Perceptual Decision Efficiency Is Modifiable and Associated with Decreased Musculoskeletal Injury Risk Among Female College Soccer Players
Abstract
1. Introduction
2. Materials and Methods
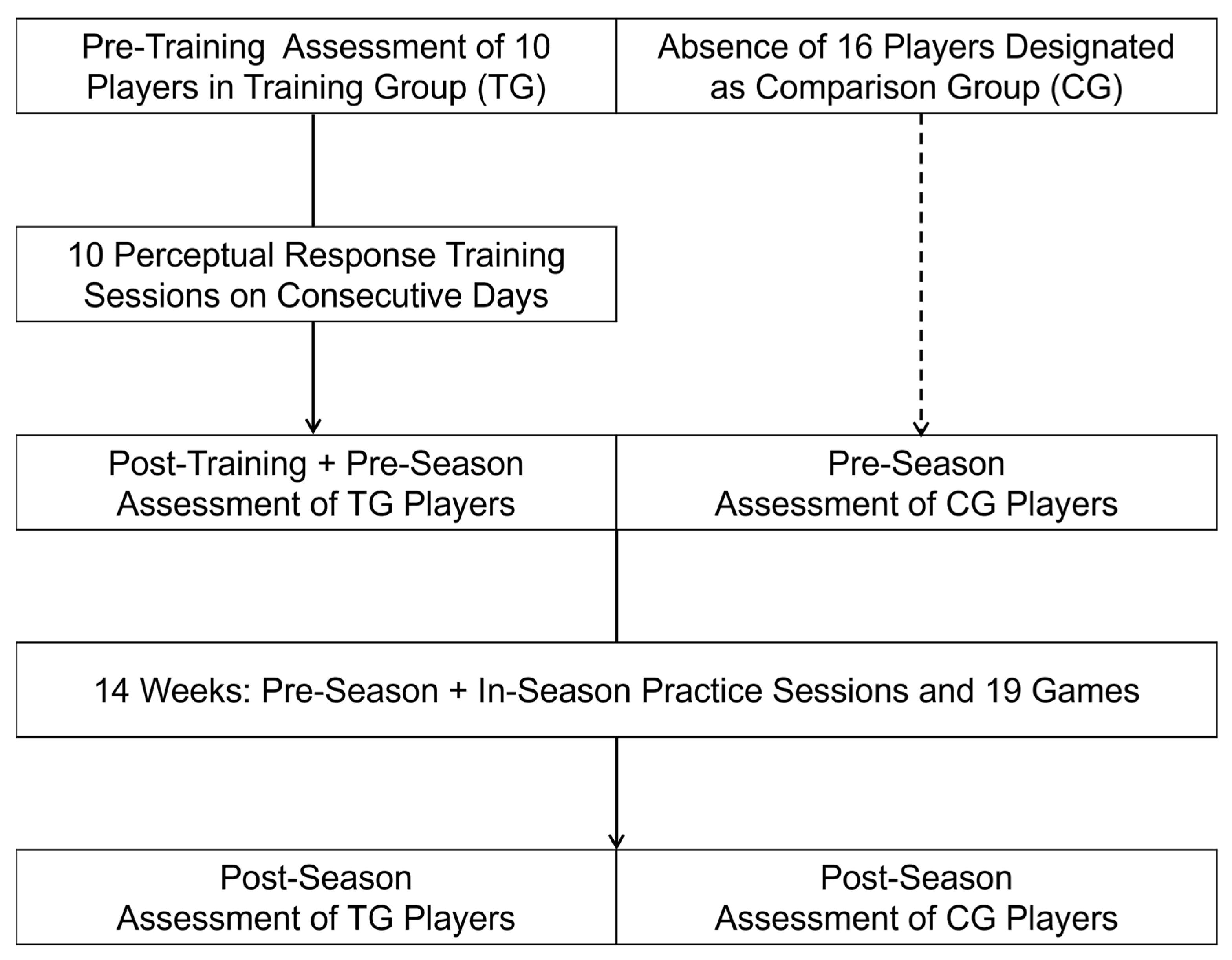
2.1. Participants and Institutional Review Board Statement
2.2. Procedures
2.3. Data Analysis
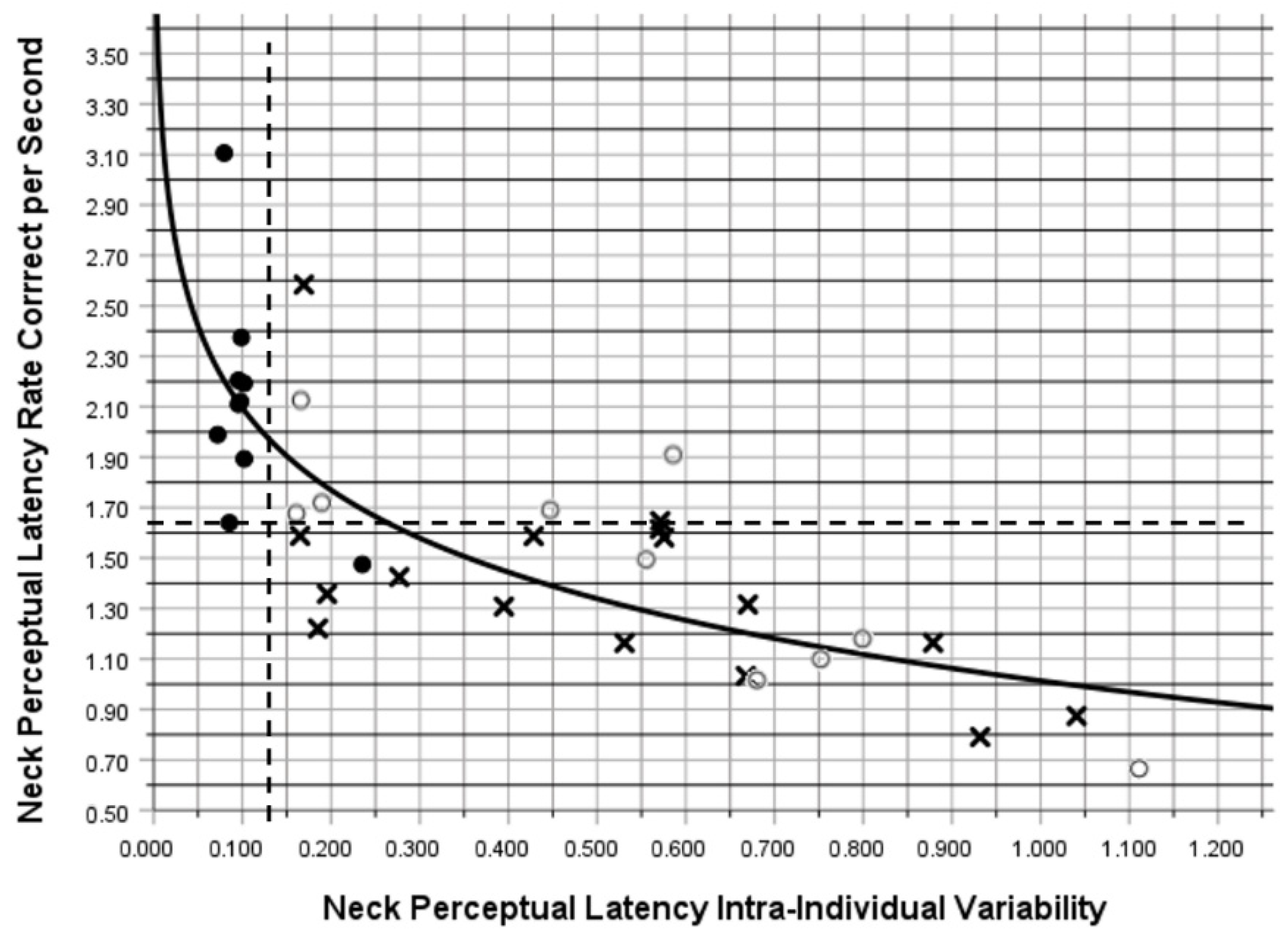
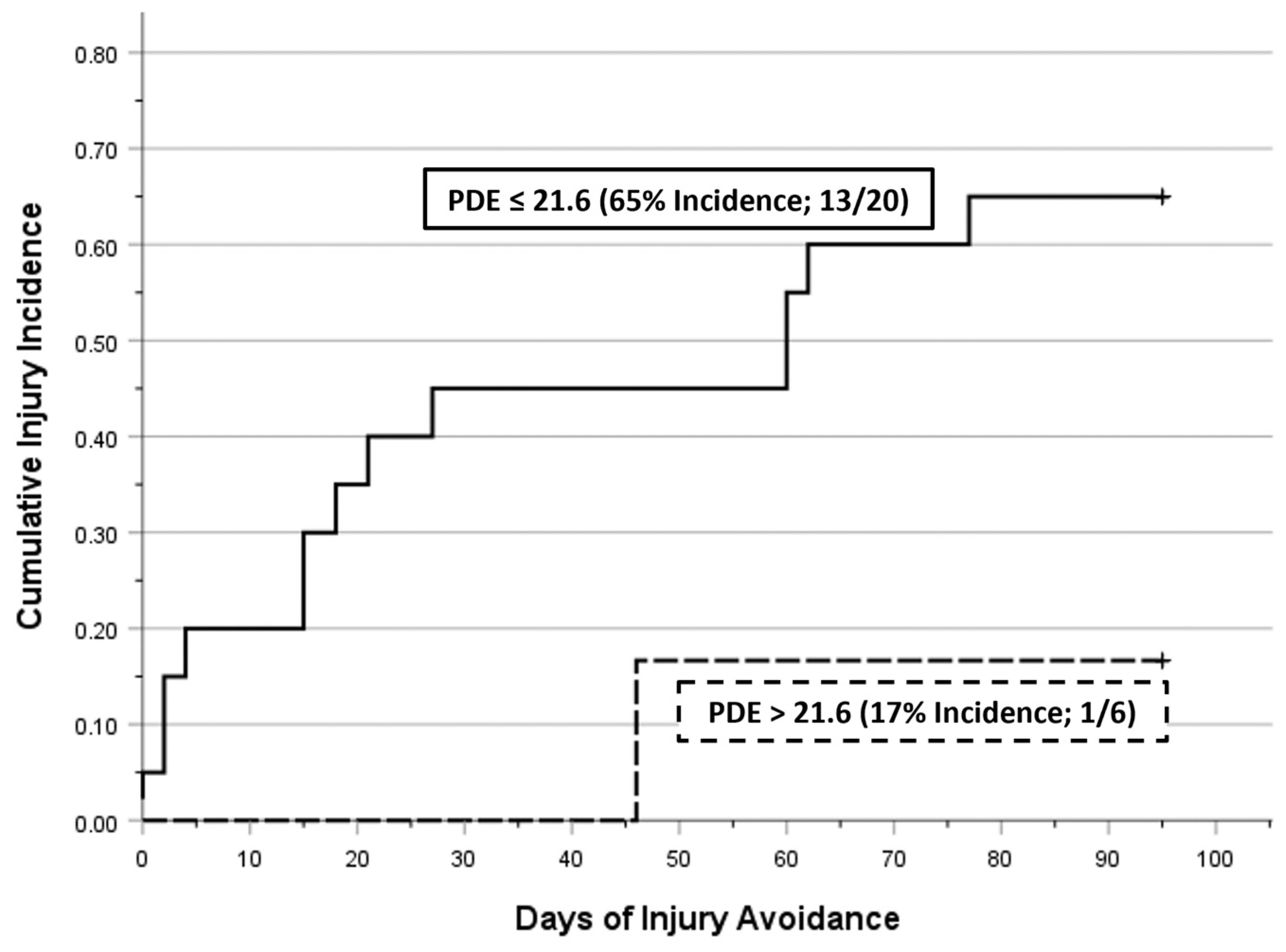
3. Results
4. Discussion
4.1. Interpretation of the Study Findings
4.2. Limitations
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crivelli, D.; Balconi, M. Neuroassessment in sports: An integrative approach for performance and potential evaluation in athletes. Front. Psychol. 2022, 13, 747852. [Google Scholar] [CrossRef]
- Gokeler, A.; McKeon, P.O.; Hoch, M.C. Shaping the functional task environment in sports injury rehabilitation: A framework to integrate perceptual-cognitive training in rehabilitation. Athl. Train. Sports Health Care 2020, 12, 283–292. [Google Scholar] [CrossRef]
- Hatfield, B.D.; Jaquess, K.J.; Lo, L.C.; Oh, H. The cognitive and affective neuroscience of superior athletic performance. In Handbook of Sport Psychology, 4th ed.; Tenenbaum, G., Eckland, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Müller, S.; Gabbett, T.; McNeil, D. Reducing injury risk and improving skill: How a psycho-perceptual-motor approach can benefit high-performance sport. Sports Health 2023, 15, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.W.; Hutchison, M.G.; Graham, S.J.; Schweizer, T.A. Brain function associated with reaction time after sport-related concussion. Brain Imaging Behav. 2021, 15, 1508–1517. [Google Scholar] [CrossRef]
- Forstmann, B.U.; Ratcliff, R.; Wagenmakers, E.-J. Sequential sampling models in cognitive neuroscience: Advantages, applications, and extensions. Ann. Rev. Psychol. 2016, 67, 641–666. [Google Scholar] [CrossRef]
- Myers, C.E.; Interian, A.; Moustafa, A.A. A practical introduction to using the drift diffusion model of decision-making in cognitive psychology, neuroscience, and health sciences. Front. Psychol. 2022, 13, 1039172. [Google Scholar] [CrossRef] [PubMed]
- Boucher, P.O.; Wang, T.; Carceroni, L.; Kane, G.; Shenoy, K.V.; Chandrasekaran, C. Initial conditions combine with sensory evidence to induce decision-related dynamics in premotor cortex. Nat. Commun. 2023, 14, 6510. [Google Scholar] [CrossRef]
- Gallivan, J.P. A motor-oriented organization of human ventral visual cortex? J. Neurosci. 2014, 34, 3119–3121. [Google Scholar] [CrossRef]
- Gokeler, A.; Tosarelli, F.; Buckthorpe, M.; Della Villa, F. Neurocognitive errors and noncontact anterior cruciate ligament injuries in professional male soccer players. J. Athl. Train. 2024, 59, 262–269. [Google Scholar] [CrossRef]
