence mediates interhemispheric integration during multiple object tracking. J. Neurophysiol. 2020, 123, 1630–1644. [Google Scholar] [CrossRef]
- De Schotten, M.T.; Forkel, S.J. The emergent properties of the connected brain. Science 2022, 378, 505–510. [Google Scholar] [CrossRef]
- Tan, E.; Troller-Renfree, S.V.; Morales, S.; Buzzell, G.A.; McSweeney, M.; Antúnez, M.; Fox, N.A. Theta activity and cognitive functioning: Integrating evidence from resting-state and task-related developmental electroencephalography (EEG) research. Dev. Cogn. Neurosci. 2024, 67, 101404. [Google Scholar] [CrossRef]
- Feng, J.; Spence, I.; Pratt, J. Playing an action video game reduces gender differences in spatial cognition. Psychol. Sci. 2007, 18, 850–855. [Google Scholar] [CrossRef]
- Ingalhalikar, M.; Smith, A.; Parker, D.; Satterthwaite, T.D.; Elliott, M.A.; Ruparel, K.; Hakonarson, H.; Gur, R.E.; Gur, R.C.; Verma, R. Sex differences in the structural connectome of the human brain. Proc. Natl. Acad. Sci. USA 2014, 111, 823–828. [Google Scholar] [CrossRef]
- Klem, G.; Lüders, H.; Jasper, H.; Elger, C. The international federation of clinical neurophysiology. the ten-twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 3–6. [Google Scholar]
- Singh, V.; Veer, K.; Sharma, R.; Kumar, S. Comparative study of fir and iir filters for the removal of 50 hz noise from eeg signal. Int. J. Biomed. Eng. Technol. 2016, 22, 250–257. [Google Scholar] [CrossRef]
- Lee, T.-W.; Girolami, M.; Sejnowski, T.J. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 1999, 11, 417–441. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. Eeglab: An open source toolbox for analysis of single-trial eeg dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Bigdely-Shamlo, N.; Mullen, T.; Kothe, C.; Su, K.-M.; Robbins, K.A. The prep pipeline: Standardized preprocessing for large-scale eeg analysis. Front. Neuroinform. 2015, 9, 16. [Google Scholar] [CrossRef]
- Shoka, A.; Dessouky, M.; El-Sherbeny, A.; El-Sayed, A. Literature review on eeg preprocessing, feature extraction, and classifications techniques. Menoufia J. Electron. Eng. Res. 2019, 28, 292–299. [Google Scholar] [CrossRef]
- Helfrich, R.F.; Knepper, H.; Nolte, G.; Strüber, D.; Rach, S.; Herrmann, C.S.; Schneider, T.R.; Engel, A.K. Selective modulation of interhemispheric functional connectivity by hd-tacs shapes perception. PLoS Biol. 2014, 12, e1002031. [Google Scholar] [CrossRef]
- Lachaux, J.-P.; Rodriguez, E.; Martinerie, J.; Varela, F.J. Measuring phase synchrony in brain signals. Hum. Brain Mapp. 1999, 8, 194–208. [Google Scholar] [CrossRef]
- Mormann, F.; Lehnertz, K.; David, P.; Elger, C.E. Mean phase coherence as a measure for phase synchronization and its application to the eeg of epilepsy patients. Phys. D Nonlinear Phenom. 2000, 144, 358–369. [Google Scholar] [CrossRef]
- Lowet, E.; Roberts, M.J.; Bonizzi, P.; Karel, J.; De Weerd, P. Quantifying neural oscillatory synchronization: A comparison between spectral coherence and phase-locking value approaches. PloS ONE 2016, 11, e0146443. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, G.; Chang, W.; Huang, W.; Yuan, Y. Eeg-based multi-frequency band functional connectivity analysis and the application of spatio-temporal features in emotion recognition. Biomed. Signal Process. Control 2023, 79, 104157. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for meg/eeg analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, W.; Tian, C.; Xu, P.; Yao, D. Brain oscillations and electroencephalography scalp networks during tempo perception. Neurosci. Bull. 2013, 29, 731–736. [Google Scholar] [CrossRef]
- Xia, M.; Wang, J.; He, Y. Brainnet viewer: A network visualization tool for human brain connectomics. PLoS ONE 2013, 8, e68910. [Google Scholar] [CrossRef]
