Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia
Abstract
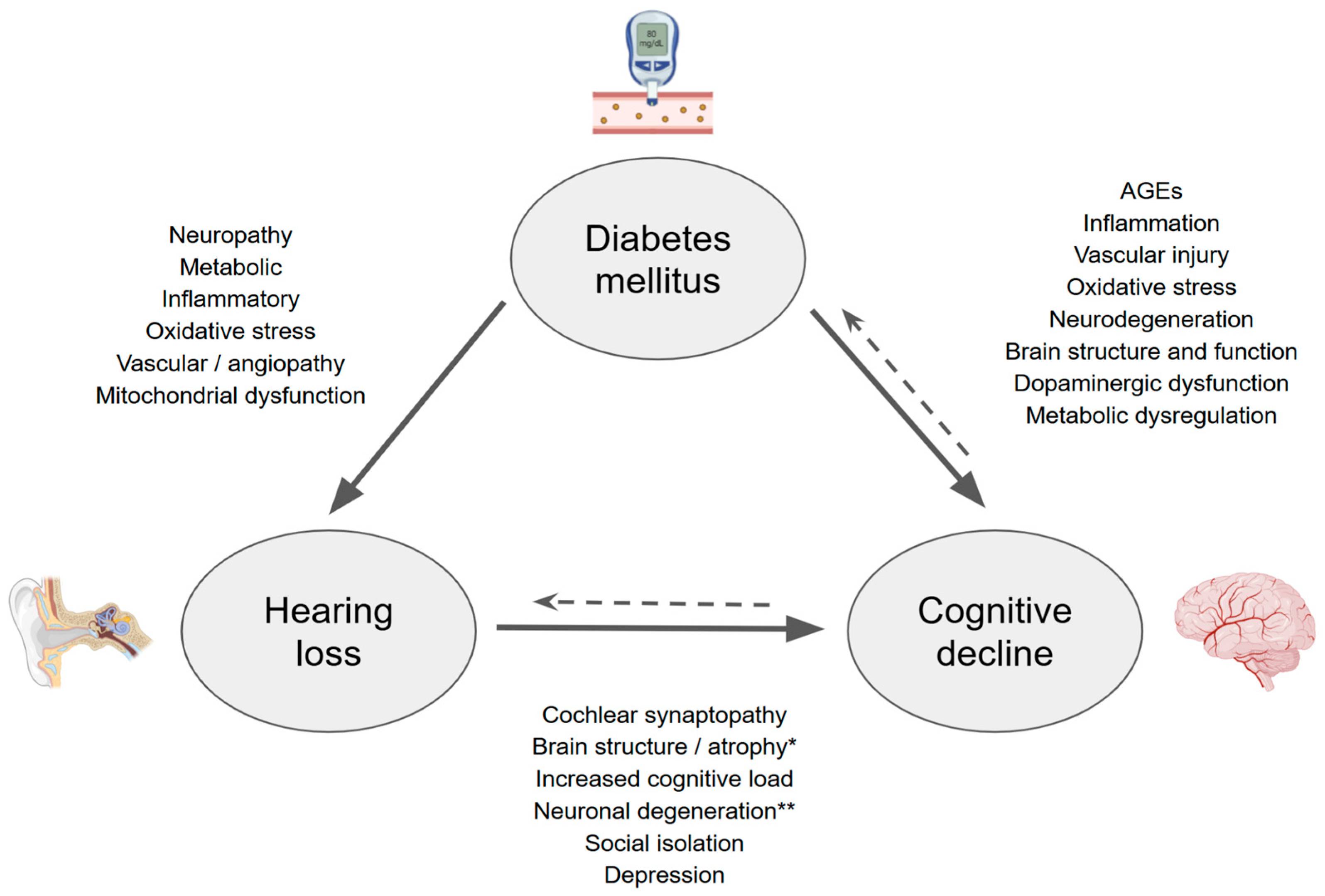
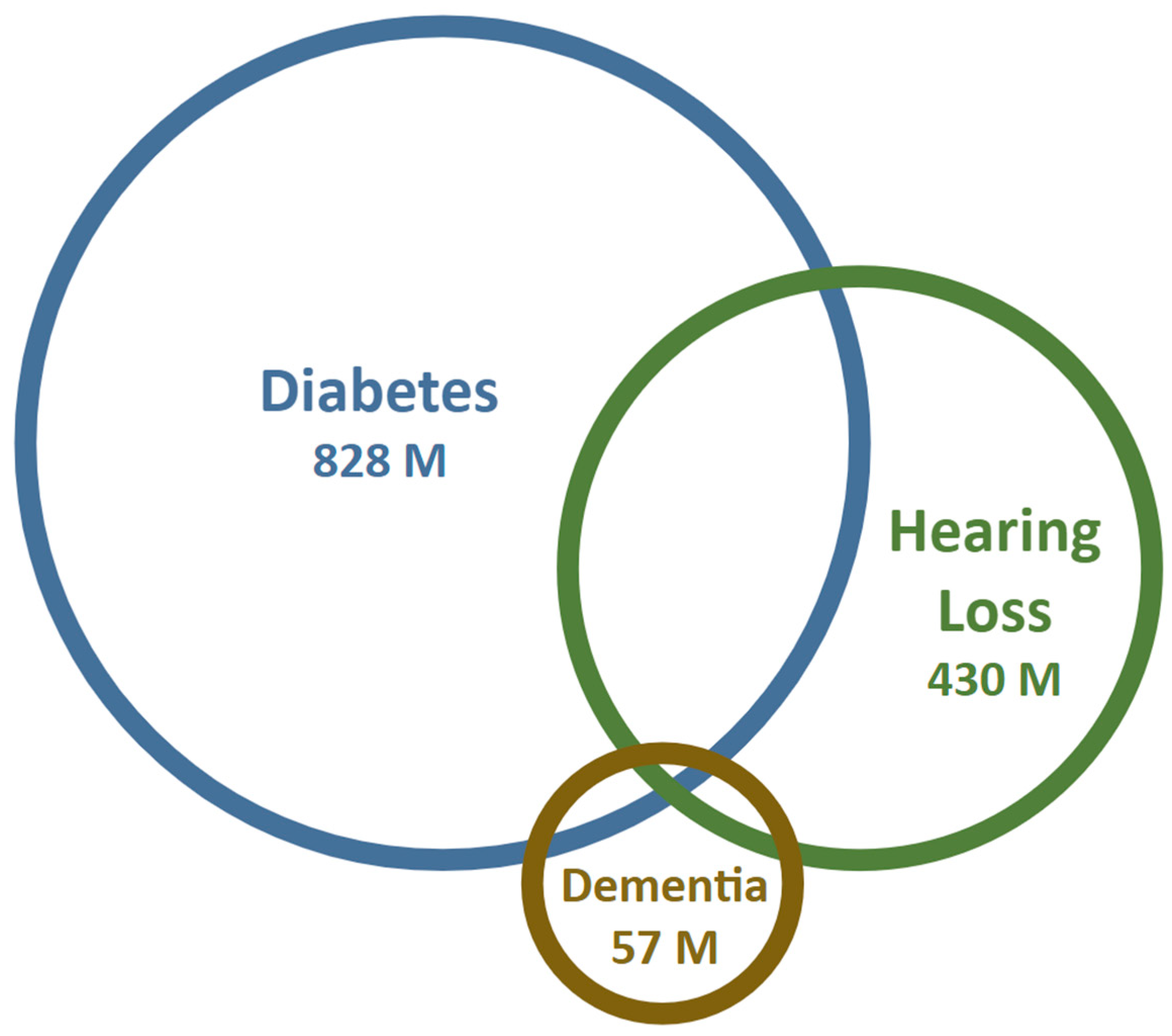
:1. Introduction
2. Hearing Loss and Its Relationship with Cognition
3. What About Hearing Loss and Dementia?
| Study | Sample (Control/Diabetic) | Age (Control/Diabetic) | Incidence of Hearing Loss (Control/Diabetic) |
|---|---|---|---|
| ElSherif M et al. (2024) [48] | 40/42 | 49.7 ± 6.4/50.7 ± 6.4 | 0%/9.5% |
| Alizadeh Y et al. (2022) [49] | 105/315 | 60.0 ± 8.8/59.8 ± 8.2 | Control: 10.2% Diabetic: 11.6% (no DR) * 12.9% (mild–mod NPDR) 34.2% (severe NPDR/PDR) |
| Mishra A & Poorey (2019) [50] | 50/50 | Age-matched | 18%/74% |
| Li J et al. (2018) [51] | 43/51 | 54.4 ± 10.1/56.1 ± 10.1 | 25.6%/45.1% |
| Adebola S et al. (2016) [52] | 90/97 | 58.8 ± 14.7/58.9 ± 14.9 | 8.9%/21.5% |
| Bamanie A & Al-Noury K (2011) [53] | 87/109 | 45.7/47.9 | 39.1%/69.7% |
| Mozaffari M et al. (2010) [54] | 80/80 | 45.1/45 | 20%/45% |
