The Neural Correlates of Chewing Gum—A Neuroimaging Review of Its Effects on Brain Activity
Abstract
1. Introduction
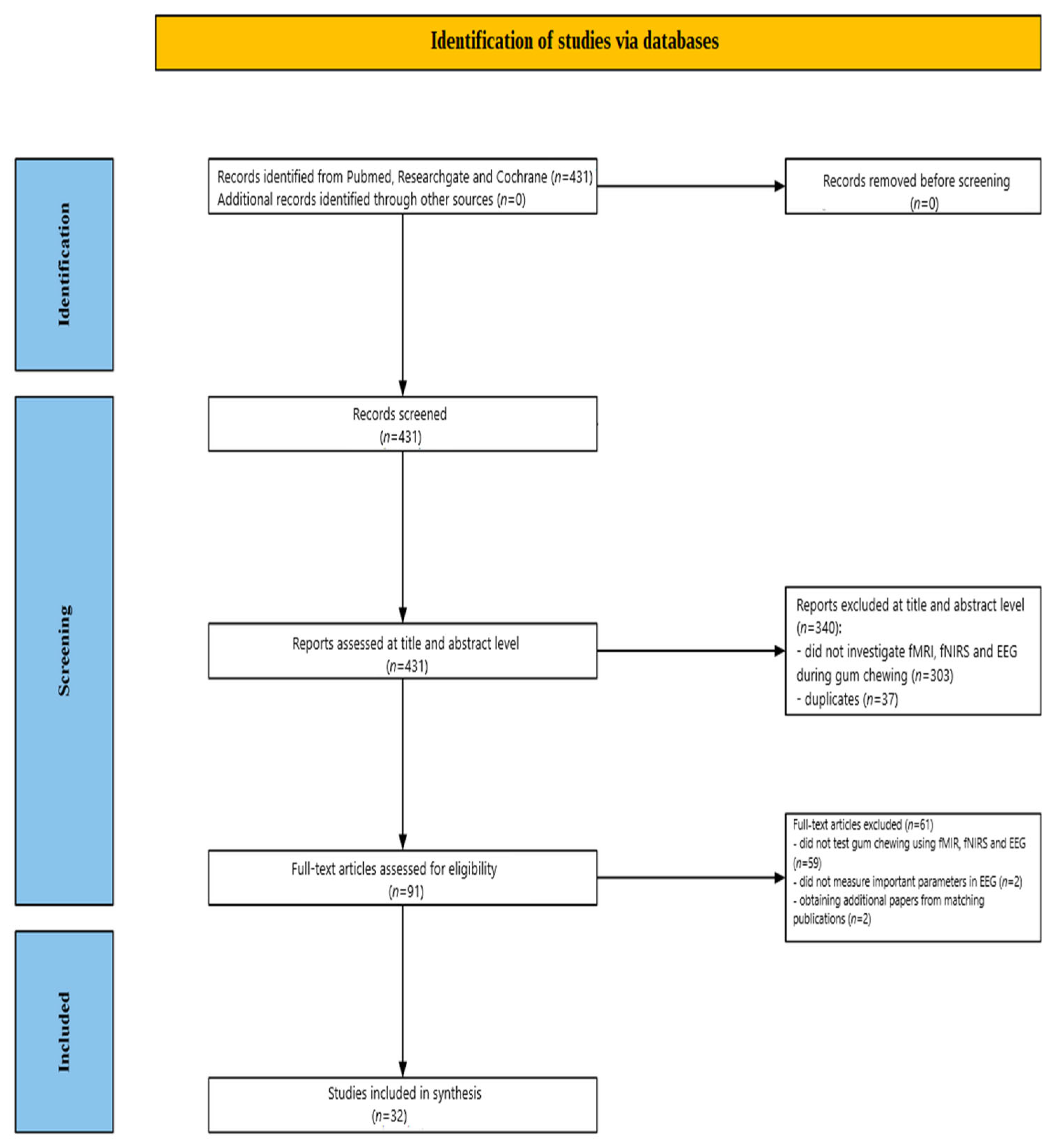
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection Criteria
2.3. Screening Process
2.3.1. Title and Abstract Screening
2.3.2. Full-Text Assessment
3. Results
3.1. Summary of Included Studies
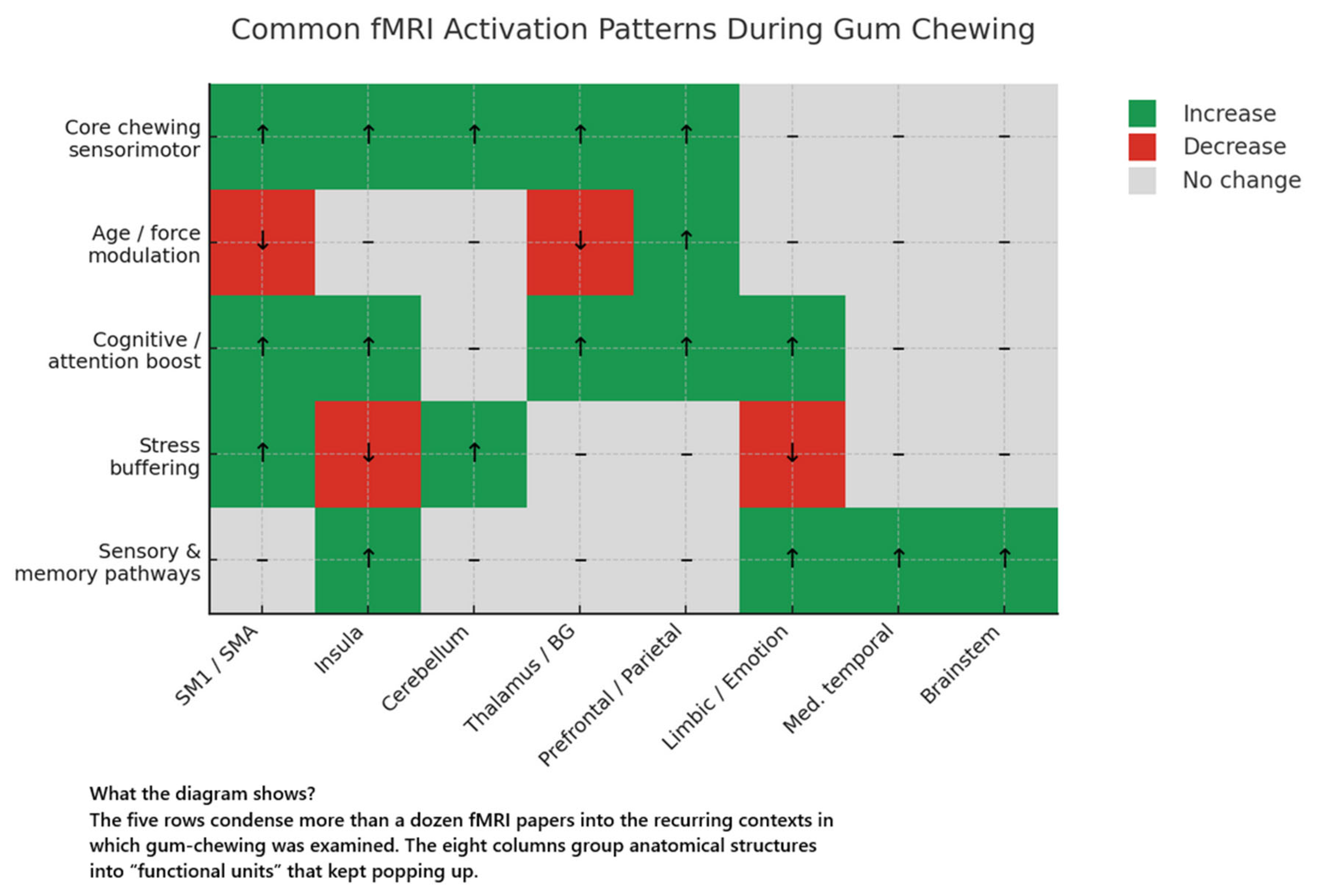
3.1.1. fMRI Studies
Chewing Paradigms Employed Across the fMRI Literature
Participants Characteristics
fMRI Methodologies
- Scanner Hardware and Sequences
- Task Design
- Motion control and preprocessing
Canonical Chewing-Related Activation Pattern
Modulators of Brain Activation
- Mechanical load
- Laterality, handedness, and chewing-side preference
- Concurrent motor or cognitive load
- Affective context
- Ageing
- Habitual mint-gum use
- Prolonged mastication
3.1.2. Network-Level Organisation
3.1.3. Temporal Dynamics of the Chewing Cycle
3.1.4. fMRI—Demographic Factors
3.1.5. Summary of Quantitative Effects
3.1.6. fNIRS Studies
Participants Characteristics
Chewing Paradigms Employed Across the fNIRS Literature
Cortical Hemodynamic Responses to Mastication
Peripheral Physiological Correlates
- Cardiovascular dynamics
- Autonomic balance
- Cranio-facial muscle activity
- Cerebrovascular correlates
- Neurochemical and nociceptive markers
- Central–autonomic integration
Behavioural, Cognitive, and Affective Outcomes
- Palatability effects
- Stress attenuation
- Cognitive performance
- Frequency and dual-task factors
- Analgesic outcome
Methodological Considerations for fNIRS Chewing Gum Studies
fNIRS—Demographic Factors
Synthesis
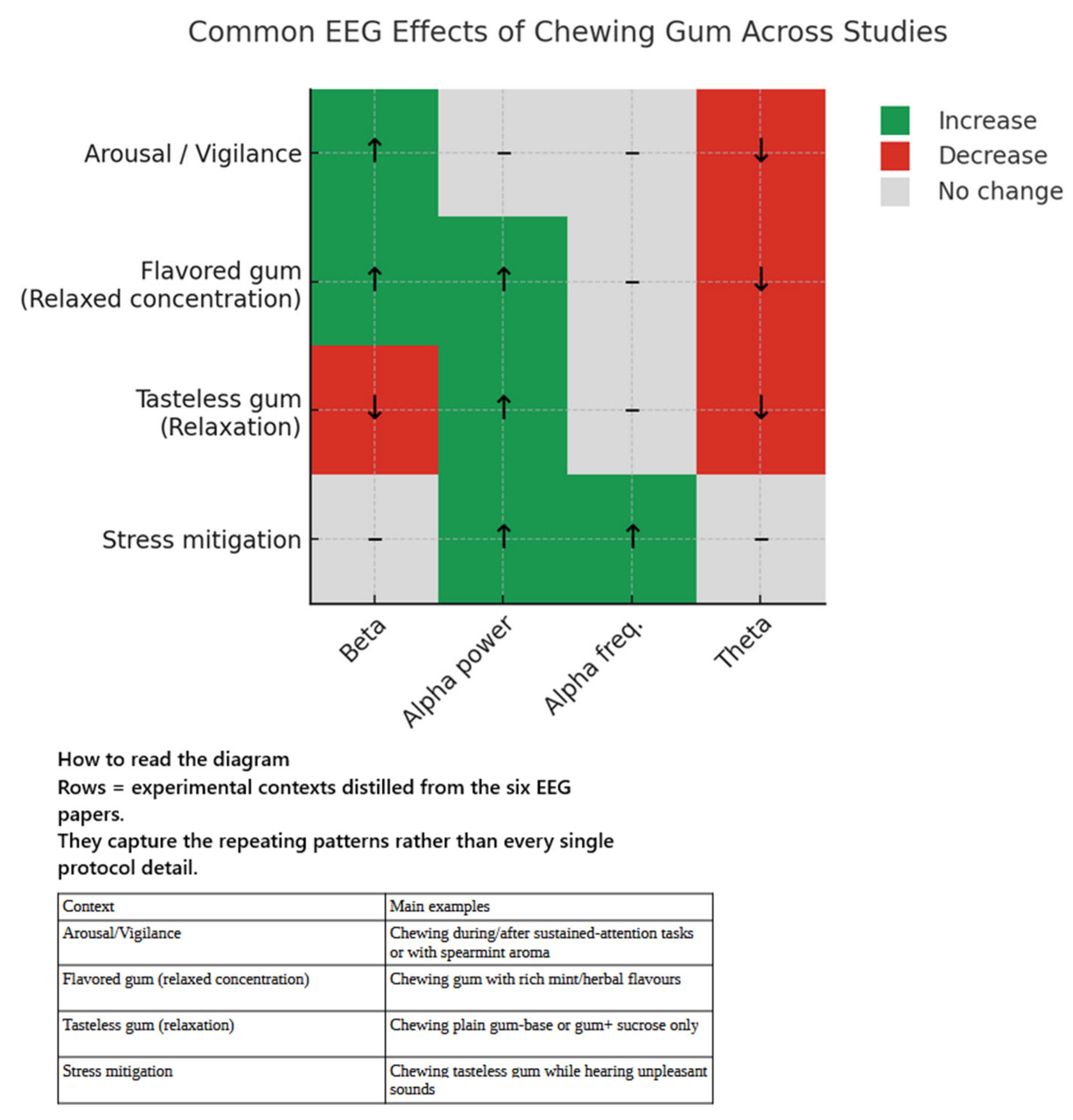
3.1.7. EEG Studies
Chewing Paradigms Employed Across the EEG Literature
Participant Characteristics
Electroencephalographic Outcomes
Behavioural and Autonomic Correlates
EEG—Demographic Factors
4. Discussion
4.1. fMRI Outcomes
4.1.1. Sensorimotor Activation and Modulatory Variables
4.1.2. Higher-Order Cortical Regions and Cognitive Correlates
4.1.3. Medial Temporal Lobe and Memory-Related Activation
4.1.4. Task Interference and Motor Resource Allocation
4.1.5. Dynamics of Chewing and Temporal Segmentation
4.1.6. Unresolved Questions About Central Pattern Generators
4.2. fNIRS Outcomes
4.2.1. Flavour and Emotional Valence
4.2.2. Motor Control vs. Cognitive/Emotional Processing
4.2.3. Chewing Frequency and PFC Activation
4.2.4. Cognitive Performance Outcomes
4.2.5. Stress, Emotion, and Comfort
4.2.6. Pain Modulation and the Serotonergic System
4.2.7. Influence of Dual Tasks and Movement
4.2.8. The Role of Gum Chewing on fNIRS Results in the Context of Brain Disorders
4.3. EEG Outcomes
4.4. Chewing Gum and Alpha Oscillations: Mechanisms and Functional Significance
4.4.1. Psychological Interpretations
4.4.2. Theoretical Models Integrating Alpha’s Role