- Liu, M.; Yu, C.; Shi, J.; Xu, Y.; Li, Z.; Huang, J.; Si, Z.; Yao, L.; Yin, K.; Zhao, Z. Effects of one-week bilateral cerebellar itbs on resting-state functional brain network and multi-task attentional performance in healthy individuals: A randomized, sham-controlled trial. NeuroImage 2024, 295, 120648. [Google Scholar] [CrossRef]
- Sim, C.H.; Gan, F.F.; Chang, T.C. Outlier labeling with boxplot procedures. J. Am. Stat. Assoc. 2005, 100, 642–652. [Google Scholar] [CrossRef]
- Kwak, S.K.; Kim, J.H. Statistical data preparation: Management of missing values and outliers. Korean J. Anesthesiol. 2017, 70, 407. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of shapiro-wilk, kolmogorov-smirnov, lilliefors and anderson-darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Liu, M.; Yu, C.; Tian, M.; Shi, J.; Xu, Y.; Li, Z.; Si, Z.; Yang, X.; Yang, X.; Huang, J.; et al. Inducing long-term plastic changes and visual attention enhancement via one-week cerebellar crus ii intermittent theta burst stimulation (itbs): An EEG study. IEEE J. Biomed. Health Inform. 2025, 1–12. [Google Scholar] [CrossRef]
- Scholl, B.J. What have we learned about attention from multiple object tracking (and vice versa). In Computation, Cognition, and Pylyshyn; MIT Press: Cambridge, MA, USA, 2009; pp. 49–78. [Google Scholar]
- Cavanagh, P.; Alvarez, G.A. Tracking multiple targets with multifocal attention. Trends Cogn. Sci. 2005, 9, 349–354. [Google Scholar] [CrossRef]
- Merritt, P.; Hirshman, E.; Wharton, W.; Stangl, B.; Devlin, J.; Lenz, A. Evidence for gender differences in visual selective attention. Personal. Individ. Differ. 2007, 43, 597–609. [Google Scholar] [CrossRef]
- Alvarez, G.A.; Franconeri, S.L. How many objects can you track?: Evidence for a resource-limited attentive tracking mechanism. J. Vis. 2007, 7, 14. [Google Scholar] [CrossRef]
- Halpern, D.F. Sex Differences in Cognitive Abilities; Psychology Press: East Sussex, UK, 2000. [Google Scholar]
- Green, C.S.; Li, R.; Bavelier, D. Perceptual learning during action video game playing. Top. Cogn. Sci. 2010, 2, 202–216. [Google Scholar] [CrossRef]
- Che, X.; Zhang, Y.; Lin, J.; Zhang, K.; Yao, W.; Lan, J.; Li, J. Two-dimensional and three-dimensional multiple object tracking learning performance in adolescent female soccer players: The role of flow experience reflected by heart rate variability. Physiol. Behav. 2023, 258, 114009. [Google Scholar] [CrossRef]
- Jordan, K.; Wüstenberg, T.; Heinze, H.-J.; Peters, M.; Jäncke, L. Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia 2002, 40, 2397–2408. [Google Scholar] [CrossRef]
- Hirnstein, M.; Hugdahl, K.; Hausmann, M. Cognitive sex differences and hemispheric asymmetry: A critical review of 40 years of research. Laterality Asymmetries Body Brain Cogn. 2019, 24, 204–252. [Google Scholar] [CrossRef]
- Vaquero, E.; Cardoso, M.; Vazque, M.; Gomez, C. Gender differences in event-related potentials during visual-spatial attention. Int. J. Neurosci. 2004, 114, 541–557. [Google Scholar] [CrossRef]
- Ramos-Loyo, J.; González-Garrido, A.A.; Llamas-Alonso, L.A.; Sequeira, H. Sex differences in cognitive processing: An integrative review of electrophysiological findings. Biol. Psychol. 2022, 172, 108370. [Google Scholar] [CrossRef]
- Doesburg, S.M.; Green, J.J.; McDonald, J.J.; Ward, L.M. Theta modulation of inter-regional gamma synchronization during auditory attention control. Brain Res. 2012, 1431, 77–85. [Google Scholar] [CrossRef]
- Jiang, Y.; He, S.; Zhang, J. The adaptive flexibility of rhythmic attentional sampling in attending to multiple targets. J. Exp. Psychol. Gen. 2024, 153, 26. [Google Scholar] [CrossRef]
