Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia
Abstract
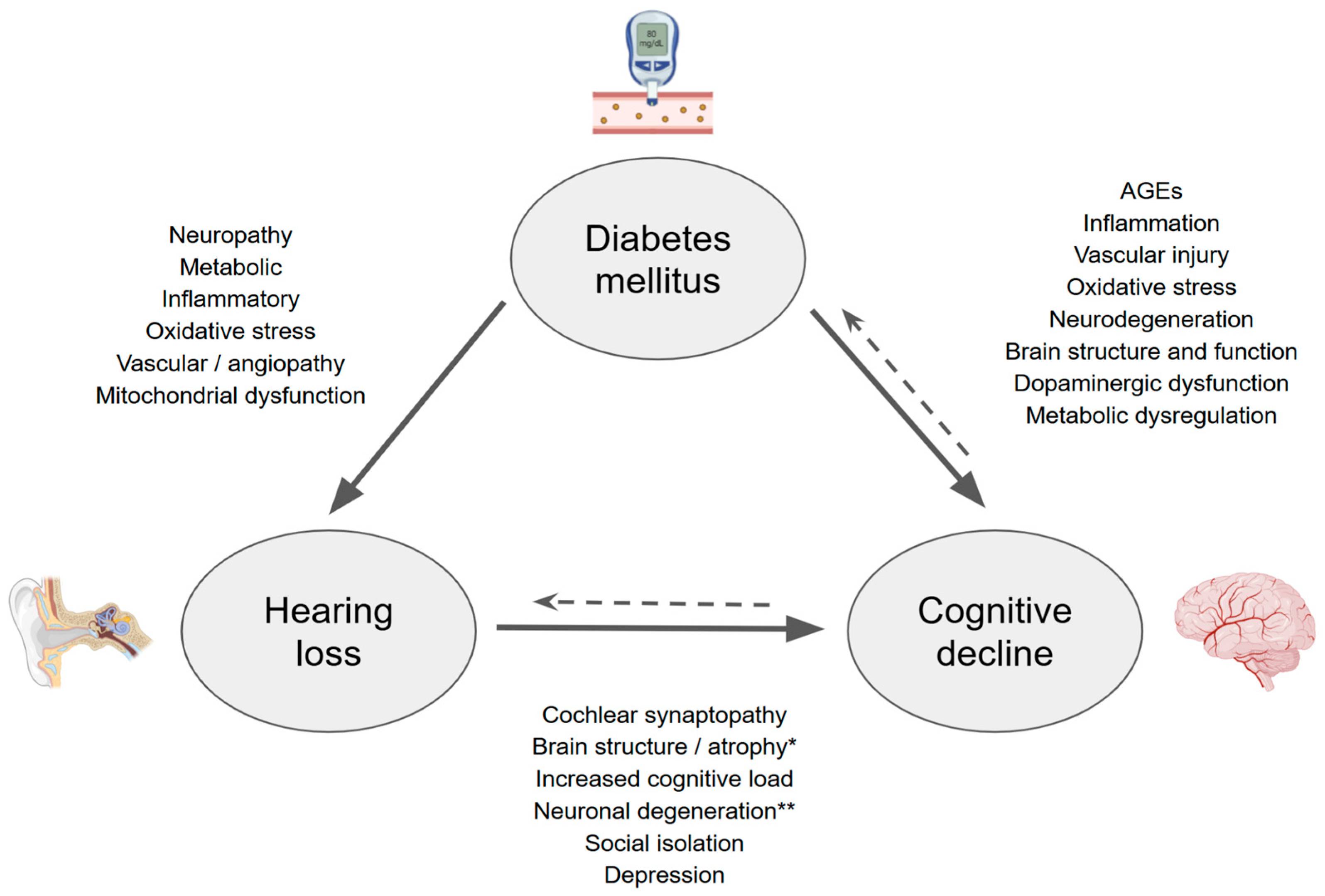
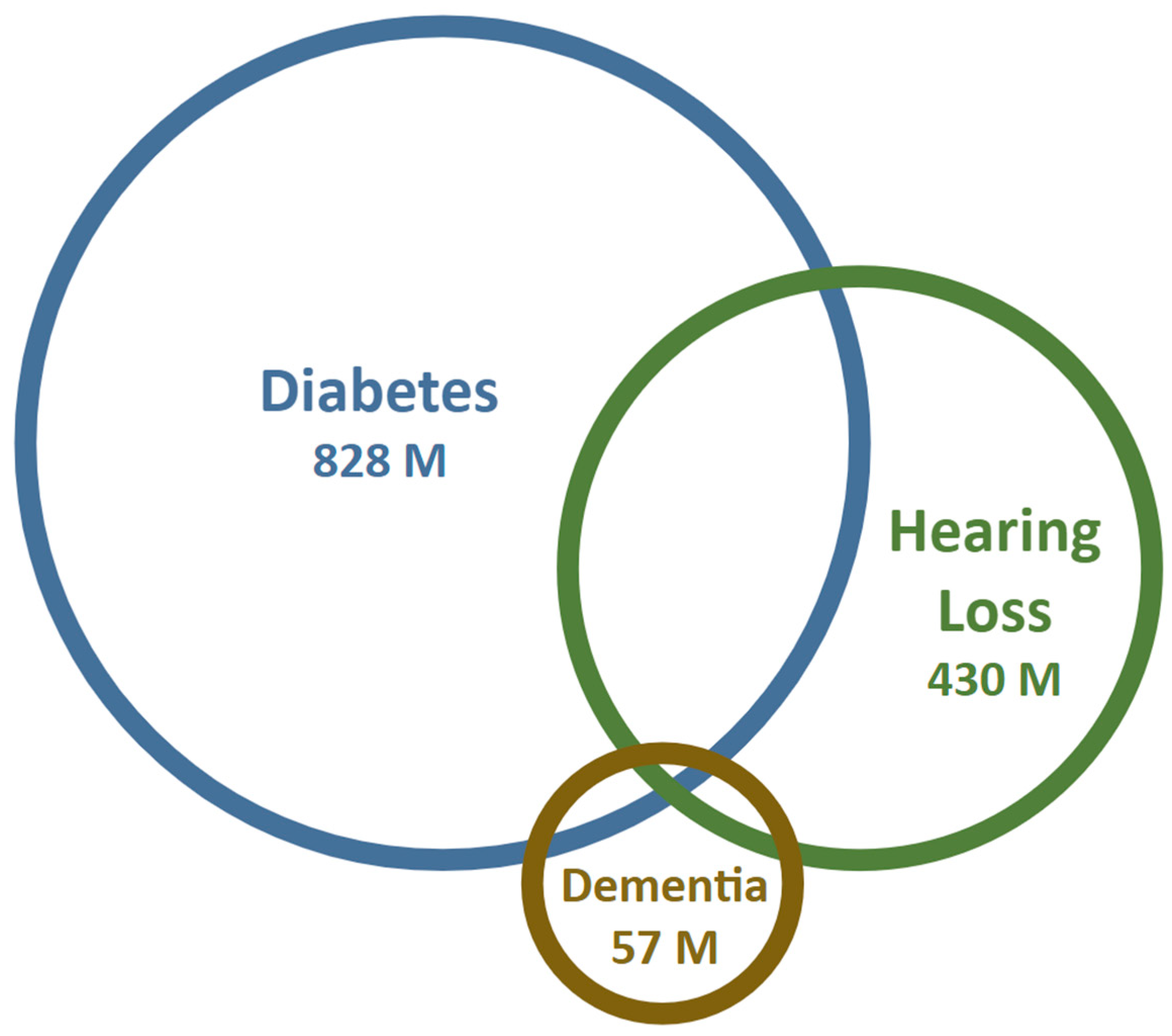
1. Introduction
2. Hearing Loss and Its Relationship with Cognition
3. What About Hearing Loss and Dementia?
| Study | Sample (Control/Diabetic) | Age (Control/Diabetic) | Incidence of Hearing Loss (Control/Diabetic) |
|---|---|---|---|
| ElSherif M et al. (2024) [48] | 40/42 | 49.7 ± 6.4/50.7 ± 6.4 | 0%/9.5% |
| Alizadeh Y et al. (2022) [49] | 105/315 | 60.0 ± 8.8/59.8 ± 8.2 | Control: 10.2% Diabetic: 11.6% (no DR) * 12.9% (mild–mod NPDR) 34.2% (severe NPDR/PDR) |
| Mishra A & Poorey (2019) [50] | 50/50 | Age-matched | 18%/74% |
| Li J et al. (2018) [51] | 43/51 | 54.4 ± 10.1/56.1 ± 10.1 | 25.6%/45.1% |
| Adebola S et al. (2016) [52] | 90/97 | 58.8 ± 14.7/58.9 ± 14.9 | 8.9%/21.5% |
| Bamanie A & Al-Noury K (2011) [53] | 87/109 | 45.7/47.9 | 39.1%/69.7% |
| Mozaffari M et al. (2010) [54] | 80/80 | 45.1/45 | 20%/45% |
| Aladag I et al. (2009) [55] | 37/63 | 47.5/46.6 | 48.6%/44% |
| Mitchell P et al. (2009) [56] | 1648/210 | 69.7/70.5 | 38.2%/50% |
| Sakuta H et al. (2007) [57] | 596/103 | 52.9 ± 1.0 | 45.2%/60.2% |
4. Diabetes Mellitus and Hearing Loss
5. Diabetes Mellitus and Cognitive Decline
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD Results. Institute for Health Metrics and Evaluation. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 23 May 2025).