- “Gating by Inhibition” Framework: Alpha oscillations are thought to actively gate information flow in the brain by inhibiting neural processing in specific pathways or regions [118]. Jensen and Mazaheri formalised this idea, proposing that alpha activity reflects functional inhibition of cortical areas not currently required for the task at hand [118]. From this perspective, increased alpha power is not a passive byproduct of idling but an active process for filtering out distractions or irrelevant inputs. Chewing gum’s induction of alpha can thus be interpreted as a gating mechanism: rhythmic alpha oscillations suppress extraneous sensory input and stress-related signals, effectively “closing the gate” on noise and allowing the brain to operate more efficiently on pertinent matters (or to rest without intrusion). This inhibition is likely mediated by GABAergic interneurons, which generate rhythmic inhibitory postsynaptic potentials that periodically hyperpolarise cortical neurons. The gating by inhibition model aligns well with the gum-chewing findings—as one chews, alpha elevations in networks unrelated to the mastication task indicate those regions are being functionally inhibited, which can conserve resources and promote a stable, calm focus. Notably, when gum is chewed during a cognitive activity, alpha may increase in areas not directly engaged by the task, potentially preventing distraction by suppressing activity in task-irrelevant regions.
- Alpha as an “Idling” Rhythm: Historically, alpha waves have been considered the brain’s default idling oscillation during wakefulness in the absence of demanding cognitive processing. Berger’s classic observation showed that alpha dominates the EEG during quiet rest (e.g., eyes-closed relaxation) and diminishes with mental engagement. In the context of chewing gum, part of the alpha increase may reflect an idling of certain cognitive systems. Chewing is a habitual, automatic motor behaviour that requires minimal conscious effort, allowing large portions of the cortex to enter an idle mode. For example, if no complex task is being performed whilst chewing, the visual cortex may exhibit strong alpha activity (akin to an eyes-closed state) due to a lack of critical visual input. Gum chewing facilitates reversion to a default, idling oscillatory state. However, modern interpretations emphasise that “idling” is not passive or inefficient; rather, it reflects the brain actively disengaging certain networks. Alpha, as previously noted, increases in regions not engaged by the current behaviour [118]—the neural correlate of an idling state. Thus, what was historically understood as an idle rhythm can be reinterpreted as the brain actively inhibiting unnecessary processing. Chewing-induced alpha may therefore represent a functional idling: the brain is awake and primed but in a low-engagement, energy-conserving mode. This state is adaptive for relaxation and recovery, and may also set a favourable baseline for switching to focused processing, as the brain in an alpha-rich idle state can rapidly desynchronise and reallocate resources upon salient stimulus or task demands.