| Aladag I et al. (2009) [55] | 37/63 | 47.5/46.6 | 48.6%/44% |
| Mitchell P et al. (2009) [56] | 1648/210 | 69.7/70.5 | 38.2%/50% |
| Sakuta H et al. (2007) [57] | 596/103 | 52.9 ± 1.0 | 45.2%/60.2% |
4. Diabetes Mellitus and Hearing Loss
5. Diabetes Mellitus and Cognitive Decline
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD Results. Institute for Health Metrics and Evaluation. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 23 May 2025).
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 23 May 2025).
- Mittal, J.; Mittal, R.; Lemos, J.R.N.; Hirani, K.; Keith, G.; Lacey, M.; Assayed, A. Diabetes mellitus, hearing loss, and therapeutic interventions: A systematic review of insights from preclinical animal models. PLoS ONE 2024, 19, e0305617. [Google Scholar] [CrossRef]
- Lee, H.J.; Joo, Y.H.; Han, K.D.; Park, K.H. Association between hearing loss and cognitive disorder: A nationwide population-based study. Yonsei Med. J. 2021, 62, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Ando, F.; Shimokata, H.; Uchida, Y.; Otsuka, R.; Nakamura, A.; Tange, C.; Iwata, K.; Nishita, Y.; Suzuki, H.; et al. Smaller hippocampal volume and degraded peripheral hearing among Japanese community dwellers. Front. Aging Neurosci. 2018, 10, 319. [Google Scholar]
- Davatzikos, C.; An, Y.; Doshi, J.; Resnick, S.M.; Lin, F.R.; Deal, J.A.; Erus, G.; Armstrong, N.M.; Ferrucci, L. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 794–802. [Google Scholar]
- Wang, L.; Feng, J.; Wang, H.-F.; Li, Y.; Ma, Y.-H.; Rolls, E.T.; Zhang, W.; Kang, J.; Cheng, W.; Yu, J.-T. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. eBioMedicine 2022, 86, 104336. [Google Scholar] [CrossRef]
- WHO. World Report on Hearing. Available online: https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/highlighting-priorities-for-ear-and-hearing-care (accessed on 10 April 2025).