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 23 May 2025).
- Mittal, J.; Mittal, R.; Lemos, J.R.N.; Hirani, K.; Keith, G.; Lacey, M.; Assayed, A. Diabetes mellitus, hearing loss, and therapeutic interventions: A systematic review of insights from preclinical animal models. PLoS ONE 2024, 19, e0305617. [Google Scholar] [CrossRef]
- Lee, H.J.; Joo, Y.H.; Han, K.D.; Park, K.H. Association between hearing loss and cognitive disorder: A nationwide population-based study. Yonsei Med. J. 2021, 62, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Ando, F.; Shimokata, H.; Uchida, Y.; Otsuka, R.; Nakamura, A.; Tange, C.; Iwata, K.; Nishita, Y.; Suzuki, H.; et al. Smaller hippocampal volume and degraded peripheral hearing among Japanese community dwellers. Front. Aging Neurosci. 2018, 10, 319. [Google Scholar]
- Davatzikos, C.; An, Y.; Doshi, J.; Resnick, S.M.; Lin, F.R.; Deal, J.A.; Erus, G.; Armstrong, N.M.; Ferrucci, L. Association of midlife hearing impairment with late-life temporal lobe volume loss. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 794–802. [Google Scholar]
- Wang, L.; Feng, J.; Wang, H.-F.; Li, Y.; Ma, Y.-H.; Rolls, E.T.; Zhang, W.; Kang, J.; Cheng, W.; Yu, J.-T. Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology. eBioMedicine 2022, 86, 104336. [Google Scholar] [CrossRef]
- WHO. World Report on Hearing. Available online: https://www.who.int/teams/noncommunicable-diseases/sensory-functions-disability-and-rehabilitation/highlighting-priorities-for-ear-and-hearing-care (accessed on 10 April 2025).
- Frank, L. Age-Related Hearing Loss. N. Engl. J. Med. 2024, 390, 1505–1512. [Google Scholar] [CrossRef]
- Tsai Do, B.S.; Bush, M.L.; Weinreich, H.M.; Schwartz, S.R.; Anne, S.; Adunka, O.F.; Bender, K.; Bold, K.M.; Brenner, M.J.; Hashmi, A.Z.; et al. Clinical practice guideline: Age-related hearing loss. Otolaryngol. Head Neck Surg. 2024, 170 (Suppl. S2), S1–S54. [Google Scholar] [CrossRef]
- Ganek, H.V.; Madubueze, A.; Merritt, C.E.; Bhutta, Z.A. Prevalence of Hearing Loss in Children Living in Low- And Middle-Income Countries Over the Last 10 years: A Systematic Review. Dev. Med. Child Neurol. 2023, 65, 600–610. [Google Scholar] [CrossRef]
- Dubno, J.R.; Wilson, R.H.; Wingfield, A.; Humes, L.E.; Gordon-Salant, S.; Gates, G.A.; Cacace, A.T.; Lister, J.J.; Cruickshanks, K.J. Central presbycusis: A review and evaluation of the evidence. J. Am. Acad. Audiol. 2012, 23, 635–666. [Google Scholar] [CrossRef]
- Delgado, C.; Torrente, M.C.; Moreno-Gómez, F.N.; Leiva, A.; Marcenaro, B.; Belkhiria, C.; Martin, S.S.; Vergara, R.; Delano, P.H. Speech Perception and Dichotic Listening Are Associated with Hearing Thresholds and Cognition, Respectively, in Unaided Presbycusis. Front. Aging Neurosci. 2022, 14, 786330. [Google Scholar] [CrossRef]
- Meimaroglou, S.; Eleftheriadis, N.; Iliadou, V.M. Better education required for professionals in healthcare regarding auditory processing disorder. Eur. Arch. Otorhinolaryngol. 2025, 282, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Steel, K.P.; Schmiedt, R.A.; Schulte, B.A.; Lewis, M.A.; Harris, K.C.; Lang, H.; Dubno, J.R.; Vaden, K.I. Translational and interdisciplinary insights into presbyacusis: A multidimensional disease. Hear Res. 2021, 402, 108109. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.L.; Fritzsch, B.; Yamoah, E.N.; Zine, A. Age-related hearing loss: Sensory and neural etiology and their interdependence. Front. Aging Neurosci. 2022, 14, 814528. [Google Scholar] [CrossRef] [PubMed]
- Moser, T.; Starr, A. Auditory Neuropathy--Neural Synaptic Mechanisms. Nat. Rev. Neurol. 2016, 12, 135–149. [Google Scholar] [CrossRef]
- Rance, G.; Starr, A. Pathophysiological mechanisms and functional hearing consequences of auditory neuropathy. Brain 2015, 138 Pt 11, 3141–3158. [Google Scholar] [CrossRef]