- Cardoso, F.d.S.L.; Neves, J.A.; Roca, A.; Teoldo, I. The association between perceptual-cognitive processes and response time in decision making in young soccer players. J. Sports Sci. 2021, 39, 926–935. [Google Scholar] [CrossRef]
- van Maarseveen, M.J.; Oudejans, R.R.; Mann, D.L.; Savelsbergh, G.J. Perceptual-cognitive skill and the in situ performance of soccer players. Q. J. Exp. Psychol. 2018, 71, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Walton, C.C.; Keegan, R.J.; Martin, M.; Hallock, H. The potential role for cognitive training in sport: More research needed. Front. Psychol. 2018, 9, 1121. [Google Scholar] [CrossRef]
- Cossich, V.R.; Carlgren, D.; Holash, R.J.; Katz, L. Technological breakthroughs in sport: Current practice and future potential of artificial intelligence, virtual reality, augmented reality, and modern data visualization in performance analysis. Appl. Sci. 2023, 13, 12965. [Google Scholar] [CrossRef]
- Chang, Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Dekker, E.; Morris-Binelli, K.; Piggott, B.; Hoyne, G.; Christensen, W.; Fadde, P.; Zaichkowsky, L.; Brenton, J.; Hambrick, D.Z. Attributes of expert anticipation should Inform the design of virtual reality simulators to accelerate learning and transfer of skill. Sports Med. 2023, 53, 301–309. [Google Scholar] [CrossRef]
- Mansouri, F.A.; Buckley, M.J.; Tanaka, K. Mapping causal links between prefrontal cortical regions and intra-individual behavioral variability. Nat. Commun. 2024, 15, 140. [Google Scholar] [CrossRef]
- Brosnan, M.B.; Sabaroedin, K.; Silk, T.; Genc, S.; Newman, D.P.; Loughnane, G.M.; Fornito, A.; O’Connell, R.G.; Bellgrove, M.A. Evidence accumulation during perceptual decisions in humans varies as a function of dorsal frontoparietal organization. Nat. Hum. Behav. 2020, 4, 844–855. [Google Scholar] [CrossRef]
- Domenech, P.; Dreher, J.-C. Decision threshold modulation in the human brain. J. Neurosci. 2010, 30, 14305–14317. [Google Scholar] [CrossRef]
- Herz, D.M.; Zavala, B.A.; Bogacz, R.; Brown, P. Neural correlates of decision thresholds in the human subthalamic nucleus. Curr. Biol. 2016, 26, 916–920. [Google Scholar] [CrossRef]
- Khilkevich, A.; Lohse, M.; Low, R.; Orsolic, I.; Bozic, T.; Windmill, P.; Mrsic-Flogel, T.D. Brain-wide dynamics linking sensation to action during decision-making. Nature 2024, 634, 890–900. [Google Scholar] [CrossRef]
- Lo, C.-C.; Xiao-Jing, W. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci. 2006, 9, 956. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.G.; Shadlen, M.N.; Wong-Lin, K.; Kelly, S.P. Bridging neural and computational viewpoints on perceptual decision-making. Trends Neurosci. 2018, 41, 838–852. [Google Scholar] [CrossRef]
- Stine, G.M.; Trautmann, E.M.; Jeurissen, D.; Shadlen, M.N. A neural mechanism for terminating decisions. Neuron 2023, 111, 2601–2613.e5. [Google Scholar] [CrossRef]