- Frank, L. Age-Related Hearing Loss. N. Engl. J. Med. 2024, 390, 1505–1512. [Google Scholar] [CrossRef]
- Tsai Do, B.S.; Bush, M.L.; Weinreich, H.M.; Schwartz, S.R.; Anne, S.; Adunka, O.F.; Bender, K.; Bold, K.M.; Brenner, M.J.; Hashmi, A.Z.; et al. Clinical practice guideline: Age-related hearing loss. Otolaryngol. Head Neck Surg. 2024, 170 (Suppl. S2), S1–S54. [Google Scholar] [CrossRef]
- Ganek, H.V.; Madubueze, A.; Merritt, C.E.; Bhutta, Z.A. Prevalence of Hearing Loss in Children Living in Low- And Middle-Income Countries Over the Last 10 years: A Systematic Review. Dev. Med. Child Neurol. 2023, 65, 600–610. [Google Scholar] [CrossRef]
- Dubno, J.R.; Wilson, R.H.; Wingfield, A.; Humes, L.E.; Gordon-Salant, S.; Gates, G.A.; Cacace, A.T.; Lister, J.J.; Cruickshanks, K.J. Central presbycusis: A review and evaluation of the evidence. J. Am. Acad. Audiol. 2012, 23, 635–666. [Google Scholar] [CrossRef]
- Delgado, C.; Torrente, M.C.; Moreno-Gómez, F.N.; Leiva, A.; Marcenaro, B.; Belkhiria, C.; Martin, S.S.; Vergara, R.; Delano, P.H. Speech Perception and Dichotic Listening Are Associated with Hearing Thresholds and Cognition, Respectively, in Unaided Presbycusis. Front. Aging Neurosci. 2022, 14, 786330. [Google Scholar] [CrossRef]
- Meimaroglou, S.; Eleftheriadis, N.; Iliadou, V.M. Better education required for professionals in healthcare regarding auditory processing disorder. Eur. Arch. Otorhinolaryngol. 2025, 282, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Steel, K.P.; Schmiedt, R.A.; Schulte, B.A.; Lewis, M.A.; Harris, K.C.; Lang, H.; Dubno, J.R.; Vaden, K.I. Translational and interdisciplinary insights into presbyacusis: A multidimensional disease. Hear Res. 2021, 402, 108109. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.L.; Fritzsch, B.; Yamoah, E.N.; Zine, A. Age-related hearing loss: Sensory and neural etiology and their interdependence. Front. Aging Neurosci. 2022, 14, 814528. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Starr, A. Auditory Neuropathy--Neural Synaptic Mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Rance, G.; Starr, A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain 2015, 138 Pt 11, 3141–3158. [Google Scholar] [CrossRef]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Primary neural degeneration in noise-exposed human cochleas: Correlations with outer hair cell loss and word-discrimination scores. J. Neurosci. 2021, 41, 4439–4447. [Google Scholar] [CrossRef]
- Liberman, M.C.; Epstein, M.J.; Cleveland, S.S.; Wang, H.; Maison, S.F. Toward a differential diagnosis of hidden hearing loss in humans. PLoS ONE 2016, 11, e0162726. [Google Scholar] [CrossRef]
- Shearer, A.E.; Hildebrand, M.S.; Schaefer, A.M. Genetic Hearing Loss Overview; StatPearls: Petersburg, FL, USA, 2023. [Google Scholar]
- Moverman, D.J.; Liberman, L.D.; Kraemer, S.; Corfas, G.; Liberman, M.C. Ultrastructure of noise-induced cochlear synaptopathy. Sci. Rep. 2023, 13, 19456. [Google Scholar] [CrossRef]
- Suthakar, K.; Liberman, M.C. Auditory-nerve responses in mice with noise-induced cochlear synaptopathy. J. Neurophysiol. 2021, 126, 2027–2038. [Google Scholar] [CrossRef]
- Ding, D.; Salvi, R.; Chen, G.-D.; Auerbach, B.D.; Lobarinas, E.; Radziwon, K.; Sun, W.; Wang, J. Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front. Neurosci. 2017, 10, 621. [Google Scholar]
- Harris, K.C.; Dias, J.W.; McClaskey, C.M.; Rumschlag, J.; Prisciandaro, J.; Dubno, J.R. Afferent loss, GABA, and central gain in older adults: Associations with speech recognition in noise. J. Neurosci. 2022, 42, 7201–7212. [Google Scholar] [CrossRef]
- Harris, K.C.; Dias, J.W.; Lang, H.; Panganiban, C.; McClaskey, C.M.; Noble, K.V.; Kerouac, L.B.; Rumschlag, J.A. Age-related central gain with degraded neural synchrony in the auditory brainstem of mice and humans. Neurobiol. Aging 2022, 115, 50–59. [Google Scholar]