- Inhibition-Timing Hypothesis: Building upon the above concepts, Klimesch and colleagues proposed an “inhibition-timing” model of alpha oscillations [123]. They posit that alpha rhythms reflect periodic windows of cortical inhibition, precisely timed to regulate neuronal firing. The oscillation’s phase and amplitude modulations serve as a timing mechanism for neural excitability. Notably, alpha power often increases (event-related synchronisation, ERS) in situations requiring top-down control, such as when a person must withhold a response or endure a delay—conditions in which the brain imposes inhibition to prevent premature or irrelevant activity. Thus, alpha ERS is interpreted as an active top-down inhibitory control process. The timing aspect of the hypothesis emphasises that the rhythmic nature of alpha (e.g., ~10 cycles per second) creates alternating periods of inhibition and disinhibition in neuronal populations, effectively dividing time into discrete windows where processing is suppressed versus allowed. Empirical support for this comes from findings that alpha phase coherence between brain regions increases during tasks requiring coordination, with phase lags consistent with neural transmission delays [123]. In practical terms, the inhibition-timing model means that alpha oscillations can temporally organise neural firing. Applied to chewing gum, it can be hypothesised that the act of chewing engages such inhibitory timing mechanisms to optimise neural processing. The brain might use alpha oscillations to periodically inhibit irrelevant sensory inputs or motor impulses in synchrony with the chewing cycle. For instance, if an individual is chewing while also performing a task, alpha could help “schedule” processing by providing brief inhibitory epochs that protect the ongoing task from interference at regular intervals. Even in the absence of an external task, the very act of rhythmic chewing could entrain alpha-timed inhibition across sensorimotor and associational areas, which would manifest as the observed alpha power increase. The inhibition-timing framework therefore enriches the explanation by suggesting that chewing-induced alpha is not just a byproduct of relaxation, but also an indicator that the brain is actively timing its inhibitory control of neural processes. This could be one reason why the alpha state during chewing still permits responsiveness and does not equate to sleep—the timing of inhibition is organised in an optimal way to maintain readiness.
- Predictive Coding Model: Recent theoretical work has linked alpha oscillations to predictive coding, a mechanism by which the brain continually predicts incoming sensory inputs and minimises surprise. In predictive coding models, higher-level neuronal regions send predictions (or suppressing signals) to lower-level sensory areas to eliminate expected input, allowing the brain to focus on unexpected or novel information. Alpha-band activity has been proposed as a carrier of these top-down predictions, acting to silence or filter out expected stimuli [124]. This framework elegantly accounts for the increase in alpha power observed during gum chewing. Chewing is a self-generated, rhythmic act that produces highly predictable sensory consequences (e.g., the feel of jaw movement, the taste and texture of gum, etc.). The brain quickly learns this pattern and can anticipate the sensory feedback from each chew. According to predictive coding theory, the brain sends inhibitory predictions to the somatosensory and other relevant cortices to dampen responses to expected chewing-related input. Alpha oscillations are a plausible mechanism for implementing this inhibitory prediction—by oscillating in phase with the predicted sensory events, they could suppress neural responses at the optimal moment. The result would be an overall increase in alpha power during chewing, reflecting the fact that much of the incoming information (from the chewing motions) is predicted and gated out. This minimises “surprise” or prediction error, contributing to the subjective feeling that the act becomes automatic or mindless. In short, the predictive coding model suggests that chewing-induced alpha is a sign that the brain’s internal models are effectively accounting for the sensory consequences of chewing, thereby silencing redundant inputs. This perspective complements the gating-by-inhibition framework: it suggests that alpha-mediated predictive suppression is the specific means in which the brain closes the gate on expected information. By doing so, the brain remains in a steady, relaxed state (since nothing unexpected is happening during rhythmic chewing), yet it is still prepared to detect any deviance. If, for instance, an unexpected stimulus occurs, alpha would momentarily diminish to allow for error detection. In this manner, alpha oscillations during chewing exemplify the brain’s predictive regulation of its sensory environment.
4.5. The Effects of Flavours in Chewing Gum—Additional Changes in Brain Activity and Possible Therapeutic Potential
5. The Need for Further Investigation into the Therapeutic Potential of Gum Chewing
6. Limitations and Future Directions
6.1. fMRI Studies
6.1.1. Sampling and Generalisability
6.1.2. Task Design and Experimental Control
6.1.3. Motion and Spatial Resolution Constraints
6.1.4. Temporal Resolution
6.1.5. Analytical Scope
6.1.6. Behavioural and Physiological Coupling
6.1.7. Longitudinal and Translational Relevance
6.2. fNIRS Studies
6.3. EEG Studies
6.4. Control of Psychological and Physiological States
6.5. The Unresolved Question—How Long to Chew Gum to Cause Lasting Changes in the Brain?
6.6. Gum Chewing—Safety Issues
7. Societal and Scientific Justification for Research on Gum-Induced Brain Activity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.H.; Lee, H.; Jang, S.H.; Noeske, R.; Choi, C.; Chang, Y.; Choi, Y.H. Effect of chewing hard material on boosting brain antioxidant levels and enhancing cognitive function. Front. Syst. Neurosci. 2024, 18, 1489919. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Zhou, Q.; Niwa, M.; Kubo, K.Y. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1687. [Google Scholar] [CrossRef]
- Chen, H.; Iinuma, M.; Onozuka, M.; Kubo, K.Y. Chewing Maintains Hippocampus—Dependent Cognitive Function. Int. J. Med. Sci. 2015, 12, 502–509. [Google Scholar] [CrossRef]
- Chuhuaicura, P.; Dias, F.J.; Arias, A.; Lezcano, M.F.; Fuentes, R. Mastication as a protective factor of the cognitive decline in adults: A qualitative systematic review. Int. Dent. J. 2019, 69, 334–340. [Google Scholar] [CrossRef]
- Lopez-Chaichio, L.; Padial-Molina, M.; O’Valle, F.; Gil-Montoya, J.A.; Catena, A.; Galindo-Moreno, P. Oral health and healthy chewing for healthy cognitive ageing: A comprehensive narrative review. Gerodontology 2021, 38, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mujib, B.R.A.; Grover, S.; Vinayak, V.; Mittal, S.; Kumar, M. Chewing Gum and Oral Health. Indian J. Contemp. Dent. 2013, 1, 72–74. [Google Scholar] [CrossRef]
- Dodds, M.W. The oral health benefits of chewing gum. J. Ir. Dent. Assoc. 2012, 58, 253–261. [Google Scholar] [PubMed]
- Wilkinson, L.; Scholey, A.; Wesnes, K. Chewing gum selectively improves aspects of memory in healthy volunteers. Appetite 2002, 38, 235–236, Erratum in Appetite 2003, 40, 373. [Google Scholar] [CrossRef]
- Stephens, R.; Tunney, R.J. Role of glucose in chewing gum-related facilitation of cognitive function. Appetite 2004, 43, 211–213. [Google Scholar] [CrossRef]
- Baker, J.R.; Bezance, J.B.; Zellaby, E.; Aggleton, J.P. Chewing gum can produce context-dependent effects upon memory. Appetite 2004, 43, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Miles, C. Chewing gum and context-dependent memory: The independent roles of chewing gum and mint flavour. Br. J. Psychol. 2008, 99 Pt 2, 293–306. [Google Scholar] [CrossRef]
- Onyper, S.V.; Carr, T.L.; Farrar, J.S.; Floyd, B.R. Cognitive advantages of chewing gum. Now you see them, now you don’t. Appetite 2011, 57, 321–328. [Google Scholar] [CrossRef]