- Wu, P.Z.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Primary neural degeneration in noise-exposed human cochleas: Correlations with outer hair cell loss and word-discrimination scores. J. Neurosci. 2021, 41, 4439–4447. [Google Scholar] [CrossRef]
- Liberman, M.C.; Epstein, M.J.; Cleveland, S.S.; Wang, H.; Maison, S.F. Toward a differential diagnosis of hidden hearing loss in humans. PLoS ONE 2016, 11, e0162726. [Google Scholar] [CrossRef]
- Shearer, A.E.; Hildebrand, M.S.; Schaefer, A.M. Genetic Hearing Loss Overview; StatPearls: Petersburg, FL, USA, 2023. [Google Scholar]
- Moverman, D.J.; Liberman, L.D.; Kraemer, S.; Corfas, G.; Liberman, M.C. Ultrastructure of noise-induced cochlear synaptopathy. Sci. Rep. 2023, 13, 19456. [Google Scholar] [CrossRef]
- Suthakar, K.; Liberman, M.C. Auditory-nerve responses in mice with noise-induced cochlear synaptopathy. J. Neurophysiol. 2021, 126, 2027–2038. [Google Scholar] [CrossRef]
- Ding, D.; Salvi, R.; Chen, G.-D.; Auerbach, B.D.; Lobarinas, E.; Radziwon, K.; Sun, W.; Wang, J. Inner hair cell loss disrupts hearing and cochlear function leading to sensory deprivation and enhanced central auditory gain. Front. Neurosci. 2017, 10, 621. [Google Scholar]
- Harris, K.C.; Dias, J.W.; McClaskey, C.M.; Rumschlag, J.; Prisciandaro, J.; Dubno, J.R. Afferent loss, GABA, and central gain in older adults: Associations with speech recognition in noise. J. Neurosci. 2022, 42, 7201–7212. [Google Scholar] [CrossRef]
- Harris, K.C.; Dias, J.W.; Lang, H.; Panganiban, C.; McClaskey, C.M.; Noble, K.V.; Kerouac, L.B.; Rumschlag, J.A. Age-related central gain with degraded neural synchrony in the auditory brainstem of mice and humans. Neurobiol. Aging 2022, 115, 50–59. [Google Scholar]
- Rüttiger, L.; Singer, W.; Klose, U.; Wolpert, S.; Hofmeier, B.; Refat, F.; Wertz, J.; Hinrichs, P.; Saemisch, J.; Knipper, M. Functional biomarkers that distinguish between tinnitus with and without hyperacusis. Clin. Transl. Med. 2021, 11, e378. [Google Scholar]
- Peelle, J.E.; Wingfield, A. The neural consequences of age-related hearing loss. Trends Neurosci. 2016, 39, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Slade, K.; Plack, C.J.; Nuttall, H.E. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. 2020, 43, 810–821. [Google Scholar] [CrossRef]
- Doshi, J.; Ferrucci, L.; Metter, E.; Kraut, M.; Resnick, S.; An, Y.; Lin, F.; Davatzikos, C.; Goh, J. Association of hearing impairment with brain volume changes in older adults. Neuroimage 2014, 90, 84–92. [Google Scholar]
- Vernooij, M.W.; Ikram, M.A.; Bos, D.; Metselaar, M.; Goedegebure, A.; Roshchupkin, G.V.; Rigters, S.C.; de Jong, R.J.B. Hearing impairment is associated with smaller brain volume in aging. Front. Aging Neurosci. 2017, 9, 2. [Google Scholar]
- Belkhiria, C.; Vergara, R.C.; Martín, S. Cingulate Cortex Atrophy Associated Hearing Loss Presbycusis Cochlear Amplifier Dysfunction. Front. Aging Neurosci. 2019, 11, 97. [Google Scholar] [CrossRef]
- Martinez, M.; Delano, P.H.; Andrade, M.; Leiva, A.; Marcenaro, B.; Belkhiria, C.; Vergara, R.C.; Martin, S.S.; Delgado, C. Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front. Aging Neurosci. 2020, 12, 102. [Google Scholar]
- Edden, R.A.; Li, F.; Wu, L.; Zong, W.; Ma, W.; Li, N.; Li, X.; Hui, S.C.; Ren, F.; Dai, Z.; et al. Neurochemical and functional reorganization of the cognitive-ear link underlies cognitive impairment in presbycusis. Neuroimage 2023, 268, 119861. [Google Scholar]
- Vergara, R.; Delgado, C.; Delano, P.H.; García, X.; Vidal, V.; Cerda, M.; Navarro, C.F.; Leiva, A.; Martínez, M.; Belkhiria, C.; et al. Cochlear dysfunction as an early biomarker of cognitive decline in normal hearing and mild hearing loss. Alzheimers Dement. 2024, 16, e12467. [Google Scholar]