- Rüttiger, L.; Singer, W.; Klose, U.; Wolpert, S.; Hofmeier, B.; Refat, F.; Wertz, J.; Hinrichs, P.; Saemisch, J.; Knipper, M. Functional biomarkers that distinguish between tinnitus with and without hyperacusis. Clin. Transl. Med. 2021, 11, e378. [Google Scholar]
- Peelle, J.E.; Wingfield, A. The neural consequences of age-related hearing loss. Trends Neurosci. 2016, 39, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Slade, K.; Plack, C.J.; Nuttall, H.E. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. 2020, 43, 810–821. [Google Scholar] [CrossRef]
- Doshi, J.; Ferrucci, L.; Metter, E.; Kraut, M.; Resnick, S.; An, Y.; Lin, F.; Davatzikos, C.; Goh, J. Association of hearing impairment with brain volume changes in older adults. Neuroimage 2014, 90, 84–92. [Google Scholar]
- Vernooij, M.W.; Ikram, M.A.; Bos, D.; Metselaar, M.; Goedegebure, A.; Roshchupkin, G.V.; Rigters, S.C.; de Jong, R.J.B. Hearing impairment is associated with smaller brain volume in aging. Front. Aging Neurosci. 2017, 9, 2. [Google Scholar]
- Belkhiria, C.; Vergara, R.C.; Martín, S. Cingulate Cortex Atrophy Associated Hearing Loss Presbycusis Cochlear Amplifier Dysfunction. Front. Aging Neurosci. 2019, 11, 97. [Google Scholar] [CrossRef]
- Martinez, M.; Delano, P.H.; Andrade, M.; Leiva, A.; Marcenaro, B.; Belkhiria, C.; Vergara, R.C.; Martin, S.S.; Delgado, C. Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front. Aging Neurosci. 2020, 12, 102. [Google Scholar]
- Edden, R.A.; Li, F.; Wu, L.; Zong, W.; Ma, W.; Li, N.; Li, X.; Hui, S.C.; Ren, F.; Dai, Z.; et al. Neurochemical and functional reorganization of the cognitive-ear link underlies cognitive impairment in presbycusis. Neuroimage 2023, 268, 119861. [Google Scholar]
- Vergara, R.; Delgado, C.; Delano, P.H.; García, X.; Vidal, V.; Cerda, M.; Navarro, C.F.; Leiva, A.; Martínez, M.; Belkhiria, C.; et al. Cochlear dysfunction as an early biomarker of cognitive decline in normal hearing and mild hearing loss. Alzheimers Dement. 2024, 16, e12467. [Google Scholar]
- Zhao, F.; Gao, M.; Feng, T.; Shen, J.; Zheng, Y.; Liang, J.; Yang, H. Cognitive reserve disorder in age-related hearing loss: Cognitive cortical compensatory to auditory perceptual processing. Cereb. Cortex 2023, 33, 9616–9626. [Google Scholar]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: Review. JAMA 2019, 322, 1589. [Google Scholar] [CrossRef]
- Oh, E.S. Dementia. Ann. Intern. Med. 2024, 177, ITC161–ITC176. [Google Scholar] [CrossRef]
- Cao, Q.; Tan, C.C.; Xu, W.; Hu, H.; Cao, X.-P.; Dong, Q.; Tan, L. The prevalence of dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Aguilar-Navarro, S.G.; de la Peña, J.E.; Gutierez-Gutierez, L.; Suerna-Hernandez, A.; Gonzelez-Figueroa, E.; Juarez-Cedillo, T.; Garcia-Cruz, J.C. Prevalence of dementia and main subtypes in Mexico: The study on aging and dementia in Mexico (SADEM). J. Alzheimers Dis. 2022, 89, 931–941. [Google Scholar]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54 (Suppl. S5), S4–S9. [Google Scholar]
- Ren, Z.; Wang, X.-D.; Tang, Y.; Lv, Y.; Huang, G.; Niu, J.; Gang, B.; Meng, X.; Cai, P.; Zeng, Y.; et al. The updated prevalence and risk factors of dementia in old adults in China: A cross-sectional study. J. Alzheimers Dis. 2024, 102, 1209–1223. [Google Scholar]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Lee, M.K.; Rah, Y.C.; Park, S.; Kim, B.; Choi, J.; Han, K. Association between the severity of hearing loss and the risk of dementia within the 2010–2017 national insurance service survey in South Korea. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Mandavia, R.; Omar, R.; Pavlou, M.; Lin, F.; Schilder, A.; Proctor, D.; Yu, R.-C.; Gonzalez, S.C.; Lewis, G.; et al. Adult-onset hearing loss and incident cognitive impairment and dementia—A systematic review and meta-analysis of cohort studies. Ageing Res. Rev. 2024, 98, 102346. [Google Scholar]