- Houcan, Z.; Li, W. Effects of Chewing Gum onLearning and Memory. China J. Health Psychol. 2007, 15, 518–520. [Google Scholar]
- Tucha, L.; Koerts, J. Gum Chewing and Cognition: An Overview. Neurosci. Med. 2012, 3, 243–250. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Iinuma, M.; Chen, H. Mastication as a Stress-Coping Behavior. BioMed Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef] [PubMed]
- Yaman-Sözbir, Ş.; Ayaz-Alkaya, S.; Bayrak-Kahraman, B. Effect of chewing gum on stress, anxiety, depression, self-focused attention, and academic success: A randomized controlled study. Stress Health 2019, 35, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Sasaki-Otomaru, A.; Sakuma, Y.; Mochizuki, Y.; Ishida, S.; Kanoya, Y.; Sato, C. Effect of regular gum chewing on levels of anxiety, mood, and fatigue in healthy young adults. Clin. Pract. Epidemiol. Ment. Health CP EMH 2011, 7, 133–139, Erratum in Clin. Pract. Epidemiol. Ment. Health CP EMH 2012, 8, 46. [Google Scholar] [CrossRef]
- Allen, A.P.; Smith, A.P. Effects of chewing gum and time-on-task on alertness and attention. Nutr. Neurosci. 2012, 15, 176–185. [Google Scholar] [CrossRef]
- Allen, A.P.; Smith, A.P. Chewing gum: Cognitive performance, mood, well-being, and associated physiology. BioMed Res. Int. 2015, 2015, 654806. [Google Scholar] [CrossRef]
- Smith, A. Effects of chewing gum on mood, learning, memory and performance of an intelligence test. Nutr. Neurosci. 2009, 12, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.J.; Muneem, M.; Miles, C. Chewing gum benefits sustained attention in the absence of task degradation. Nutr. Neurosci. 2013, 16, 153–159. [Google Scholar] [CrossRef]
- Tänzer, U.; von Fintel, A.; Eikermann, T. Chewing gum and concentration performance. Psychol. Rep. 2009, 105, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Effects of chewing gum on cognitive function, mood and physiology in stressed and non-stressed volunteers. Nutr. Neurosci. 2010, 13, 7–16. [Google Scholar] [CrossRef]
- Ginns, P.; Kim, T.; Zervos, E. Chewing gum while studying: Effects on alertness and test performance. Appl. Cogn. Psychol. 2018, 33, 214–224. [Google Scholar] [CrossRef]
- Onozuka, M.; Fujita, M.; Watanabe, K.; Hirano, Y.; Niwa, M.; Nishiyama, K.; Saito, S. Mapping Brain Region Activity during Chewing: A Functional Magnetic Resonance Imaging Study. J. Dent. Res. 2002, 81, 743–746. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, H.; Liu, G.; Jin, Z.; Wang, L.; Ma, J.; Li, H. Analysis of brain activity involved in chewing-side preference during chewing: An fMRI study. J. Oral Rehabil. 2015, 42, 27–33. [Google Scholar] [CrossRef]
- Jang, S.H.; Kwon, H.C.; Kwon, H.G.; Jang, W.H. The cortical effect of chewing gum during hand movements: A functional MRI study. Somatosens. Mot. Res. 2015, 32, 110–113. [Google Scholar] [CrossRef]
- Choi, Y.H.; Jang, W.H.; Im, S.U.; Song, K.B.; Lee, H.K.; Lee, H.D.; Seo, Y.S.; Jang, S.H. The brain activation pattern of the medial temporal lobe during chewing gum: A functional MRI study. Neural Regen. Res. 2017, 12, 812–814. [Google Scholar]
- Lotze, M.; Domin, M.; Kordass, B. Symmetry of fMRI activation in the primary sensorimotor cortex during unilateral chewing. Clin. Oral Investig. 2017, 21, 967–973. [Google Scholar] [CrossRef]
- Quintero, A.; Ichesco, E.; Myers, C.; Schutt, R.; Gerstner, G.E. Brain activity and human unilateral chewing: An FMRI study. J. Dent. Res. 2013, 92, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Miyamoto, T. A fronto-parietal network for chewing of gum: A study on human subjects with functional magnetic resonance imaging. Neurosci. Lett. 2004, 360, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Bracco, P.; Anastasi, G.; Piancino, M.G.; Frongia, G.; Milardi, D.; Favaloro, A.; Bramanti, P. Hemispheric prevalence during chewing in normal right-handed and left-handed subjects: A functional magnetic resonance imaging preliminary study. Cranio 2010, 28, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, X.; Liu, J.; Zhou, X. Gum chewing inhibits the sensory processing and the propagation of stress-related information in a brain network. PLoS ONE 2013, 8, e57111. [Google Scholar] [CrossRef]
- Hirano, Y.; Obata, T.; Takahashi, H.; Tachibana, A.; Kuroiwa, D.; Takahashi, T.; Ikehira, H.; Onozuka, M. Effects of chewing on cognitive processing speed. Brain Cogn. 2013, 81, 376–381. [Google Scholar] [CrossRef]
- Han, P.; Penzler, M.; Jonathan, W.; Hummel, T. Frequent minty chewing gum use is associated with increased trigeminal sensitivity: An fMRI study. Brain Res. 2020, 1730, 146663. [Google Scholar] [CrossRef]
- Algin, O.; Kocak, O.M.; Gokcekuyu, Y.; Turker, K.S. Demonstration of chewing-related areas in the brain via functional magnetic resonance imaging. Pol. J. Radiol. 2023, 88, e65–e74. [Google Scholar] [CrossRef]
- Onozuka, M.; Fujita, M.; Watanabe, K.; Hirano, Y.; Niwa, M.; Nishiyama, K.; Saito, S. Age-related changes in brain regional activity during chewing: A functional magnetic resonance imaging study. J. Dent. Res. 2003, 82, 657–660. [Google Scholar] [CrossRef]
- Li, F.; Shen, H.; Li, B.; Zhou, X.; Hu, D. Multiple Neural Networks Involved in Chewing Gum: A Functional MRI Study Using Group-ICA. In Advances in Cognitive Neurodynamics (II); Wang, R., Gu, F., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Viggiano, A.; Manara, R.; Conforti, R.; Paccone, A.; Secondulfo, C.; Lorusso, L.; Sbordone, L.; Di Salle, F.; Monda, M.; Tedeschi, G.; et al. Mastication induces long-term increases in blood perfusion of the trigeminal principal nucleus. Neuroscience 2015, 311, 75–80. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Sakuramoto, A.; Suzuki, T.; Sakagami, J.; Shiramizu, M.; Tachibana, Y.; Kishimoto, H.; Ono, Y.; Ono, T. Emotional modulation of cortical activity during gum chewing: A functional near-infrared spectroscopy study. Front. Neurosci. 2022, 16, 964351. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Tachibana, Y.; Sakagami, J.; Zhang, M.; Urade, M.; Ono, T. Flavor-Enhanced Modulation of Cerebral Blood Flow during Gum Chewing. PLoS ONE 2013, 8, e66313. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Ono, T.; Sakagami, J.; Hori, K.; Maeda, Y.; Hamasaki, T.; Nokubi, T. Influence of voluntary control of masticatory side and rhythm on cerebral hemodynamics. Clin. Oral Investig. 2011, 15, 113–118. [Google Scholar] [CrossRef]
- Nakajima, K.; Takeda, T.; Saito, M.; Konno, M.; Kawano, Y.; Suzuki, Y.; Nishino, M.; Matsuda, Y.; Ishigami, K.; Sakatani, K. Effect of Mastication Muscle Activity on Prefrontal Cortex NIRS Measurement: A Pilot Study. Adv. Exp. Med. Biol. 2020, 1232, 121–127. [Google Scholar]
- Kawakami, Y.; Takeda, T.; Konno, M.; Suzuki, Y.; Kawano, Y.; Ozawa, T.; Kondo, Y.; Sakatani, K. Relationships Between Gum Chewing and Stroop Test: A Pilot Study. Adv. Exp. Med. Biol. 2017, 977, 221–226. [Google Scholar]
- Konno, M.; Nakajima, K.; Takeda, T.; Kawano, Y.; Suzuki, Y.; Sakatani, K. Effect of Gum Chewing on PFC Activity During Discomfort Sound Stimulation. Adv. Exp. Med. Biol. 2020, 1232, 113–119. [Google Scholar] [PubMed]
- Konno, M.; Takeda, T.; Kawakami, Y.; Suzuki, Y.; Kawano, Y.; Nakajima, K.; Ozawa, T.; Ishigami, K.; Takemura, N.; Sakatani, K. Relationships Between Gum-Chewing and Stress. Adv. Exp. Med. Biol. 2016, 876, 343–349. [Google Scholar] [PubMed]
- Yokoyama, T.; Sato, M.; Natsui, S.; Kuboyama, N.; Suzuki, K.; Inaba, H.; Shibuya, K. Effect of Gum Chewing Frequency on Oxygenation of the Prefrontal Cortex. Percept. Mot. Ski. 2017, 124, 58–71. [Google Scholar] [CrossRef]
- Natsui, S.; Sato, M.; Yokoyama, T.; Inaba, H.; Kuboyama, N.; Shibuya, K. Effects of Chewing Frequency on Cerebral Blood Flow and Cognitive Function. J. Behav. Brain Sci. 2020, 10, 287–295. [Google Scholar] [CrossRef]
- Tsutsui, A.; Takeda, T.; Sakaue, T.; Togo, S.; Matsuda, Y.; Nakajima, K.; Fukuda, K.; Sakatani, K. Effects of Dual Tasks Including Gum Chewing on Prefrontal Cortex Activity. Adv. Exp. Med. Biol. 2024, 1463, 153–158. [Google Scholar]