- Zhao, F.; Gao, M.; Feng, T.; Shen, J.; Zheng, Y.; Liang, J.; Yang, H. Cognitive reserve disorder in age-related hearing loss: Cognitive cortical compensatory to auditory perceptual processing. Cereb. Cortex 2023, 33, 9616–9626. [Google Scholar]
- Albers, M.W.; Gilmore, G.C.; Kaye, J.; Murphy, C.; Wingfield, A.; Bennett, D.A.; Boxer, A.L.; Buchman, A.S.; Cruickshanks, K.J.; Devanand, D.P.; et al. At the interface of sensory and motor dysfunctions and Alzheimer’s disease. Alzheimers Dement. 2015, 11, 70–98. [Google Scholar] [CrossRef]
- Lin, F.R.; Metter, E.J.; O’Brien, R.J.; Resnick, S.M.; Zonderman, A.B.; Ferrucci, L. Hearing loss and incident dementia. Arch. Neurol. 2011, 68, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and management of dementia: Review. JAMA 2019, 322, 1589. [Google Scholar] [CrossRef]
- Oh, E.S. Dementia. Ann. Intern. Med. 2024, 177, ITC161–ITC176. [Google Scholar] [CrossRef]
- Cao, Q.; Tan, C.C.; Xu, W.; Hu, H.; Cao, X.-P.; Dong, Q.; Tan, L. The prevalence of dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2020, 73, 1157–1166. [Google Scholar] [CrossRef]
- Aguilar-Navarro, S.G.; de la Peña, J.E.; Gutierez-Gutierez, L.; Suerna-Hernandez, A.; Gonzelez-Figueroa, E.; Juarez-Cedillo, T.; Garcia-Cruz, J.C. Prevalence of dementia and main subtypes in Mexico: The study on aging and dementia in Mexico (SADEM). J. Alzheimers Dis. 2022, 89, 931–941. [Google Scholar]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lage, J.; et al. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54 (Suppl. S5), S4–S9. [Google Scholar]
- Ren, Z.; Wang, X.-D.; Tang, Y.; Lv, Y.; Huang, G.; Niu, J.; Gang, B.; Meng, X.; Cai, P.; Zeng, Y.; et al. The updated prevalence and risk factors of dementia in old adults in China: A cross-sectional study. J. Alzheimers Dis. 2024, 102, 1209–1223. [Google Scholar]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-S.; Lee, M.K.; Rah, Y.C.; Park, S.; Kim, B.; Choi, J.; Han, K. Association between the severity of hearing loss and the risk of dementia within the 2010–2017 national insurance service survey in South Korea. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Mandavia, R.; Omar, R.; Pavlou, M.; Lin, F.; Schilder, A.; Proctor, D.; Yu, R.-C.; Gonzalez, S.C.; Lewis, G.; et al. Adult-onset hearing loss and incident cognitive impairment and dementia—A systematic review and meta-analysis of cohort studies. Ageing Res. Rev. 2024, 98, 102346. [Google Scholar]
- Department of Otorhinolaryngology, Audiovestibular Medicine Unit, Alexandria University Faculty of Medicine, Alexandria, Egypt; ElSherif, M.; El Sayed Mahfouz, A.F.; Mohamed Gaber Amin, N.; Saad Kozou, H. Relation between glycated hemoglobin level and hearing loss in type 2 diabetic patients. J. Int. Adv. Otol. 2024, 20, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, Y.; Jalali, M.M.; Sehati, A. Association of different severity of diabetic retinopathy and hearing loss in type 2 diabetes mellitus. Am. J. Otolaryngol. 2022, 43, 103383. [Google Scholar] [CrossRef]
- Mishra, A.; Poorey, V.K. Clinical and audiometric assessment of hearing loss in diabetes mellitus. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 1490–1494. [Google Scholar] [CrossRef]
- Bi, J.; Liu, B.; Zhang, Y.; Li, Y.; Li, J.; Fu, X.; Zhang, L. Alteration of auditory function in type 2 diabetic and pre-diabetic patients. Acta Otolaryngol. 2018, 138, 542–547. [Google Scholar]
- Adebola, S.O.; Olamoyegun, M.A.; Sogebi, O.A.; Iwuala, S.O.; Babarinde, J.A.; Oyelakin, A.O. Otologic and audiologic characteristics of type 2 diabetics in a tertiary health institution in Nigeria. Braz. J. Otorhinolaryngol. 2016, 82, 567–573. [Google Scholar] [CrossRef]
- Bamanie, A.H.; Al-Noury, K.I. Prevalence of hearing loss among Saudi type 2 diabetic patients. Saudi Med. J. 2011, 32, 271–274. [Google Scholar]