- Shadlen, M.N.; Kiani, R. Decision making as a window on cognition. Neuron 2013, 80, 791–806. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, M.K.; Simen, P.; Nystrom, L.; Holmes, P.; Cohen, J.D. Lateralized readiness potentials reveal properties of a neural mechanism for implementing a decision threshold. PLoS ONE 2014, 9, e90943. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, R.; Brown, E.; Moehlis, J.; Holmes, P.; Cohen, J.D. The physics of optimal decision making: A formal analysis of models of performance in two-alternative forced-choice tasks. Psychol. Rev. 2006, 113, 700–765. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Lansey, J.C.; Noblett, C.N.; Sarris, C.E. Test-retest reliability of immersive virtual reality measures of perceptual-motor performance. Percept. Mot. Ski. 2023, 130, 2484–2504. [Google Scholar] [CrossRef]
- Cai, W.; Warren, S.L.; Duberg, K.; Pennington, B.; Hinshaw, S.P.; Menon, V. Latent brain state dynamics distinguish behavioral variability, impaired decision-making, and inattention. Mol. Psychiatry 2021, 26, 4944–4957. [Google Scholar] [CrossRef]
- Mennes, M.; Zuo, X.-N.; Kelly, C.; Di Martino, A.; Zang, Y.-F.; Biswal, B.; Castellanos, F.X.; Milham, M.P. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 2011, 54, 2950–2959. [Google Scholar] [CrossRef]
- Najafi, F.; Churchland, A.K. Perceptual decision-making: A field in the midst of a transformation. Neuron 2018, 100, 453–462. [Google Scholar] [CrossRef]
- Weilnhammer, V.; Stuke, H.; Standvoss, K.; Sterzer, P. Sensory processing in humans and mice fluctuates between external and internal modes. PLoS Biol. 2023, 21, e3002410. [Google Scholar] [CrossRef] [PubMed]
- Aihara, T.; Kitajo, K.; Nozaki, D.; Yamamoto, Y. How does stochastic resonance work within the human brain?—Psychophysics of internal and external noise. Chem. Phys. 2010, 375, 616–624. [Google Scholar] [CrossRef]
- Duffy, J.S.; Bellgrove, M.A.; Murphy, P.R.; O’Connell, R.G. Disentangling sources of variability in decision-making. Nat. Rev. Neurosci. 2025, 26, 247–262. [Google Scholar] [CrossRef]
- Gazzellini, S.; Napolitano, A.; Bauleo, G.; Bisozzi, E.; Lispi, M.L.; Ardu, E.; Castelli, E.; Benso, F. Time–frequency analyses of reaction times and theta/beta EEG ratio in pediatric patients with traumatic brain injury: A preliminary study. Dev. Neurorehabilit. 2017, 20, 393–407. [Google Scholar] [CrossRef]
- Gbadeyan, O.; Teng, J.; Prakash, R.S. Predicting response time variability from task and resting-state functional connectivity in the aging brain. Neuroimage 2022, 250, 118890. [Google Scholar] [CrossRef]
- Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Perri, R.L.; Di Russo, F. Executive functions and performance variability measured by event-related potentials to understand the neural bases of perceptual decision-making. Front. Hum. Neurosci. 2017, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.H.; Roberts, K.; Visscher, K.; Woldorff, M. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006, 9, 971. [Google Scholar] [CrossRef]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.K.; Walhovd, K.B. Reduced white matter integrity is related to cognitive instability. J. Neurosci. 2011, 31, 18060–18072. [Google Scholar] [CrossRef]
- Maia, P.D.; Kutz, J.N. Reaction time impairments in decision-making networks as a diagnostic marker for traumatic brain injuries and neurological diseases. J. Comput. Neurosci. 2017, 42, 323–347. [Google Scholar] [CrossRef]
- McCormick, E.M.; Kievit, R.A. Poorer white matter microstructure predicts slower and more variable reaction time performance: Evidence for a neural noise hypothesis in a large lifespan cohort. J. Neurosci. 2023, 43, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Meindertsma, T.; Arazi, A.; Donner, T.H.; Dinstein, I. The relationship between trial-by-trial variability and oscillations of cortical population activity. Sci. Rep. 2019, 9, 16901. [Google Scholar] [CrossRef] [PubMed]
- Khamechian, M.B.; Daliri, M.R.; Treue, S.; Esghaei, M. Coupled oscillations orchestrate selective information transmission in visual cortex. PNAS Nexus 2024, 3, 288. [Google Scholar] [CrossRef] [PubMed]
- Khamechian, M.B.; Daliri, M.R. Frequency modulation of cortical rhythmicity governs behavioral variability, excitability and synchrony of neurons in the visual cortex. Sci. Rep. 2022, 12, 20914. [Google Scholar] [CrossRef]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Papanicolaou, A.C.; Zouridakis, G. Aberrant whole-brain transitions and dynamics of spontaneous network microstates in mild traumatic brain injury. Front. Comput. Neurosci. 2020, 13, 90. [Google Scholar] [CrossRef]
- Antonakakis, M.; Dimitriadis, S.I.; Zervakis, M.; Micheloyannis, S.; Rezaie, R.; Babajani-Feremi, A.; Zouridakis, G.; Papanicolaou, A.C. Altered cross-frequency coupling in resting-state MEG after mild traumatic brain injury. Int. J. Psychophysiol. 2016, 102, 1–11. [Google Scholar] [CrossRef]
- Wong, J.K.; Churchill, N.W.; Graham, S.J.; Baker, A.J.; Schweizer, T.A. Altered connectivity of default mode and executive control networks among female patients with persistent post-concussion symptoms. Brain Inj. 2023, 37, 147–148. [Google Scholar] [CrossRef]
- Vandierendonck, A. A comparison of methods to combine speed and accuracy measures of performance: A rejoinder on the binning procedure. Behav. Res. Methods 2017, 49, 653–673. [Google Scholar] [CrossRef]
- He, B.J.; Zempel, J.M. Average is optimal: An inverted-U relationship between trial-to-trial brain activity and behavioral performance. PLoS Comput. Biol. 2013, 9, e1003348. [Google Scholar] [CrossRef]