- Kamiya, K.; Fumoto, M.; Kikuchi, H.; Sekiyama, T.; Mohri-Lkuzawa, Y.; Umino, M.; Arita, H. Prolonged gum chewing evokes activation of the ventral part of prefrontal cortex and suppression of nociceptive responses: Involvement of the serotonergic system. J. Med. Dent. Sci. 2010, 57, 35–43. [Google Scholar]
- Wada, M.; Hoshi, Y.; Iguchi, Y.; Kida, I. Near-infrared spectroscopic study on the effects of chewing on short-term memory. Appetite 2011, 57, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Jacob, T.J.; Smith, A.P. Effects and after-effects of chewing gum on vigilance, heart rate, EEG and mood. Physiol. Behav. 2014, 133, 244–251. [Google Scholar] [CrossRef]
- Masumoto, Y.; Morinushi, T.; Kawasaki, H.; Ogura, T.; Takigawa, M. Effects of three principal constituents in chewing gum on electroencephalographic activity. Psychiatry Clin. Neurosci. 1999, 53, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, Y.; Morinushi, T.; Kawasaki, H.; Takigawa, M. Spectral analysis of changes in electroencephalographic activity after the chewing of gum. Psychiatry Clin. Neurosci. 1998, 52, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Morinushi, T.; Masumoto, Y.; Kawasaki, H.; Takigawa, M. Effect on electroencephalogram of chewing flavored gum. Psychiatry Clin. Neurosci. 2000, 54, 645–651. [Google Scholar] [CrossRef]
- Yagyu, T.; Kondakor, I.; Kochi, K.; Koenig, T.; Lehmann, D.; Kinoshita, T.; Hirota, T.; Yagyu, T. Smell and taste of chewing gum affect frequency domain EEG source localizations. Int. J. Neurosci. 1998, 93, 205–216. [Google Scholar] [CrossRef]
- Lin, C.S.; Chang, W.J.; Fuh, J.L. Lower masticatory function relates to cognitive health and intrinsic brain network in older adults. Oral Dis. 2023, 29, 2895–2906. [Google Scholar] [CrossRef]
- Krishnamoorthy, G.; Narayana, A.I.; Balkrishanan, D. Mastication as a tool to prevent cognitive dysfunctions. Jpn. Dent. Sci. Rev. 2018, 54, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Habig, K.; Krämer, H.H.; Lautenschläger, G.; Walter, B.; Best, C. Processing of sensory, painful and vestibular stimuli in the thalamus. Brain Struct. Funct. 2023, 228, 433–447. [Google Scholar] [CrossRef]
- Ginatempo, F.; Manzo, N.; Spampinato, D.A.; Loi, N.; Burgio, F.; Rothwell, J.C.; Deriu, F. A Novel Paired Somatosensory-Cerebellar Stimulation Induces Plasticity on Cerebellar-Brain Connectivity. Cerebellum 2024, 23, 1121–1127. [Google Scholar] [CrossRef]
- Momose, T.; Nishikawa, J.; Watanabe, T.; Sasaki, Y.; Senda, M.; Kubota, K.; Funakoshi, M.; Minakuchi, S. Effect of mastication on regional cerebral blood flow in humans examined by positron-emission tomography with 15O-labelled water and magnetic resonance imaging. Arch. Oral Biol. 1997, 42, 57–61. [Google Scholar] [CrossRef]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Ohman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef]
- Duerden, E.G.; Arsalidou, M.; Lee, M.; Taylor, M.J. Lateralization of affective processing in the insula. Neuroimage 2013, 78, 159–175. [Google Scholar] [CrossRef]
- Scholey, A.; Haskell, C.; Robertson, B.; Kennedy, D.; Milne, A.; Wetherell, M. Chewing gum alleviates negative mood and reduces cortisol during acute laboratory psychological stress. Physiol. Behav. 2009, 97, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Sketchley-Kaye, K.; Jenks, R.; Miles, C.; Johnson, A.J. Chewing gum modifies state anxiety and alertness under conditions of social stress. Nutr. Neurosci. 2011, 14, 237–242. [Google Scholar] [CrossRef]
- Smith, A.P. Chewing gum, stress and health. Stress Health 2009, 25, 445–451. [Google Scholar] [CrossRef]
- Walker, J.; Hosiner, A.; Kergoat, S.; Walker, J.M.; Somoza, V. Chewing unflavored gum does not reduce cortisol levels during a cognitive task but increases the response of the sympathetic nervous system. Physiol. Behav. 2016, 154, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Torney, K.; Johnson, A.J.; Miles, C. Chewing gum and impasse-induced self-reported stress. Appetite 2009, 53, 414–417. [Google Scholar] [CrossRef]
- Hermans, E.J.; Henckens, M.J.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef]
- Liu, C.-H.; Guo, J.; Lu, S.-L.; Tang, L.-R.; Fan, J.; Wang, C.-Y.; Wang, L.; Liu, Q.-Q.; Liu, C.-Z. Increased salience network activity in patients with insomnia complaints in major depressive disorder. Front. Psychiatry 2018, 9, 93. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Namkung, H.; Kim, S.H.; Sawa, A. The Insula: An Underestimated Brain Area in Clinical Neuroscience, Psychiatry, and Neurology. Trends Neurosci. 2017, 40, 200–207, Erratum in Trends Neurosci. 2018, 41, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Takehara-Nishiuchi, K. Entorhinal cortex and consolidated memory. Neurosci. Res. 2014, 84, 27–33. [Google Scholar] [CrossRef]
- Garcia, A.D.; Buffalo, E.A. Anatomy and Function of the Primate Entorhinal Cortex. Annu. Rev. Vis. Sci. 2020, 6, 411–432. [Google Scholar] [CrossRef]
- Tozzi, F.; Guglielmo, S.; Paraciani, C.; van den Oever, M.C.; Mainardi, M.; Cattaneo, A.; Origlia, N. Involvement of a lateral entorhinal cortex engram in episodic-like memory recall. Cell Rep. 2024, 43, 114795. [Google Scholar] [CrossRef]
- Voss, J.L.; Bridge, D.J.; Cohen, N.J.; Walker, J.A. A Closer Look at the Hippocampus and Memory. Trends Cogn. Sci. 2017, 21, 577–588. [Google Scholar] [CrossRef]
- Riedel, G.; Micheau, J. Function of the hippocampus in memory formation: Desperately seeking resolution. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 835–853. [Google Scholar] [CrossRef]
- Wang, Y.W.; Ashby, F.G. A role for the medial temporal lobes in category learning. Learn. Mem. 2020, 27, 441–450. [Google Scholar] [CrossRef]
- Squire, L.R.; Zola-Morgan, S. The medial temporal lobe memory system. Science 1991, 253, 1380–1386. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.; Clark, R.E. The medial temporal lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Eichenbaum, H.; Yonelinas, A.P.; Ranganath, C. The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 2007, 30, 123–152. [Google Scholar] [CrossRef] [PubMed]
- Raslau, F.D.; Mark, I.T.; Klein, A.P.; Ulmer, J.L.; Mathews, V.; Mark, L.P. Memory part 2: The role of the medial temporal lobe. Am. J. Neuroradiol. 2015, 36, 846–849. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137 Pt 1, 12–32. [Google Scholar] [CrossRef]
- Pashler, H. Dual-task interference in simple tasks: Data and theory. Psychol. Bull. 1994, 116, 220–244. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, S.P. Intermanual coordination: From behavioural principles to neural-network interactions. Nat. Rev. Neurosci. 2002, 3, 348–359. [Google Scholar] [CrossRef]
- Serrien, D.J.; Ivry, R.B.; Swinnen, S.P. The missing link between action and cognition. Prog. Neurobiol. 2007, 82, 95–107. [Google Scholar] [CrossRef]
- Szameitat, A.J.; Schubert, T.; Müller, K.; Von Cramon, D.Y. Localization of executive functions in dual-task performance with fMRI. J. Cogn. Neurosci. 2002, 14, 1184–1199. [Google Scholar] [CrossRef]
- Lund, J.P.; Kolta, A. Generation of the central masticatory pattern and its modification by sensory feedback. Dysphagia 2006, 21, 167–174. [Google Scholar] [CrossRef]
- Nakamura, Y.; Katakura, N. Generation of masticatory rhythm in the brainstem. Neurosci. Res. 1995, 23, 1–19. [Google Scholar] [CrossRef]
- Lund, J.P.; Kolta, A. Brainstem circuits that control mastication: Do they have anything to say during speech? J. Commun. Disord. 2006, 39, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Inoue, T.; Nakamura, T.; Kawamura, Y. Characteristics of rhythmic jaw movements of the rabbit. Arch. Oral Biol. 1985, 30, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Çevik, H.; Çam, R.; Engin, M.; Yavuz, S. The effect of chewing gum on intestinal functions, postoperative pain, and early discharge after isolated coronary bypass surgery. Ann. Clin. Anal. Med. 2025, 16, 406–409. [Google Scholar]