- Mozaffari, M.; Tajik, A.; Ariaei, N.; Ali-Ehyaii, F.; Behnam, H. Diabetes mellitus and sensorineural hearing loss among non-elderly people. East. Mediterr. Health J. 2010, 16, 947–952. [Google Scholar] [CrossRef]
- Aladag, İ.; Eyibilen, A.; Güven, M.; Atış, Ö.; Erkokmaz, Ü. Role of oxidative stress in hearing impairment in patients with type two diabetes mellitus. J. Laryngol. Otol. 2009, 123, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Gopinath, B.; McMahon, C.M.; Rochtchina, E.; Wang, J.J.; Boyages, S.C.; Leeder, S.R. Relationship of Type 2 diabetes to the prevalence, incidence and progression of age-related hearing loss. Diabet. Med. 2009, 26, 483–488. [Google Scholar] [CrossRef]
- Sakuta, H.; Suzuki, T.; Yasuda, H.; Ito, T. Type 2 diabetes and hearing loss in personnel of the Self-Defense Forces. Diabetes Res Clin Pract. 2007, 75, 229–234. [Google Scholar] [CrossRef]
- Lin, F.R.; Albert, M. Hearing loss and dementia—Who is listening? Aging Ment. Health 2014, 18, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.N.; Djalilian, H.R. Approaches to mitigate mitochondrial dysfunction in sensorineural hearing loss. Ann. Biomed. Eng. 2022, 50, 1762–1770. [Google Scholar] [CrossRef]
- Tan, W.J.T.; Song, L. Role of mitochondrial dysfunction and oxidative stress in sensorineural hearing loss. Hear Res. 2023, 434, 108783. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, M.; Hato, N.; Inufusa, H.; You, F. Role of oxidative stress in sensorineural hearing loss. Int. J. Mol. Sci. 2024, 25, 4146. [Google Scholar] [CrossRef]
- Paciello, F.; Ripoli, C.; Fetoni, A.R.; Grassi, C. Redox imbalance as a common pathogenic factor linking hearing loss and cognitive decline. Antioxidants 2023, 12, 332. [Google Scholar] [CrossRef]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Juiz, J.M. Frailty syndrome and oxidative stress as possible links between age-related hearing loss and Alzheimer’s disease. Front. Neurosci. 2021, 15, 816300. [Google Scholar] [CrossRef]
- Nadhimi, Y.; Llano, D.A. Does hearing loss lead to dementia? A review of the literature. Hear Res. 2021, 402, 108038. [Google Scholar] [CrossRef]
- Weible, A.P.; Wehr, M. Amyloid pathology in the central auditory pathway of 5XFAD mice appears first in auditory cortex. J. Alzheimers Dis. 2022, 89, 1385–1402. [Google Scholar] [CrossRef]
- Na, D.; Zhang, J.; Beaulac, H.J.; Piekna-Przybylska, D.; Nicklas, P.R.; Kiernan, A.E.; White, P.M. Increased central auditory gain in 5xFAD Alzheimer’s disease mice as an early biomarker candidate for Alzheimer’s disease diagnosis. Front. Neurosci. 2023, 17, 1106570. [Google Scholar]
- Gates, G.A.; Beiser, A.; Rees, T.S.; D’Agostino, R.B.; Wolf, P.A. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer’s disease. J. Am. Geriatr. Soc. 2002, 50, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Shad, K.F.; Soubra, W.; Cordato, D.J. The auditory afferent pathway as a clinical marker of Alzheimer’s disease. J. Alzheimers Dis. 2022, 85, 47–53. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, G.; Wen, H.; Tian, X.; Wei, G.; Wang, X.; Yang, H.; Sun, S. Adjunct methods for Alzheimer’s disease detection: A review of auditory evoked potentials. J. Alzheimers Dis. 2024, 97, 1503–1517. [Google Scholar]
- McEvoy, L.K.; Bergstrom, J.; Hagler, D.J.; Wing, D.; Reas, E.T. Elevated pure tone thresholds are associated with altered microstructure in cortical areas related to auditory processing and attentional allocation. J. Alzheimers Dis. 2023, 96, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Grassi, C.; Paludetti, G.; Paciello, F.; Pisani, A.; Cocco, S.; Rinaudo, M.; Fetoni, A.R. Noise-induced auditory damage affects hippocampus causing memory deficits in a model of early age-related hearing loss. Neurobiol. Dis. 2023, 178, 106024. [Google Scholar]