- Richlan, F.; Weiß, M.; Kastner, P.; Braid, J. Virtual training, real effects: A narrative review on sports performance enhancement through interventions in virtual reality. Front. Psychol. 2023, 14, 1240790. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Fleming, L.R.; Adams, V.P.; Petty, R.J.; Carlson, L.M.; Hogg, J.A.; Acocello, S.N. Assessment and training of perceptual-motor function: Performance of college wrestlers associated with history of concussion. Brain Sci. 2024, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, G.B.; Mether, K.S.; Perrin, Z.A.; Emberton, S.L.; Carlson, L.M.; Hogg, J.A.; Acocello, S.N. Perceptual response training for reduction of injury risk among high school girls’ soccer players. Brain Sci. 2024, 14, 1091. [Google Scholar] [CrossRef] [PubMed]
- Faubert, J.; Sidebottom, L. Perceptual-cognitive training of athletes. J. Clin. Sport. Psychol. 2012, 6, 85–102. [Google Scholar] [CrossRef]
- Avedesian, J.M.; Forbes, W.; Covassin, T.; Dufek, J.S. Influence of cognitive performance on musculoskeletal injury risk: A systematic review. Am. J. Sports Med. 2022, 50, 554–562. [Google Scholar] [CrossRef]
- Kakavas, G.; Malliaropoulos, N.; Skarpas, G.; Forelli, F. The impact of concussions on neuromuscular control and anterior cruciate ligament injury risk in female soccer players: Mechanisms and prevention—A narrative review. J. Clin. Med. 2025, 14, 3199. [Google Scholar] [CrossRef]
- Kwiatkowski, A.; Weidler, C.; Habel, U.; Coverdale, N.S.; Hirad, A.A.; Manning, K.Y.; Rauscher, A.; Bazarian, J.J.; Cook, D.J.; Li, D.K. Uncovering the hidden effects of repetitive subconcussive head impact exposure: A mega-analytic approach characterizing seasonal brain microstructural changes in contact and collision sports athletes. Hum. Brain Mapp. 2024, 45, e26811. [Google Scholar] [CrossRef]
- Shamloo, F.; Kon, M.; Ritter, E.; Sereno, A.B. Quantifying the magnitude and longevity of the effect of repetitive head impacts in adolescent soccer players: Deleterious effect of long headers extend beyond a month. Neurotrauma Rep. 2023, 4, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, G.B.; Wynn, K.R.; Dill, P.W.; Acocello, S.; Carlson, L.M.; Hogg, J. Concussion history and virtual reality metrics predict core or lower extremity injury occurrence among high school athletes. Front. Sports Act. Living 2024, 6, 1374772. [Google Scholar] [CrossRef] [PubMed]
- Patridge, E.F.; Bardyn, T.P. Research electronic data capture (REDCap). J. Med. Libr. Assoc. 2018, 106, 142. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Colston, M.A.; Acocello, S.N.; Hogg, J.A.; Carlson, L.M. Subtle impairments of perceptual-motor function and well-being are detectable among military cadets and college athletes with self-reported history of concussion. Front. Sports Act. Living 2023, 5, 1046572. [Google Scholar] [CrossRef]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.; May, W.L.; Bell, M.L. Relative effect sizes for measures of risk. Commun. Stat. Theory Methods 2017, 46, 6774–6781. [Google Scholar] [CrossRef]