- Umeda, M.; Kempka, L.; Weatherby, A.; Greenlee, B.; Mansion, K. Effects of caffeinated chewing gum on muscle pain during submaximal isometric exercise in individuals with fibromyalgia. Physiol. Behav. 2016, 157, 139–145. [Google Scholar] [CrossRef]
- Jabr, L.; Altuhafy, M.; Barmak, A.B.; Rossouw, P.E.; Michelogiannakis, D. Sugar-free chewing gum versus conventional analgesic drugs for pain relief with fixed orthodontic appliances. A systematic review and meta-analysis. J. Orthod. 2023, 50, 215–228. [Google Scholar] [CrossRef]
- Guo, Q.; Liao, C.; Guan, X.; Xiao, L.; Xiang, M.; Long, S.; Liu, J.; Xiang, M. Effect of chewing gum on orthodontic pain in patients receiving fixed orthodontic treatment: A systematic review and meta-analysis. Eur. J. Med. Res. 2023, 28, 491. [Google Scholar] [CrossRef] [PubMed]
- Mando, M.; Talaat, S.; Bourauel, C. The efficacy of chewing gum in the reduction of orthodontic pain at its peak intensity: A systematic review and meta-analysis. Angle Orthod. 2023, 93, 580–590. [Google Scholar] [CrossRef]
- Aydin Sayilan, A.; Seyhan Ak, E.; Temiz, Z.; Öztekin, S.D.; Colak, S.; Pirti, O. The Effect of Gum Chewing on Abdominal Pain and Nausea Caused by Polyethylene Glycol Solution Used for Intestinal Cleansing Before Colonoscopy: An Endoscopist-Blinded, Randomized Controlled Trial. Gastroenterol. Nurs. 2020, 43, 448–455. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Roberts, A.C. Prefrontal cortex and depression. Neuropsychopharmacology 2022, 47, 225–246, Erratum in Neuropsychopharmacology 2022, 47, 609. [Google Scholar]
- Hare, B.D.; Duman, R.S. Prefrontal cortex circuits in depression and anxiety: Contribution of discrete neuronal populations and target regions. Mol. Psychiatry 2020, 25, 2742–2758. [Google Scholar] [CrossRef]
- Ho, C.S.H.; Lim, L.J.H.; Lim, A.Q.; Chan, N.H.C.; Tan, R.S.; Lee, S.H.; Ho, R.C.M. Diagnostic and Predictive Applications of Functional Near-Infrared Spectroscopy for Major Depressive Disorder: A Systematic Review. Front. Psychiatry 2020, 11, 378. [Google Scholar] [CrossRef] [PubMed]
- Smith, A. Effects of chewing gum on stress and health: A replication and investigation of dose-response. Stress Health 2013, 29, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.P.; Clayton, H. The Effects of Chewing Gum on Perceived Stress and Wellbeing in Students Under a High and Low Workload. In Human Mental Workload: Models and Applications, Proceedings of the 4th International Symposium, H-WORKLOAD 2020, Granada, Spain, 3–5 December 2020; Longo, L., Leva, M.C., Eds.; Communications in Computer and Information Science; Springer: Cham, Switzerland, 2020; Volume 1318. [Google Scholar]
- Butters, E.; Srinivasan, S.; O’Brien, J.T.; Su, L.; Bale, G. A promising tool to explore functional impairment in neurodegeneration: A systematic review of near-infrared spectroscopy in dementia. Ageing Res. Rev. 2023, 90, 101992. [Google Scholar] [CrossRef] [PubMed]
- Tzischinsky, O.; Lavie, P. Melatonin possesses time-dependent hypnotic effects. Sleep 1994, 17, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, C.H.; Ogilvie, R.D. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep 1997, 20, 724–733. [Google Scholar] [CrossRef]
- Kuo, T.B.; Chen, C.Y.; Hsu, Y.C.; Yang, C.C. EEG beta power and heart rate variability describe the association between cortical and autonomic arousals across sleep. Auton. Neurosci. 2016, 194, 32–37. [Google Scholar] [CrossRef]
- Wascher, C.A.F. Heart rate as a measure of emotional arousal in evolutionary biology. Philos. Trans. R. Soc. B 2021, 376, 20200479. [Google Scholar] [CrossRef]
- Lagopoulos, J.; Xu, J.; Rasmussen, I.; Vik, A.; Malhi, G.S.; Eliassen, C.F.; Arntsen, I.E.; Saether, J.G.; Hollup, S.; Holen, A.; et al. Increased theta and alpha EEG activity during nondirective meditation. J. Altern. Complement. Med. 2009, 15, 1187–1192. [Google Scholar] [CrossRef]
- Huff, T.; Weisbrod, L.J.; Daly, D.T. Neuroanatomy, Cranial Nerve 5 (Trigeminal). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482283/ (accessed on 30 May 2025).
- Rolls, E.T. Taste and smell processing in the brain. Handb. Clin. Neurol. 2019, 164, 97–118. [Google Scholar]
- Tucha, L.; Simpson, W. The role of time on task performance in modifying the effects of gum chewing on attention. Appetite 2011, 56, 299–301. [Google Scholar] [CrossRef]
- Tan, E.; Troller-Renfree, S.V.; Morales, S.; Buzzell, G.A.; McSweeney, M.; Antúnez, M.; Fox, N.A. Theta activity and cognitive functioning: Integrating evidence from resting-state and task-related developmental electroencephalography (EEG) research. Dev. Cogn. Neurosci. 2024, 67, 101404. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. α-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Hughes, S.W.; Crunelli, V. Thalamic mechanisms of EEG alpha rhythms and their pathological implications. Neuroscientist 2005, 11, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Knyazeva, M.G. Alpha rhythms: What they are and how they alter with aging. In Factors Affecting Neurological Aging; Academic Press: Cambridge, MA, USA, 2021; pp. 349–359. [Google Scholar]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Sadaghiani, S.; Scheeringa, R.; Lehongre, K.; Morillon, B.; Giraud, A.L.; D’Esposito, M.; Kleinschmidt, A. α-band phase synchrony is related to activity in the fronto-parietal adaptive control network. J. Neurosci. 2012, 32, 14305–14310. [Google Scholar] [CrossRef]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Chapter 3—Technical Aspects of Brain Rhythms and Speech Parameters. In Introduction to EEG- and Speech-Based Emotion Recognition; Abhang, P.A., Gawali, B.W., Mehrotra, S.C., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 51–79. [Google Scholar]
- Hirano, Y.; Onozuka, M. Chewing and attention: A positive effect on sustained attention. BioMed Res. Int. 2015, 2015, 367026. [Google Scholar] [CrossRef]
- Attar, E.T. Review of electroencephalography signals approaches for mental stress assessment. Neurosciences 2022, 27, 209–215. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition-timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- Alamia, A.; VanRullen, R. Alpha oscillations and traveling waves: Signatures of predictive coding? PLoS Biol. 2019, 17, e3000487. [Google Scholar] [CrossRef]
- Sayorwan, W.; Siripornpanich, V.; Piriyapunyaporn, T.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The effects of lavender oil inhalation on emotional states, autonomic nervous system, and brain electrical activity. J. Med. Assoc. Thail. 2012, 95, 598–606. [Google Scholar]
- Masago, R.; Matsuda, T.; Kikuchi, Y.; Miyazaki, Y.; Iwanaga, K.; Harada, H.; Katsuura, T. Effects of inhalation of essential oils on EEG activity and sensory evaluation. J. Physiol. Anthropol. Appl. Hum. Sci. 2000, 19, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Diego, M.A.; Jones, N.A.; Field, T.; Hernandez-Reif, M.; Schanberg, S.; Kuhn, C.; McAdam, V.; Galamaga, R.; Galamaga, M. Aromatherapy positively affects mood, EEG patterns of alertness and math computations. Int. J. Neurosci. 1998, 96, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sayowan, W.; Siripornpanich, V.; Hongratanaworakit, T.; Kotchabhakdi, N.; Ruangrungsi, N. The Effects of Jasmine Oil Inhalation on Brain Wave Activies and Emotions. J. Health Res. 2017, 27, 73–77. [Google Scholar]
- Lin, S.; Wang, Y.; Wu, K.; Yu, G.; Liu, C.; Su, C.; Yi, F. Study on the Effect of Mentha × piperita L. Essential Oil on Electroencephalography upon Stimulation with Different Visual Effects. Molecules 2022, 27, 4059. [Google Scholar] [CrossRef]
- Shinno, A.; Isawa, M.; Itoh, H.; Kose, N.; Ishino, H.; Nishimura, T.; Tomi, M.; Tsujii, T.; Shimada, H.; Saito, H.; et al. Effectiveness of aromatherapy evaluated with subjective and objective cognitive indicators: A clinical trial on young adults using near-infrared spectroscopy. Jpn. J. Pharm. Health Care Sci. 2012, 38, 265–271. [Google Scholar] [CrossRef]