- Department of Otorhinolaryngology, Audiovestibular Medicine Unit, Alexandria University Faculty of Medicine, Alexandria, Egypt; ElSherif, M.; El Sayed Mahfouz, A.F.; Mohamed Gaber Amin, N.; Saad Kozou, H. Relation between glycated hemoglobin level and hearing loss in type 2 diabetic patients. J. Int. Adv. Otol. 2024, 20, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Y.; Jalali, M.M.; Sehati, A. Association of different severity of diabetic retinopathy and hearing loss in type 2 diabetes mellitus. Am. J. Otolaryngol. 2022, 43, 103383. [Google Scholar] [CrossRef]
- Mishra, A.; Poorey, V.K. Clinical and audiometric assessment of hearing loss in diabetes mellitus. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 1490–1494. [Google Scholar] [CrossRef]
- Bi, J.; Liu, B.; Zhang, Y.; Li, Y.; Li, J.; Fu, X.; Zhang, L. Alteration of auditory function in type 2 diabetic and pre-diabetic patients. Acta Otolaryngol. 2018, 138, 542–547. [Google Scholar]
- Adebola, S.O.; Olamoyegun, M.A.; Sogebi, O.A.; Iwuala, S.O.; Babarinde, J.A.; Oyelakin, A.O. Otologic and audiologic characteristics of type 2 diabetics in a tertiary health institution in Nigeria. Braz. J. Otorhinolaryngol. 2016, 82, 567–573. [Google Scholar] [CrossRef]
- Bamanie, A.H.; Al-Noury, K.I. Prevalence of hearing loss among Saudi type 2 diabetic patients. Saudi Med. J. 2011, 32, 271–274. [Google Scholar]
- Mozaffari, M.; Tajik, A.; Ariaei, N.; Ali-Ehyaii, F.; Behnam, H. Diabetes mellitus and sensorineural hearing loss among non-elderly people. East. Mediterr. Health J. 2010, 16, 947–952. [Google Scholar] [CrossRef]
- Aladag, İ.; Eyibilen, A.; Güven, M.; Atış, Ö.; Erkokmaz, Ü. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J. Laryngol. Otol. 2009, 123, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Wang, J.J.; Boyages, S.C.; Leeder, S.R. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet. Med. 2009, 26, 483–488. [Google Scholar] [CrossRef]
- Sakuta, H.; Suzuki, T.; Yasuda, H.; Ito, T. Type 2 diabetes and hearing loss in personnel of the Self-Defense Forces. Diabetes Res Clin Pract. 2007, 75, 229–234. [Google Scholar] [CrossRef]
- Lin, F.R.; Albert, M. Hearing loss and dementia—Who is listening? Aging Ment. Health 2014, 18, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.N.; Djalilian, H.R. Approaches to mitigate mitochondrial dysfunction in sensorineural hearing loss. Ann. Biomed. Eng. 2022, 50, 1762–1770. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Song, L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear Res. 2023, 434, 108783. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, M.; Hato, N.; Inufusa, H.; You, F. Role of oxidative stress in sensorineural hearing loss. Int. J. Mol. Sci. 2024, 25, 4146. [Google Scholar] [CrossRef]
- Paciello, F.; Ripoli, C.; Fetoni, A.R.; Grassi, C. Redox imbalance as a common pathogenic factor linking hearing loss and cognitive decline. Antioxidants 2023, 12, 332. [Google Scholar] [CrossRef]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Juiz, J.M. Frailty syndrome and oxidative stress as possible links between age-related hearing loss and Alzheimer’s disease. Front. Neurosci. 2021, 15, 816300. [Google Scholar] [CrossRef]
- Nadhimi, Y.; Llano, D.A. Does hearing loss lead to dementia? A review of the literature. Hear Res. 2021, 402, 108038. [Google Scholar] [CrossRef]
- Weible, A.P.; Wehr, M. Amyloid pathology in the central auditory pathway of 5XFAD mice appears first in auditory cortex. J. Alzheimers Dis. 2022, 89, 1385–1402. [Google Scholar] [CrossRef]