- Liang, Z.; Li, A.; Xu, Y.; Qian, X.; Gao, X. Hearing loss and dementia: A meta-analysis of prospective cohort studies. Front. Aging Neurosci. 2021, 13, 695117. [Google Scholar] [CrossRef]
- de Oliveira, C.; Samelli, A.G.; de Paiva, K.M.; Xavier, A.J.; Valsechi, F.E.; Hillesheim, D.; D’oRsi, E. Does cognitive impairment precede self-reported poor hearing? Results from the English longitudinal study of ageing. Int. J. Audiol. 2023, 62, 787–794. [Google Scholar]
- Smeeth, L.; Ng, T.P.; Zhao, M.-H.; Nagel, G.; Ulmer, H.; Jha, A.K.; Eriksson, J.G.; Feskens, E.J.; Santos, R.; Rigo, F.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022, a pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar]
- Bruemmer, D.; Matfin, G.; American Diabetes Association Professional Practice Committee; Polsky, S.; Selvin, E.; Beverly, E.A.; McCoy, R.G.; Lingvay, I.; Khunti, K.; Gaglia, J.L.; et al. 2. Diagnosis and classification of diabetes: Standards of care in diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S27–S49. [Google Scholar]
- Uchida, Y.; Sugiura, S.; Nishita, Y.; Saji, N.; Sone, M.; Ueda, H. Age-related hearing loss cognitive decline: Potential mechanisms linking two. Auris Nasus Larynx 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Schilder, A.G.; Lasica, A.B.; Yu, R.-C.; Sheppard, J.; Ridgway, N.; Omar, R.; Costafreda, S.G. Association between adult-onset hearing loss and dementia biomarkers: A systematic review. Ageing Res. Rev. 2025, 104, 102647. [Google Scholar]
- Akinpelu, O.V.; Ibrahim, F.; Waissbluth, S.; Daniel, S.J. Histopathologic changes in the cochlea associated with diabetes mellitus—A review. Otol. Neurotol. 2014, 35, 764–774. [Google Scholar] [CrossRef]
- Fukushima, H.; Cureoglu, S.; Schachern, P.A.; Paparella, M.M.; Harada, T.; Oktay, M.F. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 934. [Google Scholar] [CrossRef]
- Akinpelu, O.V.; Mujica-Mota, M.; Daniel, S.J. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. Laryngoscope 2014, 124, 767–776. [Google Scholar] [CrossRef]
- Kim, M.-B.; Zhang, Y.; Chang, Y.; Ryu, S.; Choi, Y.; Kwon, M.-J.; Moon, I.J.; Deal, J.A.; Lin, F.R.; Guallar, E.; et al. Diabetes mellitus and the incidence of hearing loss: A cohort study. Int. J. Epidemiol. 2017, 46, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Shi, T.F.; Zhou, Z.; Jiang, W.J.; Huang, T.L.; Si, J.Q.; Li, L. Hyperglycemia-induced oxidative stress exacerbates mitochondrial apoptosis damage to cochlear stria vascularis pericytes via the ROS-mediated Bcl-2/CytC/AIF pathway. Redox Rep. 2024, 29, 2382943. [Google Scholar] [CrossRef]
- Canis, M.; Bertlich, M. Cochlear capillary pericytes. In Advances in Experimental Medicine and Biology; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 115–123. [Google Scholar]
- Lyu, A.-R.; Shin, S.-A.; Huh, Y.H.; Yu, Y.; Je, A.R.; Kim, T.-H.; Kim, E.-H.; Gajbhiye, A.; Kwon, H.-C.; Park, Y.-H.; et al. Hearing impairment in a mouse model of diabetes is associated with mitochondrial dysfunction, synaptopathy, and activation of the intrinsic apoptosis pathway. Int. J. Mol. Sci. 2021, 22, 8807. [Google Scholar] [CrossRef]
- Sheikhzadeh, M.; Bagheri, F.; Bayani, M.A.; Kami, M.; Monadi, M. Evaluation of the auditory brainstem response test in patients with type 2 diabetes mellitus. Caspian J. Intern. Med. 2024, 15, 527–534. [Google Scholar]
- Gupta, S.; Baweja, P.; Mittal, S.; Kumar, A.; Singh, K.; Sharma, R. Brainstem auditory evoked potential abnormalities in type 2 diabetes mellitus. N. Am. J. Med. Sci. 2013, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Samocha-Bonet, D.; Wu, B.; Ryugo, D.K. Diabetes mellitus and hearing loss: A review. Ageing Res. Rev. 2021, 71, 101423. [Google Scholar] [CrossRef] [PubMed]