- O’Connell, R.G.; Kelly, S.P. Neurophysiology of human perceptual decision-making. Ann. Rev. Neurosci. 2021, 44, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Buzsaki, G. The Brain from Inside Out; Oxford University Press: New York, NY, USA, 2019. [Google Scholar]
- Churchland, M.M.; Afshar, A.; Shenoy, K.V. A central source of movement variability. Neuron 2006, 52, 1085–1096. [Google Scholar] [CrossRef]
- Churchland, A.K.; Kiani, R.; Chaudhuri, R.; Wang, X.-J.; Pouget, A.; Shadlen, M.N. Variance as a signature of neural computations during decision making. Neuron 2011, 69, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.M.; Díaz, J.A. A random multiplicative model of Piéron’s law and choice reaction times. Phys. A 2021, 564, 125500. [Google Scholar] [CrossRef]
- Kucyi, A.; Esterman, M.; Capella, J.; Green, A.; Uchida, M.; Biederman, J.; Gabrieli, J.D.; Valera, E.M.; Whitfield-Gabrieli, S. Prediction of stimulus-independent and task-unrelated thought from functional brain networks. Nat. Commun. 2021, 12, 1793. [Google Scholar] [CrossRef]
- Ratcliff, R.; McKoon, G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Comput. 2008, 20, 873–922. [Google Scholar] [CrossRef]
- Selen, L.P.; Shadlen, M.N.; Wolpert, D.M. Deliberation in the motor system: Reflex gains track evolving evidence leading to a decision. J. Neurosci. 2012, 32, 2276–2286. [Google Scholar] [CrossRef]
- Gallivan, J.P.; Chapman, C.S.; Wolpert, D.M.; Flanagan, J.R. Decision-making in sensorimotor control. Nat. Rev. Neurosci. 2018, 19, 519–534. [Google Scholar] [CrossRef]
- Resulaj, A.; Kiani, R.; Wolpert, D.M.; Shadlen, M.N. Changes of mind in decision-making. Nature 2009, 461, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Cubillo, A.; Hermes, H.; Berger, E.; Winkel, K.; Schunk, D.; Fehr, E.; Hare, T.A. Intra-individual variability in task performance after cognitive training is associated with long-term outcomes in children. Dev. Sci. 2023, 26, e13252. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; McEwen, B.S.; Friston, K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog. Neurobiol. 2017, 156, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Crasta, J.E.; Nebel, M.B.; Svingos, A.; Tucker, R.N.; Chen, H.W.; Busch, T.; Caffo, B.S.; Stephens, J.; Suskauer, S.J. Rethinking recovery in adolescent concussions: Network-level functional connectivity alterations associated with motor deficits. Hum. Brain Mapp. 2023, 44, 3271–3282. [Google Scholar] [CrossRef]