- Sánchez-Vidaña, D.I.; Ngai, S.P.; He, W.; Chow, J.K.; Lau, B.W.; Tsang, H.W. The Effectiveness of Aromatherapy for Depressive Symptoms: A Systematic Review. Evid. Based Complement. Altern. Med. 2017, 2017, 5869315. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Kim, M. Effects of aromatherapy on depression: A meta-analysis of randomized controlled trials. Gen. Hosp. Psychiatry 2023, 84, 215–225. [Google Scholar] [CrossRef]
- Tan, L.; Liao, F.F.; Long, L.Z.; Ma, X.C.; Peng, Y.X.; Lu, J.M.; Qu, H.; Fu, C.G. Essential oils for treating anxiety: A systematic review of randomized controlled trials and network meta-analysis. Front. Public Health 2023, 11, 1144404. [Google Scholar] [CrossRef]
- Su, G.; Liu, F.; Yang, X.; Chen, Z.; Kang, Y.; Gao, S. The effect of inhaled aromatherapy on cognitive function in patients with cognitive impairment: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2025, 93, 20–31. [Google Scholar] [CrossRef]
- Thair, H.; Holloway, A.L.; Newport, R.; Smith, A.D. Transcranial Direct Current Stimulation (tDCS): A Beginner’s Guide for Design and Implementation. Front. Neurosci. 2017, 11, 641. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Impact of concurrent task performance on transcranial direct current stimulation (tDCS)—Induced changes in cortical physiology and working memory. Cortex 2019, 113, 37–57. [Google Scholar] [CrossRef]
- Gao, Y.; Qiu, Y.; Yang, Q.; Tang, S.; Gong, J.; Fan, H.; Wu, Y.; Lu, X. Repetitive transcranial magnetic stimulation combined with cognitive training for cognitive function and activities of daily living in patients with post-stroke cognitive impairment: A systematic review and meta-analysis. Ageing Res. Rev. 2023, 87, 101919. [Google Scholar] [CrossRef] [PubMed]
- Barbati, S.A.; Podda, M.V.; Grassi, C. Tuning brain networks: The emerging role of transcranial direct current stimulation on structural plasticity. Front. Cell. Neurosci. 2022, 16, 945777. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Cao, L.; Wang, Q.; Sheng, Y.; Yu, J.; Liang, Z. The Physiological Mechanisms of Transcranial Direct Current Stimulation to Enhance Motor Performance: A Narrative Review. Biology 2024, 13, 790. [Google Scholar] [CrossRef]
- Beynel, L.; Powers, J.P.; Appelbaum, L.G. Effects of repetitive transcranial magnetic stimulation on resting-state connectivity: A systematic review. Neuroimage 2020, 211, 116596. [Google Scholar] [CrossRef]
- Burton, C.Z.; Garnett, E.O.; Capellari, E.; Chang, S.E.; Tso, I.F.; Hampstead, B.M.; Taylor, S.F. Combined Cognitive Training and Transcranial Direct Current Stimulation in Neuropsychiatric Disorders: A Systematic Review and Meta-analysis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H. Effects of masticatory exercise on cognitive function in community-dwelling older adults. Technol. Health Care 2021, 29 (Suppl. S1), 125–131. [Google Scholar] [CrossRef]
- Matsuzaki, Y.; Nouchi, R.; Sakaki, K.; Dinet, J.; Kawashima, R. The Effect of Cognitive Training with Neurofeedback on Cognitive Function in Healthy Adults: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 843. [Google Scholar] [CrossRef]
- Nandarathana, N.; Ranjan, J.K. The Efficacy and Durability of Mindfulness-Based Cognitive Therapy in the Treatment of Anxiety and Depressive Disorders: A Systematic Review and Meta-analysis. Indian J. Psychol. Med. 2024, 29, 02537176241249375. [Google Scholar] [CrossRef]
- Martínez-Calderon, J.; Casuso-Holgado, M.J.; Muñoz-Fernandez, M.J.; Garcia-Muñoz, C.; Heredia-Rizo, A.M. Yoga-based interventions may reduce anxiety symptoms in anxiety disorders and depression symptoms in depressive disorders: A systematic review with meta-analysis and meta-regression. Br. J. Sports Med. 2023, 57, 1442–1449. [Google Scholar] [CrossRef]
- Clark, H.M.; Solomon, N.P. Age and sex differences in orofacial strength. Dysphagia 2012, 27, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; You, S.J.; Kim, J.Y.; Yeo, S.G.; Lee, J.H. Differences in orofacial muscle strength according to age and sex in East Asian healthy adults. Am. J. Phys. Med. Rehabil. 2015, 94, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiesenfeld-Hallin, Z. Sex differences in pain perception. Gend. Med. 2005, 2, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, J.A.; Davis, K.D. Deconstructing sex differences in pain sensitivity. Pain 2014, 155, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Al Sayegh, S.; Vasilatou, I.; Kumar, A.; Al Barwari, C.; Fredriksson, L.; Grigoriadis, A.; Christidis, N. Experimental pain and fatigue induced by excessive chewing. BMC Oral Health 2020, 20, 179. [Google Scholar] [CrossRef]
- Alam, M.K.; Al Shayeb, M.; Natarajan, P.M.; Abutayyem, H.; Di Blasio, M.; Marrapodi, M.M.; Cicciù, M.; Minervini, G. Association of temporomandibular disorders and other jaw anomalies in chewing gum users—A systematic review. J. Oral Facial Pain Headache 2025, 39, 35–47. [Google Scholar]
- Ryu, J.-W. Considerations in the Diagnosis and Management of Temporomandibular Disorders in Older Adults: A Narrative Review. J. Oral Med. Pain 2024, 49, 43–48. [Google Scholar] [CrossRef]
- Iwamoto, S.; Tamura, M.; Sasaki, A.; Nawano, M. Dynamics of neuronal oscillations underlying nociceptive response in the mouse primary somatosensory cortex. Sci. Rep. 2021, 11, 1667. [Google Scholar] [CrossRef]
- Teff, K. Nutritional implications of the cephalic-phase reflexes: Endocrine responses. Appetite 2000, 34, 206–213. [Google Scholar] [CrossRef]
- Helman, C.A. Chewing gum is as effective as food in stimulating cephalic phase gastric secretion. Am. J. Gastroenterol. 1988, 83, 640–642. [Google Scholar] [PubMed]
- Ouanes, J.P.; Bicket, M.C.; Togioka, B.; Tomas, V.G.; Wu, C.L.; Murphy, J.D. The role of perioperative chewing gum on gastric fluid volume and gastric pH: A meta-analysis. J. Clin. Anesth. 2015, 27, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Stephan, E.; Pardo, J.V.; Faris, P.L.; Hartman, B.K.; Kim, S.W.; Ivanov, E.H.; Daughters, R.S.; Costello, P.A.; Goodale, R.L. Functional neuroimaging of gastric distention. J. Gastrointest. Surg. 2003, 7, 740–749. [Google Scholar] [CrossRef]
- Das, A.; Myers, J.; Mathura, R.; Shofty, B.; Metzger, B.A.; Bijanki, K.; Wu, C.; Jacobs, J.; Sheth, S.A. Spontaneous neuronal oscillations in the human insula are hierarchically organized traveling waves. Elife 2022, 11, e76702. [Google Scholar] [CrossRef] [PubMed]
- Leung, L.W.; Borst, J.G. Electrical activity of the cingulate cortex. I. Generating mechanisms and relations to behavior. Brain Res. 1987, 407, 68–80. [Google Scholar] [CrossRef]
- Hetherington, M.M.; Regan, M.F. Effects of chewing gum on short-term appetite regulation in moderately restrained eaters. Appetite 2011, 57, 475–482. [Google Scholar] [CrossRef]
- Jiménez-Ten Hoevel, C.; Llauradó, E.; Valls, R.M.; Besora-Moreno, M.; Queral, J.; Solà, R.; Pedret, A. Effects of Chewing Gum on Satiety, Appetite Regulation, Energy Intake, and Weight Loss: A Systematic Review. Nutrients 2025, 17, 435. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.Y.; Tadi, P. Physiology, Obesity Neurohormonal Appetite And Satiety Control. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555906/ (accessed on 30 May 2025).
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The impact of gut hormones on the neural circuit of appetite and satiety: A systematic review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef]
- Dumanoglu, B.; Alpagat, G.; Poyraz, M.; Alan Yalim, S.; Baccioglu, A. Anaphylaxis After Consumption of Guar Gum-Containing Food: A Report of Two Cases. Cureus 2022, 14, e28022. [Google Scholar] [CrossRef]
- Gagari, E.; Zafeiratou, D.; Afantenou, S.; Panoutsopoulou, I.; Koutlas, I. Cinnamon contact hypersensitivity in the oral cavity. Clinical manifestations and diagnosis in 40 patients. J. Am. Acad. Dermatol. 2015, 72, AB78. [Google Scholar]
- Georgakopoulou, E.A. Cinnamon contact stomatitis. J. Dermatol. Case Rep. 2010, 4, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Effector mechanisms in allergic reactions. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27112/ (accessed on 30 May 2025).