- Smith, M.L.; Gosselin, F.; Schyns, P.G. Perceptual moments of conscious visual experience inferred from oscillatory brain activity. Proc. Natl. Acad. Sci. USA 2006, 103, 5626–5631. [Google Scholar] [CrossRef]
- Romei, V.; Driver, J.; Schyns, P.G.; Thut, G. Rhythmic tms over parietal cortex links distinct brain frequencies to global versus local visual processing. Curr. Biol. 2011, 21, 334–337. [Google Scholar] [CrossRef]
- Liu, L.; Luo, H. Behavioral oscillation in global/local processing: Global alpha oscillations mediate global precedence effect. J. Vis. 2019, 19, 12. [Google Scholar] [CrossRef]
- Raghavachari, S.; Kahana, M.J.; Rizzuto, D.S.; Caplan, J.B.; Kirschen, M.P.; Bourgeois, B.; Madsen, J.R.; Lisman, J.E. Gating of human theta oscillations by a working memory task. J. Neurosci. 2001, 21, 3175–3183. [Google Scholar] [CrossRef]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef]
- Asanowicz, D.; Kotlewska, I.; Panek, B. Neural underpinnings of proactive and preemptive adjustments of action control. J. Cogn. Neurosci. 2022, 34, 1590–1615. [Google Scholar] [CrossRef]
- Solomon, E.A.; Kragel, J.E.; Sperling, M.R.; Sharan, A.; Worrell, G.; Kucewicz, M.; Inman, C.S.; Lega, B.; Davis, K.A.; Stein, J.M.; et al. Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition. Nat. Commun. 2017, 8, 1704. [Google Scholar] [CrossRef]
- Knight, R.T. Distributed cortical network for visual attention. J. Cogn. Neurosci. 1997, 9, 75–91. [Google Scholar] [CrossRef]
- Fairhall, S.L.; Ishai, A. Effective connectivity within the distributed cortical network for face perception. Cereb. Cortex 2007, 17, 2400–2406. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Clayton, M.S.; Yeung, N.; Kadosh, R.C. The roles of cortical oscillations in sustained attention. Trends Cogn. Sci. 2015, 19, 188–195. [Google Scholar] [CrossRef]
- Trajkovic, J.; Veniero, D.; Hanslmayr, S.; Palva, S.; Cruz, G.; Romei, V.; Thut, G. Top-down and bottom-up interactions rely on nested brain oscillations to shape rhythmic visual attention sampling. PLoS Biol. 2025, 23, e3002688. [Google Scholar] [CrossRef]
- De Schotten, M.T.; Dell’Acqua, F.; Forkel, S.; Simmons, A.; Vergani, F.; Murphy, D.G.; Catani, M. A lateralized brain network for visuo-spatial attention. Nat. Preced. 2011, 14, 1245–1246. [Google Scholar] [CrossRef]
- Orlandi, A.; Proverbio, A.M. Left-hemispheric asymmetry for object-based attention: An erp study. Brain Sci. 2019, 9, 315. [Google Scholar] [CrossRef]
- Worden, M.S.; Foxe, J.J.; Wang, N.; Simpson, G.V. Anticipatory biasing of visuospatial attention indexed by retinotopically specific alpha-band electroencephalography increases over occipital cortex. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, RC63. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. Eeg alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Händel, B.F.; Haarmeier, T.; Jensen, O. Alpha oscillations correlate with the successful inhibition of unattended stimuli. J. Cogn. Neurosci. 2011, 23, 2494–2502. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.-Y.; Fang, F. Neural mechanisms of feature binding. Sci. China Life Sci. 2020, 63, 926–928. [Google Scholar] [CrossRef]
- Duecker, K. Harmony in Early Visual Cortex: Uncovering the Complementary Roles of Gamma & Alpha Oscillations in Local & Global Processing. Ph.D. Thesis, University of Birmingham, Birmingham, UK, 2024. [Google Scholar]
- Uhlhaas, P.J.; Pipa, G.; Neuenschwander, S.; Wibral, M.; Singer, W. A new look at gamma? high-(> 60 hz) γ-band activity in cortical networks: Function, mechanisms and impairment. Prog. Biophys. Mol. Biol. 2011, 105, 14–28. [Google Scholar] [CrossRef]