- Chanoine, J.P.; Thompson, D.M.; Lehman, A. Diabetes associated with maternally inherited diabetes and deafness (MIDD): From pathogenic variant to phenotype. Diabetes 2025, 74, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liao, L.; Dong, J.; Xu, L.; Yang, M.; Jiang, S.; Xu, C. The mutations and clinical variability in maternally inherited diabetes and deafness: An analysis of 161 patients. Front. Endocrinol. 2021, 12, 728043. [Google Scholar] [CrossRef]
- Murphy, R.; Turnbull, D.M.; Walker, M.; Hattersley, A.T. Clinical features, diagnosis and management of maternally inherited diabetes and deafness (MIDD) associated with the 3243A>G mitochondrial point mutation. Diabet. Med. 2008, 25, 383–399. [Google Scholar] [CrossRef]
- Yamasoba, T.; Oka, Y.; Tsukuda, K.; Nakamura, M.; Kaga, K. Auditory findings in patients with maternally inherited diabetes and deafness harboring a point mutation in the mitochondrial transfer RNALeu (UUR) gene. Laryngoscope 1996, 106, 49–53. [Google Scholar] [CrossRef]
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Effects of diabetes mellitus-related dysglycemia on the functions of blood–brain barrier and the risk of dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef]
- Ehtewish, H.; Arredouani, A.; El-Agnaf, O. Diagnostic, prognostic, and mechanistic biomarkers of diabetes mellitus-associated cognitive decline. Int. J. Mol. Sci. 2022, 23, 6144. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Wang, M.; Hua, S.; Liao, H.; Cao, F.; Xiong, Y. An updated meta-analysis of cohort studies: Diabetes and risk of Alzheimer’s disease. Diabetes Res. Clin. Pract. 2017, 124, 41–47. [Google Scholar] [CrossRef]
- Tan, L.; Tan, M.-S.; Xue, M.; Cao, X.-P.; Ou, Y.-N.; Xu, W.; Yu, J.-T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar]
- Gudala, K.; Bansal, D.; Schifano, F.; Bhansali, A. Diabetes mellitus and risk of dementia: A meta-analysis of prospective observational studies. J. Diabetes Investig. 2013, 4, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, Z.; Ma, C.; Wang, Z.; Wang, K. Relationship between glycemic control and cognitive impairment: A systematic review and meta-analysis. Front. Aging Neurosci. 2023, 15, 1126183. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Reijmer, Y.D. Brain changes underlying cognitive dysfunction in diabetes: What can we learn from MRI? Diabetes 2014, 63, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Janson, J.; Laedtke, T.; Parisi, J.E.; O’Brien, P.; Petersen, R.C.; Butler, P.C. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 2004, 53, 474–481. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Xu, Y.; Li, W. Type 2 diabetes is associated with increased risk of dementia, but not mild cognitive impairment: A cross-sectional study among the elderly in Chinese communities. Front. Aging Neurosci. 2022, 14, 1004954. [Google Scholar] [CrossRef]
- Chatterjee, S.; Peters, S.A.E.; Woodward, M.; Arango, S.M.; Batty, G.D.; Beckett, N.; Beiser, A.; Borenstein, A.R.; Crane, P.K.; Haan, M.N.; et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care 2016, 39, 300–307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waissbluth, S.; Delano, P.H. Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sci. 2025, 15, 669. https://doi.org/10.3390/brainsci15070669
Waissbluth S, Delano PH. Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sciences. 2025; 15(7):669. https://doi.org/10.3390/brainsci15070669
Chicago/Turabian StyleWaissbluth, Sofia, and Paul H. Delano. 2025. "Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia" Brain Sciences 15, no. 7: 669. https://doi.org/10.3390/brainsci15070669
APA StyleWaissbluth, S., & Delano, P. H. (2025). Dissecting the Interactions of Diabetes Mellitus and Hearing Loss with Cognitive Decline and Dementia. Brain Sciences, 15(7), 669. https://doi.org/10.3390/brainsci15070669