- Na, D.; Zhang, J.; Beaulac, H.J.; Piekna-Przybylska, D.; Nicklas, P.R.; Kiernan, A.E.; White, P.M. Increased central auditory gain in 5xFAD Alzheimer’s disease mice as an early biomarker candidate for Alzheimer’s disease diagnosis. Front. Neurosci. 2023, 17, 1106570. [Google Scholar]
- Gates, G.A.; Beiser, A.; Rees, T.S.; D’Agostino, R.B.; Wolf, P.A. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J. Am. Geriatr. Soc. 2002, 50, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Shad, K.F.; Soubra, W.; Cordato, D.J. The auditory afferent pathway as a clinical marker of Alzheimer’s disease. J. Alzheimers Dis. 2022, 85, 47–53. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, G.; Wen, H.; Tian, X.; Wei, G.; Wang, X.; Yang, H.; Sun, S. Adjunct methods for Alzheimer’s disease detection: A review of auditory evoked potentials. J. Alzheimers Dis. 2024, 97, 1503–1517. [Google Scholar]
- McEvoy, L.K.; Bergstrom, J.; Hagler, D.J.; Wing, D.; Reas, E.T. Elevated pure tone thresholds are associated with altered microstructure in cortical areas related to auditory processing and attentional allocation. J. Alzheimers Dis. 2023, 96, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Grassi, C.; Paludetti, G.; Paciello, F.; Pisani, A.; Cocco, S.; Rinaudo, M.; Fetoni, A.R. Noise-induced auditory damage affects hippocampus causing memory deficits in a model of early age-related hearing loss. Neurobiol. Dis. 2023, 178, 106024. [Google Scholar]
- Liang, Z.; Li, A.; Xu, Y.; Qian, X.; Gao, X. Hearing loss and dementia: A meta-analysis of prospective cohort studies. Front. Aging Neurosci. 2021, 13, 695117. [Google Scholar] [CrossRef]
- de Oliveira, C.; Samelli, A.G.; de Paiva, K.M.; Xavier, A.J.; Valsechi, F.E.; Hillesheim, D.; D’oRsi, E. Does cognitive impairment precede self-reported poor hearing? Results from the English longitudinal study of ageing. Int. J. Audiol. 2023, 62, 787–794. [Google Scholar]
- Smeeth, L.; Ng, T.P.; Zhao, M.-H.; Nagel, G.; Ulmer, H.; Jha, A.K.; Eriksson, J.G.; Feskens, E.J.; Santos, R.; Rigo, F.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022, a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar]
- Bruemmer, D.; Matfin, G.; American Diabetes Association Professional Practice Committee; Polsky, S.; Selvin, E.; Beverly, E.A.; McCoy, R.G.; Lingvay, I.; Khunti, K.; Gaglia, J.L.; et al. 2. Diagnosis and classification of diabetes: Standards of care in diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-related hearing loss cognitive decline: Potential mechanisms linking two. Auris Nasus Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Schilder, A.G.; Lasica, A.B.; Yu, R.-C.; Sheppard, J.; Ridgway, N.; Omar, R.; Costafreda, S.G. Association between adult-onset hearing loss and dementia biomarkers: A systematic review. Ageing Res. Rev. 2025, 104, 102647. [Google Scholar]
- Akinpelu, O.V.; Ibrahim, F.; Waissbluth, S.; Daniel, S.J. Histopathologic changes in the cochlea associated with diabetes mellitus—A review. Otol. Neurotol. 2014, 35, 764–774. [Google Scholar] [CrossRef]
- Fukushima, H.; Cureoglu, S.; Schachern, P.A.; Paparella, M.M.; Harada, T.; Oktay, M.F. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 934. [Google Scholar] [CrossRef]
- Akinpelu, O.V.; Mujica-Mota, M.; Daniel, S.J. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope 2014, 124, 767–776. [Google Scholar] [CrossRef]
- Kim, M.-B.; Zhang, Y.; Chang, Y.; Ryu, S.; Choi, Y.; Kwon, M.-J.; Moon, I.J.; Deal, J.A.; Lin, F.R.; Guallar, E.; et al. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int. J. Epidemiol. 2017, 46, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shi, T.F.; Zhou, Z.; Jiang, W.J.; Huang, T.L.; Si, J.Q.; Li, L. Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway. Redox Rep. 2024, 29, 2382943. [Google Scholar] [CrossRef]
- Canis, M.; Bertlich, M. Cochlear capillary pericytes. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 115–123. [Google Scholar]