- Lempke, L.B.; Lynall, R.C. The state of the science for potential contributors to musculoskeletal injury following concussion: Mechanisms, gaps, and clinical considerations. Musculoskelet. Sci. Pract. 2025, 75, 103219. [Google Scholar] [CrossRef]
- Dolman, K.E.; Staines, R.S.; Mughal, S.; Brown, K.E.; Meehan, S.K.; Staines, W.R. Long-term effects of concussion on attention, sensory gating and motor learning. Exp. Brain Res. 2025, 243, 30. [Google Scholar] [CrossRef]
- Hayes, K.D.; Khan, M.E.; Graham, K.R.; Staines, W.R.; Meehan, S.K. Persistent adaptations in sensorimotor interneuron circuits in the motor cortex with a history of sport-related concussion. Exp. Brain Res. 2025, 243, 5. [Google Scholar] [CrossRef]
- Wilke, J.; Groneberg, D.; Banzer, W.; Giesche, F. Perceptual–cognitive function and unplanned athletic movement task performance: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 7481. [Google Scholar] [CrossRef]
- Müller, S.; Vallence, A.-M.; Winstein, C. Investigation of perceptual-motor behavior across the expert athlete to disabled patient skill continuum can advance theory and practical application. J. Mot. Behav. 2018, 50, 697–707. [Google Scholar] [CrossRef]
- Harris, D.J.; Bird, J.M.; Smart, P.A.; Wilson, M.R.; Vine, S.J. A framework for the testing and validation of simulated environments in experimentation and training. Front. Psychol. 2020, 11, 605. [Google Scholar] [CrossRef]

| Distribution Skew (S-W p) | Mean (Std Dev) | Difference | ||||
|---|---|---|---|---|---|---|
| Virtual Reality Metric | Pre-Training | Post-Training | Pre-Training | Post-Training | p | d |
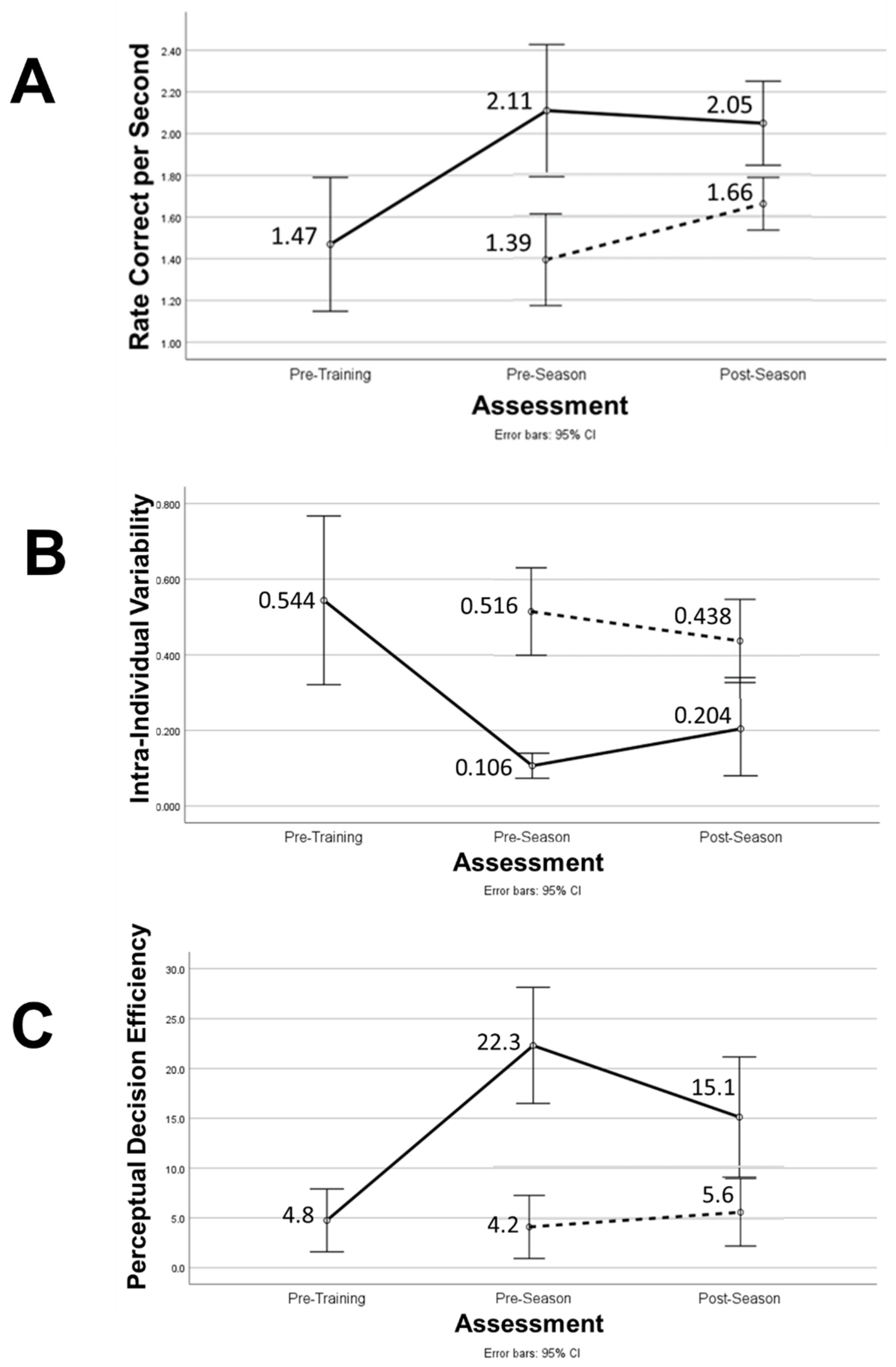
| Rate Correct per Second | −0.327 (0.813) | 1.013 (0.309) | 1.47 (0.45) | 2.11 (0.44) | 0.012 | 1.00 |
| Intra-Individual Variability | 0.243 (0.484) | 2.880 (<0.001) | 0.544 (0.312) | 0.106 (0.046) | 0.002 | 1.34 |
| Perceptual Decision Efficiency | 1.025 (0.024) | 0.169 (0.181) | 4.75 (4.41) | 22.30 (8.13) | <0.001 | 1.75 |
| Distribution Skew (S-W p) | Geometric Mean (Loge) | Difference | ||||
|---|---|---|---|---|---|---|
| Virtual Reality Metric | Pre-Training | Post-Training | Pre-Training | Post-Training | p | d |
| Rate Correct per Second | −0.932 (0.367) | 0.280 (0.692) | 1.40 (0.34) | 2.07 (0.73) | 0.013 | 0.98 |
| Intra-Individual Variability | −0.607 (0.003) | 2.372 (<0.001) | 0.448 (−0.80) | 0.101 (−2.30) | <0.001 | 1.77 |
| Perceptual Decision Efficiency | 0.065 (0.530) | −1.885 (0.008) | 3.12 (1.14) | 20.60 (3.03) | <0.001 | 1.57 |
| Distribution Skew (S-W p) Original Data | Distribution Skew (S-W p) Loge Data | |||
|---|---|---|---|---|
| Virtual Reality Metric | Pre-Season Skew (S-W p) | Post-Season Skew (S-W p) | Pre-Season Skew (S-W p) | Post-Season Skew (S-W p) |
| Rate Correct per Second | 0.711 (0.389) | 0.301 (0.849) | −0.114 (0.981) | −0.108 (0.934) |
| Intra-Individual Variability | 0.892 (0.001) | 0.786 (0.012) | 0.128 (0.016) | −0.144 (0.032) |