- Canolty, R.T.; Edwards, E.; Dalal, S.S.; Soltani, M.; Nagarajan, S.S.; Kirsch, H.E.; Berger, M.S.; Barbaro, N.M.; Knight, R.T. High gamma power is phase-locked to theta oscillations in human neocortex. Science 2006, 313, 1626–1628. [Google Scholar] [CrossRef]
- Sternshein, H.; Agam, Y.; Sekuler, R. Eeg correlates of attentional load during multiple object tracking. PloS ONE 2011, 6, e22660. [Google Scholar] [CrossRef]
- Rouhinen, S.; Siebenhühner, F.; Palva, J.M.; Palva, S. Spectral and anatomical patterns of large-scale synchronization predict human attentional capacity. Cereb. Cortex 2020, 30, 5293–5308. [Google Scholar] [CrossRef]
- Palva, J.M.; Monto, S.; Kulashekhar, S.; Palva, S. Neuronal synchrony reveals working memory networks and predicts individual memory capacity. Proc. Natl. Acad. Sci. USA 2010, 107, 7580–7585. [Google Scholar] [CrossRef]
- Xu, T.; Xu, L.; Zhang, H.; Ji, Z.; Li, J.; Bezerianos, A.; Wang, H. Effects of rest-break on mental fatigue recovery based on eeg dynamic functional connectivity. Biomed. Signal Process. Control 2022, 77, 103806. [Google Scholar] [CrossRef]
- Ren, L.; Wu, L.; Feng, T.; Liu, X. A new method for inducing mental fatigue: A high mental workload task paradigm based on complex cognitive abilities and time pressure. Brain Sci. 2025, 15, 541. [Google Scholar] [CrossRef]
- Neubauer, A.C.; Fink, A. Intelligence and neural efficiency. Neurosci. Biobehav. Rev. 2009, 33, 1004–1023. [Google Scholar] [CrossRef]
- Maniglia, M.R.; Souza, A.S. Age differences in the efficiency of filtering and ignoring distraction in visual working memory. Brain Sci. 2020, 10, 556. [Google Scholar] [CrossRef]
- Werrmann, M.; Niedeggen, M. Age-specific effects of visual feature binding. Brain Sci. 2023, 13, 1389. [Google Scholar] [CrossRef]
- Liu, M.; Xu, Y.; Li, Z.; Si, Z.; Yang, X.; Yang, X.; Zhao, Z. Kolmogorov-arnold networks for time series granger causality inference. arXiv 2025, arXiv:2501.08958. [Google Scholar]
- Papaioannou, O.; Crespo, L.P.; Clark, K.; Ogbuagu, N.N.; Alliende, L.M.; Silverstein, S.M.; Erickson, M.A. Is cortical theta-gamma phase-amplitude coupling memory-specific? Brain Sci. 2022, 12, 1131. [Google Scholar] [CrossRef]




| Condition | Males | Females | FDR-Corrected p-Values |
|---|---|---|---|
| Mean accuracy | |||
| 2T-W | 0.9419 | 0.9265 | 0.3114 |
| 4T-W | 0.8199 | 0.7848 | 0.2452 |
| 2T-B | 0.9600 | 0.9112 | 0.0092 |
| 4T-B | 0.8051 | 0.7495 | 0.1575 |
| Mean click time (seconds) | |||
| 2T-W | 1.1587 | 1.3499 | 0.0017 |
| 4T-W | 0.9112 | 1.0903 | 0.0010 |
| 2T-B | 1.1261 | 1.3384 | 0.0010 |
| 4T-B | 0.9077 | 1.1002 | 0.0010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yang, X.; Si, Z.; Liu, M.; Li, Z.; Yang, X.; Zhao, Z. From Gamma Coherence to Theta-Phase Synchronization: Task-Dependent Interhemispheric Integration in Boundary-Free Multiple-Object Tracking. Brain Sci. 2025, 15, 722. https://doi.org/10.3390/brainsci15070722
Xu Y, Yang X, Si Z, Liu M, Li Z, Yang X, Zhao Z. From Gamma Coherence to Theta-Phase Synchronization: Task-Dependent Interhemispheric Integration in Boundary-Free Multiple-Object Tracking. Brain Sciences. 2025; 15(7):722. https://doi.org/10.3390/brainsci15070722
Chicago/Turabian StyleXu, Yunfang, Xiaoxiao Yang, Zhengye Si, Meiliang Liu, Zijin Li, Xinyue Yang, and Zhiwen Zhao. 2025. "From Gamma Coherence to Theta-Phase Synchronization: Task-Dependent Interhemispheric Integration in Boundary-Free Multiple-Object Tracking" Brain Sciences 15, no. 7: 722. https://doi.org/10.3390/brainsci15070722
APA StyleXu, Y., Yang, X., Si, Z., Liu, M., Li, Z., Yang, X., & Zhao, Z. (2025). From Gamma Coherence to Theta-Phase Synchronization: Task-Dependent Interhemispheric Integration in Boundary-Free Multiple-Object Tracking. Brain Sciences, 15(7), 722. https://doi.org/10.3390/brainsci15070722