- Lyu, A.-R.; Shin, S.-A.; Huh, Y.H.; Yu, Y.; Je, A.R.; Kim, T.-H.; Kim, E.-H.; Gajbhiye, A.; Kwon, H.-C.; Park, Y.-H.; et al. Hearing impairment in a mouse model of diabetes is associated with mitochondrial dysfunction, synaptopathy, and activation of the intrinsic apoptosis pathway. Int. J. Mol. Sci. 2021, 22, 8807. [Google Scholar] [CrossRef]
- Sheikhzadeh, M.; Bagheri, F.; Bayani, M.A.; Kami, M.; Monadi, M. Evaluation of the auditory brainstem response test in patients with type 2 diabetes mellitus. Caspian J. Intern. Med. 2024, 15, 527–534. [Google Scholar]
- Gupta, S.; Baweja, P.; Mittal, S.; Kumar, A.; Singh, K.; Sharma, R. Brainstem auditory evoked potential abnormalities in type 2 diabetes mellitus. N. Am. J. Med. Sci. 2013, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Wu, B.; Ryugo, D.K. Diabetes mellitus and hearing loss: A review. Ageing Res. Rev. 2021, 71, 101423. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, J.P.; Thompson, D.M.; Lehman, A. Diabetes associated with maternally inherited diabetes and deafness (MIDD): From pathogenic variant to phenotype. Diabetes 2025, 74, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liao, L.; Dong, J.; Xu, L.; Yang, M.; Jiang, S.; Xu, C. The mutations and clinical variability in maternally inherited diabetes and deafness: An analysis of 161 patients. Front. Endocrinol. 2021, 12, 728043. [Google Scholar] [CrossRef]
- Murphy, R.; Turnbull, D.M.; Walker, M.; Hattersley, A.T. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet. Med. 2008, 25, 383–399. [Google Scholar] [CrossRef]
- Yamasoba, T.; Oka, Y.; Tsukuda, K.; Nakamura, M.; Kaga, K. Auditory findings in patients with maternally inherited diabetes and deafness harboring a point mutation in the mitochondrial transfer RNALeu (UUR) gene. Laryngoscope 1996, 106, 49–53. [Google Scholar] [CrossRef]
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Effects of diabetes mellitus-related dysglycemia on the functions of blood–brain barrier and the risk of dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef]
- Ehtewish, H.; Arredouani, A.; El-Agnaf, O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int. J. Mol. Sci. 2022, 23, 6144. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Wang, M.; Hua, S.; Liao, H.; Cao, F.; Xiong, Y. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Tan, L.; Tan, M.-S.; Xue, M.; Cao, X.-P.; Ou, Y.-N.; Xu, W.; Yu, J.-T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, Z.; Ma, C.; Wang, Z.; Wang, K. Relationship between glycemic control and cognitive impairment: A systematic review and meta-analysis. Front. Aging Neurosci. 2023, 15, 1126183. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reijmer, Y.D. Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes 2014, 63, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Xu, Y.; Li, W. Type 2 diabetes is associated with increased risk of dementia, but not mild cognitive impairment: A cross-sectional study among the elderly in Chinese communities. Front. Aging Neurosci. 2022, 14, 1004954. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.N.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waissbluth, S.; Delano, P.H. Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sci. 2025, 15, 669. https://doi.org/10.3390/brainsci15070669
Waissbluth S, Delano PH. Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sciences. 2025; 15(7):669. https://doi.org/10.3390/brainsci15070669
Chicago/Turabian StyleWaissbluth, Sofia, and Paul H. Delano. 2025. "Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia" Brain Sciences 15, no. 7: 669. https://doi.org/10.3390/brainsci15070669
APA StyleWaissbluth, S., & Delano, P. H. (2025). Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sciences, 15(7), 669. https://doi.org/10.3390/brainsci15070669