| Perceptual Decision Efficiency | 0.927 (0.001) | 1.099 (<0.001) | −0.153 (0.082) | 0.122 (0.128) |
| Mean (Std Dev) | Group X Session Interaction | Group Difference | |||||
|---|---|---|---|---|---|---|---|
| Virtual Reality Metric | Group | Pre-Season | Post-Season | p | ηp2 | p | ηp2 |
| Perceptual Decision Efficiency | TG | 22.31 (3.02) | 12.71 (2.54) | 0.017 | 0.216 | <0.001 | 0.717 |
| CG | 4.23 (1.11) | 4.39 (1.48) | |||||
| Rate Correct per Second | TG | 2.11 (0.72) | 2.03 (0.70) | 0.063 | 0.137 | <0.001 | 0.510 |
| CG | 1.39 (0.29) | 1.64 (0.49) | |||||
| Intra-Individual Variability | TG | 0.106 (0.046) | 0.204 (0.174) | 0.147 | 0.085 | <0.001 | 0.503 |
| CG | 0.516 (0.279) | 0.438 (0.232) | |||||
| Geometric Mean (Loge) | Group X Session Interaction | Group Difference | |||||
|---|---|---|---|---|---|---|---|
| Virtual Reality Metric | Group | Pre-Season | Post-Season | p | ηp2 | p | ηp2 |
| Perceptual Decision Efficiency | TG | 20.60 (3.03) | 12.71 (2.54) | 0.038 | 0.168 | <0.001 | 0.680 |
| CG | 3.06 (1.12) | 4.39 (1.48) | |||||
| Rate Correct per Second | TG | 2.07 (0.73) | 2.03 (0.70) | 0.038 | 0.168 | <0.001 | 0.511 |
| CG | 1.34 (0.29) | 1.64 (0.49) | |||||
| Intra-Individual Variability | TG | 0.101 (−2.30) | 0.160 (−1.83) | 0.073 | 0.128 | <0.001 | 0.653 |
| CG | 0.437 (−0.83) | 0.374 (−0.98) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkerson, G.B.; Gullion, A.J.; McMahan, K.L.; Brooks, L.T.; Colston, M.A.; Carlson, L.M.; Hogg, J.A.; Acocello, S.N. Perceptual Decision Efficiency Is Modifiable and Associated with Decreased Musculoskeletal Injury Risk Among Female College Soccer Players. Brain Sci. 2025, 15, 721. https://doi.org/10.3390/brainsci15070721
Wilkerson GB, Gullion AJ, McMahan KL, Brooks LT, Colston MA, Carlson LM, Hogg JA, Acocello SN. Perceptual Decision Efficiency Is Modifiable and Associated with Decreased Musculoskeletal Injury Risk Among Female College Soccer Players. Brain Sciences. 2025; 15(7):721. https://doi.org/10.3390/brainsci15070721
Chicago/Turabian StyleWilkerson, Gary B., Alejandra J. Gullion, Katarina L. McMahan, Lauren T. Brooks, Marisa A. Colston, Lynette M. Carlson, Jennifer A. Hogg, and Shellie N. Acocello. 2025. "Perceptual Decision Efficiency Is Modifiable and Associated with Decreased Musculoskeletal Injury Risk Among Female College Soccer Players" Brain Sciences 15, no. 7: 721. https://doi.org/10.3390/brainsci15070721
APA StyleWilkerson, G. B., Gullion, A. J., McMahan, K. L., Brooks, L. T., Colston, M. A., Carlson, L. M., Hogg, J. A., & Acocello, S. N. (2025). Perceptual Decision Efficiency Is Modifiable and Associated with Decreased Musculoskeletal Injury Risk Among Female College Soccer Players. Brain Sciences, 15(7), 721. https://doi.org/10.3390/brainsci15070721