- Alshammari, A.; Almotairy, N.; Kumar, A.; Grigoriadis, A. Effect of malocclusion on jaw motor function and chewing in children: A systematic review. Clin. Oral Investig. 2022, 26, 2335–2351. [Google Scholar] [CrossRef] [PubMed]
- Mäkinen, K.K. Gastrointestinal Disturbances Associated with the Consumption of Sugar Alcohols with Special Consideration of Xylitol: Scientific Review and Instructions for Dentists and Other Health-Care Professionals. Int. J. Dent. 2016, 2016, 5967907. [Google Scholar] [CrossRef]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Yang, D.; Yang, S.; Zhao, Y.; Jiang, M.; Wang, X.; Zhao, L.; Liu, Q.; Lu, Z.; et al. Tooth loss and the risk of cognitive decline and dementia: A meta-analysis of cohort studies. Front. Neurol. 2023, 14, 1103052. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hirayama, A.; Hosoe, N.; Furube, M.; Hirano, S. Effects of soft-diet feeding on BDNF expression in hippocampus of mice. Bull. Tokyo Dent. Coll. 2008, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Okihara, H.; Ogawa, T.; Ishidori, H.; Misawa, E.; Kato, C.; Ono, T. Pre-Adolescent Diet Normalization Restores Cognitive Function in Young Mice. J. Clin. Med. 2023, 12, 3642. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hirayama, A. Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Res. 2001, 902, 255–263. [Google Scholar] [CrossRef]

| Reference | Sample Characteristics | Experimental Paradigm | Main Findings |
|---|---|---|---|
| [26] | 17 (10M, 7F, aged 20–31) | Chewing moderately hard vs. hard gum (1 Hz) | Bilateral activation in M1, S1, SMA, insula, thalamus, cerebellum; SMA and insula more active with moderately hard gum; cerebellum more active with hard gum. |
| [27] | 16 (10F, 6M, aged 23–38) | Chewing based on chewing-side preference | Contralateral SI/MI activation; left CSP → right substantia nigra; right CSP → left cerebellum; activation in IFG, IPL, and left insula. |
| [28] | 9 (6M, 3F, aged 22–31) | Finger movement with and without chewing | Reduced SM1 activation when chewing added (1296 vs. 2090 voxels); significant decrease in M1 and S1 activation. |
| [29] | 8 (4M, 4F, aged 20–29) | Chewing tasteless gum on right molars | Activation in left hippocampus, entorhinal cortex (BA28), and parahippocampal cortex (BA36); no significant perirhinal cortex activation. |
| [30] | 15 (6F, mean age 25.3) | Unilateral chewing (left and right) | Bilateral activation in S1, S2, M1, SMA, cingulate, insula, thalamus, cerebellum; symmetrical patterns; lateralisation index < 0.055. |
| [31] | 29 (15M, 14F, mean age 24.0) | Right-side chewing, segmental timing analysis | Activation in M1, SMA, cerebellum, caudate, cingulate gyrus; early chewing → frontal regions; later chewing → cerebellum and superior temporal gyrus. |
| [32] | 12 (6M, 6F, aged 20–28) | Gum chewing vs. sham chewing | SMA, PMA, insula, SPL, frontal and parietal lobes more active in real chewing than sham chewing. |
| [33] | 10 (5R, 5L-handed, ~28.2 years) | Chewing soft vs. hard bolus in varied posture | Right-handed → right hemisphere dominance; left-handed → left hemisphere; soft bolus activated more cortical areas; bilateral SMA, M1, S1, Broca’s, insula. |
| [34] | 16 (6F, mean age 22.7) | Stress (noise) with/without chewing | Chewing reduced activation in STS and AI during noise; reduced AI–dACC connectivity; gum disrupted transmission of stress-related signals. |
| [35] | 17 (8F, aged 20–34) | Attention Network Task with/without chewing | Chewing improved reaction time; increased ACC and frontal gyrus activity; anterior cerebellum showed reduced activity during chewing. |
| [36] | 29 (13F, mean age 23) | Odor perception in gum users vs. non-users | High-frequency gum users → more trigeminal activation (midcingulate, SMA, pre/postcentral); low-users → more hippocampus and OFC activity. |
| [37] | 32 (11M, 21F, aged 18–50) | Spontaneous vs. controlled chewing + rosary task | Cerebellum activated during rhythmic chewing and rosary pulling; not during voluntary chewing; M1, S1, and premotor areas activated in all chewing tasks. |
| [38] | 27 (3 age groups: YA, MA, EA) | Gum chewing across age groups | M1, thalamus, cerebellum activation decreased with age; right prefrontal activation increased with age; no age effects in SMA or insula. |
| [39] | 38 (from 60, aged 18–35) | Natural chewing vs. rest using Group ICA | ICA revealed 3 networks: (1) sensorimotor, (2) cognitive–emotional (ACC, BA9/10), (3) syntax-related (IFG, BA47); chewing engages multiple functional systems. |
| [40] | 18 (9M, 9F, aged 19–28) | 1 h chewing with ASL perfusion MRI | Increased blood flow in right trigeminal nucleus (Vp); correlated with chewing-side preference and masseter muscle volume. |
| Reference | Sample Characteristics | Experimental Paradigm | Main Findings |
|---|---|---|---|
| [41] | 36 (19M, 17F, mean age 28) | Chewing palatable vs. unpalatable gum | Higher left DLPFC/frontopolar activation with unpalatable gum; no muscle/HR differences |
| [42] | 25 (13M, 12F, mean age 27) | Chewing 3 gums (C, T, TO); TCD + NIRS | TO-gum → ↑ DO2Hb and MCAV; effects lasted 2 min post-task; no EMG differences |
| [43] | 25 (11M, 14F, mean age 27.3) | Free vs. controlled chewing (right side, rhythm) | ↑ MCAV during all chewing; no differences between conditions; muscle activation varied |
| [44] | 8 (mean age 25.3) | Left-side gum chewing; dual-distance probes | ↑ Total-Hb in PFC; superficial muscle activity affected signals in deviated probe |
| [45] | 14 (7M, 7F, mean age 26.9) | Stroop test with/without chewing | ↑ Oxy-Hb in left DLPFC with gum; faster RTs, unchanged accuracy |
| [46] | 12 (10M, 2F, mean age 24.0) | Negative sound exposure ± gum | ↑ PFC activation with gum; enhanced alpha waves; ↑ HR; reduced discomfort |
| [47] | 11 (9M, 2F, mean age 26.8) | Negative sound exposure ± gum | ↑ PFC activation and alpha waves with gum; ↓ STAI anxiety, ↑ VAS comfort |
| [48] | 11 (9F, 2M, mean age 20.9) | Chewing at 3 speeds (30, 70, 110 CPM) | ↑ Oxy-Hb with chewing; highest at 110 CPM; ↓ Deoxy-Hb; no change in total-Hb |
| [49] | 11 (9F, 2M, mean age 20.5) | Uchida-Kraepelin test + chewing (3 speeds) | ↑ Oxy-Hb at 110 CPM; no cognitive performance improvements |
| [50] | 11 males (mean age 29.5) | Gum + walking + pleasant sounds | ↑ PFC activity in gum and walking + gum; ↑ pleasantness in VAS |
| [51] | 10 (5M, 5F, aged 26–37) | 20 min chewing; serotonin + pain analysis | ↑ Oxy-Hb in ventral PFC; ↓ pain reflex; ↑ blood serotonin; no deoxy-Hb change |
| [52] | 30 (11F, 19M, mean age 23.7) | Serial recall task pre/post chewing | ↑ Oxy-Hb during chewing; no change in recall accuracy or reaction time |
| Reference | Sample Characteristics | Experimental Paradigm | Main Findings |
|---|---|---|---|
| [46] | 12 healthy adults (10M, 2F; mean age 24.0 years) | Block-design eyes-closed listening to unpleasant IADS-2 sounds without gum (NS) versus with tasteless gum (NS + Gum) | Alpha-wave appearance rate fell during NS (42.78%) but rose significantly when chewing gum (44.43%; p = 0.0227, Cohen d = 0.85) |
| [47] | 11 healthy adults (9M, 2F; mean age 26.8 years) | Identical unpleasant-sound blocks without vs. with gum under eyes-closed resting instructions | Alpha-wave appearance rate increased from 44.00% (NS) to 47.10% (NS + Gum; p < 0.05), showing chewing-related attenuation of stress-induced alpha suppression |
| [53] | 40 right-handed adults | Vigilance task with chewing vs. no chewing, 4 sessions (baseline, during, post) | ↓ Reaction times, ↑ correct detections during chewing; ↑ beta power (F7, T3) post-chewing; ↑ HR during chewing; alertness maintained; EEG and HR effects were temporary |
| [54] | 20 (11M, 9F), aged 24–34 | Chewing gum base, gum + spearmint, gum + sucrose; aroma and ingestion control | Gum base: ↑ beta, ↓ theta → arousal; Spearmint: ↑ beta, ↓ alpha → stimulation; Gum + sucrose: ↑ theta, ↓ beta → relaxation; aroma mirrored spearmint effects; sucrose alone = no change |
| [55] | 11 (7M, 4F), aged 24–32 | Resting EEG vs. post-chewing spearmint gum for 3 min | ↑ Alpha in most brain regions (O, T, F, P); interpreted as arousal and alertness effect; bilateral activation; no hemispheric asymmetry |
| [56] | 9 (6M, 3F), aged 27–33 | Chewing flavoured vs. unflavoured gum; inhaling flavoured oil | Flavoured gum: ↑ alpha and beta, ↓ theta → arousal; Unflavoured gum: ↑ alpha, ↓ beta → relaxation; oil had similar effects as flavoured gum, showing influence of both flavour and mastication |
| [57] | 20 males, mean age 24.9 ± 4.9 | Chewing gum base, flavoured gum, theanine gum; EEG source localisation + VAS ratings | Flavoured gum → anterior/right source shift (↑ alpha-2, beta-2); gum base → posterior/left; ↑ Global Field Power for delta-theta, alpha-2, beta-1 after flavoured gum; ↑ refreshment and comfort ratings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, J.; Malinowska, A. The Neural Correlates of Chewing Gum—A Neuroimaging Review of Its Effects on Brain Activity. Brain Sci. 2025, 15, 657. https://doi.org/10.3390/brainsci15060657
Chmiel J, Malinowska A. The Neural Correlates of Chewing Gum—A Neuroimaging Review of Its Effects on Brain Activity. Brain Sciences. 2025; 15(6):657. https://doi.org/10.3390/brainsci15060657
Chicago/Turabian StyleChmiel, James, and Agnieszka Malinowska. 2025. "The Neural Correlates of Chewing Gum—A Neuroimaging Review of Its Effects on Brain Activity" Brain Sciences 15, no. 6: 657. https://doi.org/10.3390/brainsci15060657
APA StyleChmiel, J., & Malinowska, A. (2025). The Neural Correlates of Chewing Gum—A Neuroimaging Review of Its Effects on Brain Activity. Brain Sciences, 15(6), 657. https://doi.org/10.3390/brainsci15060657







